Abstract
KAP1 is a universal corepressor for Kruppel-associated box zinc finger proteins. In this study, expression level of KAP1 and its association with drug resistance and expression of P-gp and BCRP in epithelial ovarian cancer were investigated. Immunohistological staining of KAP1 in cancer and matched paraneoplastic tissues was evaluated in 242 patients with epithelial ovarian cancer. Immunohistological staining of P-gp and BCRP were also evaluated, and the associations with the expression of KAP1 in epithelial ovarian cancer were investigated. MTT assay for cell proliferation and clonogenic survival assay were applied to determine the effect of KAP1 on the sensitivity of DDP, through up-regulating the level of KAP1 expression of SKOV3 using KAP1 plasmid and down-regulating the level of KAP1 expression of SKOV3/DDP using siRNA. The results demonstrated that the expression levels of KAP1 in cancer tissues were higher than matched paraneoplastic tissues (t = 21.39, P<0.001). The patients with higher KAP1 expression often had drug resistance, and the level of KAP1 expression was positively correlated with the expression of P-gp and BCRP (P = 0.07 and P<0.001 respectively). Up-regulated the expression of KAP1 in SKOV3 cell line induced the up-regulated expression of BCRP and P-gp, increasing the resistance of chemotherapeutic drug, and down-regulated the expression of KAP1 got opposite effects. KAP1 expression correlated with aggressive clinical features in ovarian cancer, maybe through regulating the expression of P-gp and BCRP.
Keywords: KAP1, Ovarian cancer, P-gp, BCRP, drug resistance
Introduction
Ovarian cancer is one of the most fatal malignancies in women and is the leading cause of gynecological cancer deaths [1]. About 22,240 women were diagnosed with invasive epithelial ovarian cancer in the United States in 2013 [2]. Because ovarian cancer locate deep within the pelvis and is difficult to touch, as well as it has no early typical symptoms and effective diagnostic methods, most patients with ovarian cancer are diagnosed at the advanced stage with metastatic functions leading to its poor prognosis [3]. Therefore, better targeted therapies and biomarkers for diagnosis or prognosis are urgently needed [4,5].
In spite of advances in treatment of ovarian cancer over the past decade, the cure rate has improved modestly [6], which may be explained by resistance to chemotherapeutic treatment. Decreased drug uptake, activation of detoxifying systems, activation of DNA repair mechanisms, intracellular changes that raise the apoptotic threshold, alteration of target proteins, or increased drug efflux, individually or synergistically attributed to multidrug resistance (MDR) [7]. P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) were the best characterized efflux pumps that mediate MDR and belonged to the ATP-binding cassette (ABC) protein superfamily [8,9]. By utilizing ATPs, these proteins acquire the ability of pumping a wide variety of compounds out of the cell, which inducing MDR [10].
KRAB-associated protein 1 (KAP1, also known as TRIM28 and TIF1b), is a well-known transcriptional corepressor of Kruppel-associated box zinc finger protein [11]. KAP1 is an essential partner in several multiple-protein complexes and involves in a wide range of biological processes [12]. It is critical for epigenetic stability during mouse oocyte to embryo transition [13] and convergent extension of extra-embryonic tissues [14]. Moreover, the CBF-A/KAP1/FTS-1 complex activates FSP-1 transcription and subsequently epithelial-mesenchymal transition [15]. Additionally, phosphorylation of KAP1-Serine 824 (Ser824) by ataxia-telangiectasia-mutated (ATM) kinase leads to increased expression of KAP1 target genes controlling DNA damage repair in response to genotoxic stress [16,17], but there was no papers about the correlation of KAP1 and drug resistance.
Although a growing number of studies have demonstrated the function of KAP1, few reports have shown the expression status of KAP1 in ovarian cancer and any clinical significance associated with KAP1 expression. We have found that KAP1 was expressed highly in ovarian cancer tissues [18], in this paper we increased the number of cases to evaluate it, and detected the correlation of KAP1 and drug resistance. We reported here KAP1 was high expressed in ovarian cancer tissues. Then we found that KAP1 was positively correlated with P-gp and BCRP, indicating that KAP1 could regulate the expression of P-gp and BCRP leading to the drug resistance and poor survival.
Materials and methods
Patients and tissue samples
242 ovarian epithelial cancers and matched paraneoplastic tissues were obtained from the Department of Pathology, Tianjin Cancer Hospital, Tianjin Medical University of 2005-2009. All tissues were examined by specialists to make a final diagnosis using the World Health Organization criteria. The classification of cancer stage and grade was according to the International Federation of Gynecology and Obstetrics (FIGO, 2009).
Drug resistance related clinical and pathological parameters
Clinicopathological data were collected including age, histology type, ascites, metastases status, tumor grade and chemotherapy scheme. There were 198 patients receiving chemotherapy (Taxo/cisplatin or paclitaxel/cisplatin). All patients’ characteristics were summarized in Table 1. Concerning the chemotherapy response, 198 patients were divided into three groups, chemotherapy resistant group (62 cases), partial sensitive group (51 cases) and sensitive group (85 cases) according to the guideline of National Comprehensive Cancer Network (NCCN) (recurrence during the chemotherapy period or within 6 months after the chemotherapy was define as drug resistance group; recurrence between 6 to 12 months was partial sensitive group after the chemotherapy; recurrence beyond 12 months after the chemotherapy or didn’t recurrence was sensitive group).
Table 1.
Patients’ characteristics
| Variables | Sensitive | Partial Sensitive | Resistance | |
|---|---|---|---|---|
|
| ||||
| n=85 | n=51 | n=62 | ||
| Age (years) | <55 | 37 | 27 | 32 |
| ≥55 | 48 | 24 | 30 | |
| Stage | Early (stageI-II) | 51 | 25 | 24 |
| Advanced (stageIII-IV) | 34 | 26 | 38 | |
| Grade | I | 22 | 7 | 4 |
| II | 28 | 15 | 18 | |
| II | 35 | 29 | 40 | |
| Ascites | No | 56 | 22 | 28 |
| Yes | 39 | 29 | 34 | |
| Metastases | Negative | 52 | 25 | 12 |
| Positive | 33 | 26 | 50 | |
| Histology type | Serous | 51 | 39 | 20 |
| Endometrioid | 18 | 10 | 34 | |
| Mucinous | 10 | 2 | 2 | |
| Clear cell | 6 | 0 | 6 | |
Antibodies
The primary antibodies, rabbit anti-KAP1 (Santa Cruz Biotechnology), mouse anti-P-gp (Santa Cruz Biotechnology), rabbit anti-BCRP (Santa Cruz Biotechnology), mouse anti-β-actin (Santa Cruz Biotechnology) were used in this study. The secondary antibodies, anti-mouse (Santa Cruz Biotechnology), and anti-rabbit (Zhongshan Goldbridge Biotechnology) were purchased for western blot.
Immunohistochemistry staining and evaluation
Tissue sections were deparaffinized and rehydrated with xylene and graded in alcohol solutions. After washed with PBS, 3% hydrogen peroxide was used to endogenous quench peroxidase activity, and 10 mM citrate buffer (pH 6.0) was used for antigen exposure, boiled for 3 min in an autoclave sterilizer followed by cooling at room temperature for more than 20 min. Then sections were incubated with primary antibody KAP1 (1:75 dilution in antibody diluent, Zhongshan Goldbridge Biotechnology CO., Ltd, Beijing, China) for overnight at 4°C. After rinsing with PBS, sections were incubated with PV6001 or PV6002 (Zhongshan Goldbridge Biotechnology CO., Ltd, Beijing, China) for 30 min at 37°C and stained with DAB for 1 to 2 min. The slides were counterstained with hematoxylin, dehydrated with ethanol, cleared with xylene, and mounted in neutral gum. Control sections were incubated with PBS instead of a primary antibody.
For KAP1 evaluation, the method used in our previous paper was utilized [39]. High-power fields in serial sections were chosen from each slice, scored them, and estimated the mean percentage of chromatic cells. Patients with KAP1 expression levels of ≤50% in tumor tissues were assigned to the low-expression group (n = 105), whereas those with values >50% were assigned to the high-expression group (n = 137). The cutoff between these two groups was defined by the mean value of KAP1 expression in cancerous tissue.
For P-gp and BCRP, both the percentage and intensity were considered in a semi-quantitative assessment. The percentage of positive cells was scored as 0 (0% positive cells), 1 (1-25% positive cells), 2 (26-50% positive cells), 3 (50-75% positive cells), or 4 (>75% positive cells). The intensity of immunostaining was also scored as 0 (negative), 1 (weak), 2 (intermediate) and 3 (strong). The intensity score (0-3) was multiplied by the percentage score (0-4) and a final score was assigned 0 (negative), 1-4 (weak expression), 5-8 (moderate expression), and 8-12 (strong expression). For statistical analysis, samples with scores of 0-4 were considered to show low expression, while those with scores of 5-12 were considered to show high expression.
Plasmids and cell infection
Sequence encoding human KAP1 protein was cloned into the EcoRI-BamHI sites of pCDH-puro-vector. The KAP1 siRNA sequence 5’-GCGATCTGGTTATGTGCAATT-3’, and the control siRNA sequence was synthesized by Shanghai Genechem Co., Ltd (Shanghai, China). The siRNAs were synthesized and subcloned into a lentiviral siRNA vector Plko.1-Amp/puromycin. The day before transfection, 1×105 cells were placed in 35 mm dishes in DMEM supplemented with 10% fetal bovine serum and without antibiotics. The transfected cells were cultured for 48 h, and expression protein level were confirmed with Western-blot analysis in cells.
Western blot
Total protein was obtained using a lysis buffer (1% SDS, 10 Mm Tris-Hcl, pH 7.6, 20 μg/ml aprotinin, 20 μg/ml leupeptin and 1 mM AEBSF) and the protein concentration was measured with Bradford method. Protein were separated on a 10% SDS-PAGE gel and blotted onto a PVDF membrane, which was blocked and incubated with primary and secondary. β-actin was used as an internal control. The bands for samples were analyzed with a gel imaging system (Kodak).
MTT assay for cell viability and proliferation
Stable infected cells were treated with different concentrations of cisplatin, dacarbazine, paclitaxel or staurosporin for 48 h, cell survival detected by MTT normalized to untreated cells.
Seventy-two hours after transfection, siKAP1 and control cells were seeded in sextuplicate in 96 well plates, at a density of 3,000 cells/well and incubated for 0, 24, 48, 72 and 96 hours in normal medium and medium contain cisplatin (DDP) to detect cell proliferation. At the end of incubation, 20 µL of 5 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, Sigma, St. Louis, USA) were added to each well. The plates were incubated in a humidified incubator at 37°C, under 5% CO2 for 4 hours, following which 150 µL dimethyl sulfoxide was added. The plates were gently agitated until the formazan was completely dissolved, and the absorbance was measured at 490 nm wavelength.
Clonogenic survival assay
Viable cells (1000/well) were seeded on 3.5 cm dish and incubated for 7-14 days, and then fixed with methanol and stained with gentian violet. Colonies containing more than 50 cells were scored as surviving cells. Each surviving fractions were corrected using these cell survivals.
Statistical methods
SPSS 16.0 was used to evaluate the data. Paired t test was used to assess the expression level of KAP1 in cancer and paraneoplastic tissues. The χ2 test was used to assess the association of KAP1 expression with P-gp and BCRP. The standard two-tailed t-test was performed to compare the differences of the KAP1 protein in different groups. The significance level was defined as P<0.05.
Results
Higher expression of KAP1 protein was detected in human ovarian cancer tissues than matched paraneoplastic tissues
Immunohistochemistry (IHC) was applied to investigate the expression of KAP1, which showed that KAP1 was expressed in both tumor cells and some mesenchymal cells, and localized to cell nucleus (Figure 1). Then we compared the expression level of KAP1 in ovarian cancer tissues and matched paraneoplastic tissues, the results showed that the expression level of KAP1 was higher in ovarian cancer samples than matched paraneoplastic tissues (Figure 1C; t = 21.39, P<0.001). And it also showed that the level of KAP1 in adjacent normal tissues was higher than cancer tissues in the same breast cancer sample (Figure 1B).
Figure 1.
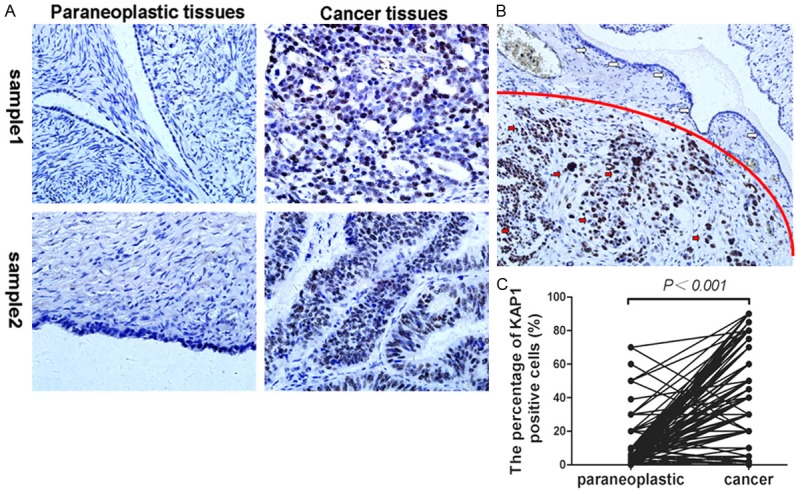
Expression of KAP1 in human non-tumor ovarian tissues and ovarian cancer. A: Representative pictures about KAP1 expression in ovarian cancer tissues and matched paraneoplastic tissues of two cases; B: The expression level of KAP1 in non-tumor cells was lower than tumor cells in the one sample, upper portion was non-tumor cells (white arrow), and under part was cancer cells (red arrow); C: Statistics showed the expression level of KAP1 in cancer tissues was higher than matched paraneoplastic ovarian tissues. (IHC, 400×).
The correlation of KAP1 expression and drug resistance of patients
Now that we have found that the patients with higher level of KAP1 expression often had poor prognosis [18], and the patients with resistance to chemotherapeutic treatment also had poor prognosis, so we thought whether KAP1 could affect chemotherapeutic resistance. In our study there were 198 patients receiving chemotherapy, which were divided into three groups, chemotherapy resistant group (62 cases), partial sensitive group (51 cases) and sensitive group (85 cases). Then we compared the expression level of KAP1 in these three groups. The results showed that the expression level of KAP1 was highest in chemotherapy resistant group, and lowest in sensitive group, and the differences between every group were statistically significant (Figure 2, all P<0.05).
Figure 2.
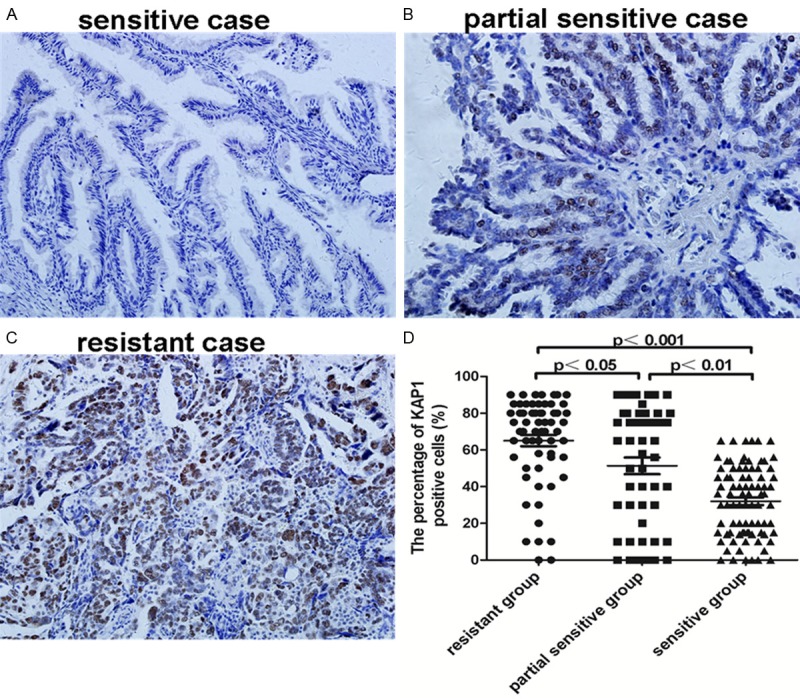
Expression of KAP1 in 198 patients receiving chemotherapy. A: Representative picture in chemotherapy sensitive case; B: Representative picture in chemotherapy partial sensitive case; C: Representative picture in chemotherapy resistance case; D: Statistics showed the expression level of KAP1 in three groups receiving chemotherapy. (IHC, 200×).
The correlation of KAP1 and BCRP expression
Now that the patients with higher level of KAP1 expression often had poor prognosis [18], and the patients with resistance to chemotherapeutic treatment also had poor prognosis, so we thought whether KAP1 could affect chemotherapeutic resistance. Then we detected the expression of BCRP and P-gp, which were important proteins that mediated MDR. The results showed that BCRP were expressed in tumor cells, as well as some mesenchymal cells, and localized to cell cytoplasm and/or membrane (Figure 3A). Importantly, there was a significantly positive correlation between the expression of KAP1 and BCRP, the patients with higher KAP1 expression often had higher BCRP expression (Figure 3B).
Figure 3.
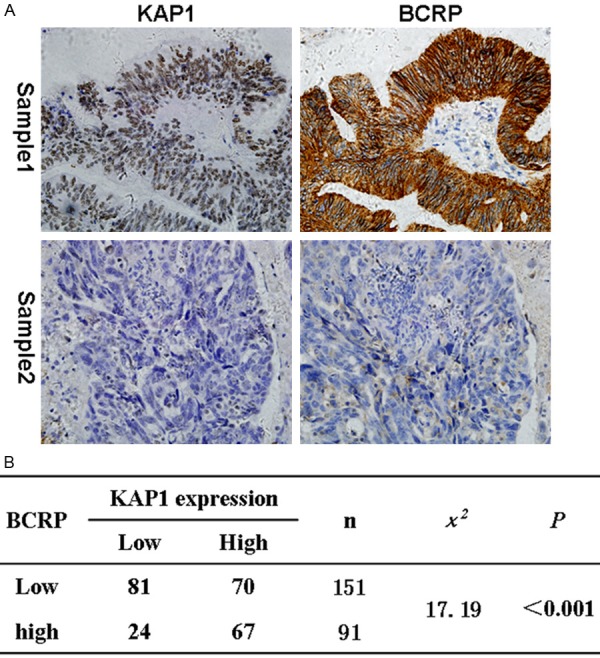
The correlation of KAP1 and BCRP expression. A: Representative IHC pictures about KAP1 and BCRP expression in ovarian cancer tissues (KAP1 and BCRP all higher expressed in sample 1, and all lower in sample 2); B: Statistics showed that there was a positive correlation between the expression level of KAP1 and BCRP. (IHC, 400×).
The correlation of KAP1 and p-gp expression
Then we detected the expression of P-gp, which also was an important protein that mediated MDR. The results showed that a positive correlation between the expression of KAP1 and p-gp was also found, although there is no statistical significance (Figure 4). So we speculated that KAP1 maybe regulate the expression of multi-drug resistance-related proteins.
Figure 4.
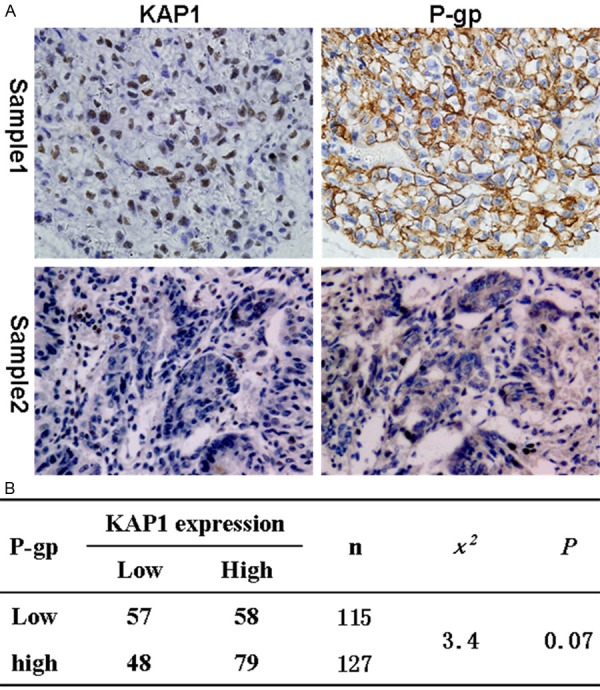
The correlation of KAP1and p-gp expression. A: Representative IHC pictures about KAP1 and P-gp expression in ovarian cancer tissues (KAP1 and p-gp all higher expressed in sample 1, and all lower in sample 2); B: Statistics showed a correlation between the expression level of KAP1 and P-gp. (IHC, 400×).
The expression of KAP1 could regulate the sensibility of chemotherapeutic drugs
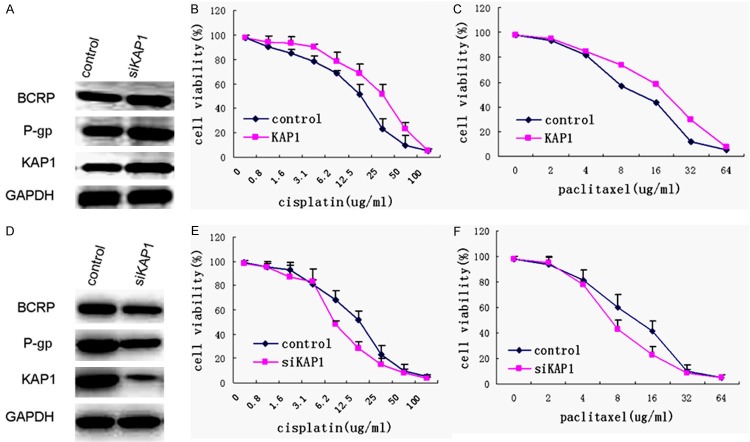
Since the previous results have shown that there were positive correlations between the expression level of KAP1 and BCRP and P-gp, so we speculated that KAP1 maybe regulate the expression of multi-drug resistance-related proteins. First we overexpressed KAP1 in SKOV3 cells, and the results showed that higher level KAP1 could up-regulate the expression of BCRP and P-gp (Figure 5A). Meanwhile, we detected the drug sensitivity in these cell lines. Therefore, SKOV3-KAP1 cell line and control cells were treated with different concentrations of the chemotherapeutic drug cisplatin and paclitaxel, and cell survival was determined normalized to untreated cells respectively after treatment 48 h. The results showed that the cells overexpressed KAP1 were less sensitive to both cisplatin and paclitaxel. In particular, in SKOV3-KAP1 cells the IC-50 value of cisplatin was 28 μg/ml, and the IC-50 value of paclitaxel was 17 μg/ml approximately, but the IC-50 value of cisplatin was 15 μg/ml, and the IC-50 value of paclitaxel was 8 μg/ml approximately in control cells (Figure 5B and 5C).
Figure 5.
The expression level of KAP1 could affect the sensibility of chemotherapeutic drugs. A: Western blot showed that overexpression KAP1 up-regulated the expression level of BCRP and P-gp; B: MTT cell proliferation assay demonstrated that up-regulated KAP1 expression could significantly decrease the cisplatin sensitivity of SKOV3; C: MTT cell proliferation assay demonstrated that up-regulated KAP1 expression could significantly decrease the paclitaxel sensitivity of SKOV3; D: Western blot showed that siKAP1 down-regulated the expression level of BCRP and P-gp; E: siKAP1 could significantly increase the cisplatin sensitivity of SKOV3; F: siKAP1 could significantly increase the paclitaxel sensitivity of SKOV3.
Furthermore, we down-regulated KAP1 in SKOV3 cells using siRNA, and lower levels of BCRP and P-gp were also detected (Figure 5D). We found that the siKAP1cells were more sensitive to both cisplatin and paclitaxel. And in siKAP1 cells the IC-50 value of cisplatin was 6.5 μg/ml, and the IC-50 value of paclitaxel was 5 μg/ml approximately (Figure 5E and 5F).
The cell line SKOV3/DDP, resistance to DDP, with higher KAP1 expression, was interfered with siKAP1 (Figure 6A) in our study to detect the different drug sensibility between SKOV3/DDP -siKAP1 and control cells. The MTT cell proliferation assay demonstrated that down-regulated KAP1 expression could significantly increase the sensitivity of SKOV3/DDP (Figure 6B, P<0.01). After treatment of DDP, the stable KAP1 knockdown group in SKOV3/DDP cell line exhibited a 6-7-fold decrease in cell growth based on the focus formation assay (P<0.01) (Figure 6C). These results suggested that down-regulated expression of KAP1 in SKOV3/DDP cell line induce the down-regulated expression of BCRP and P-gp, and increase the sensitivity of chemotherapeutic drugs.
Figure 6.
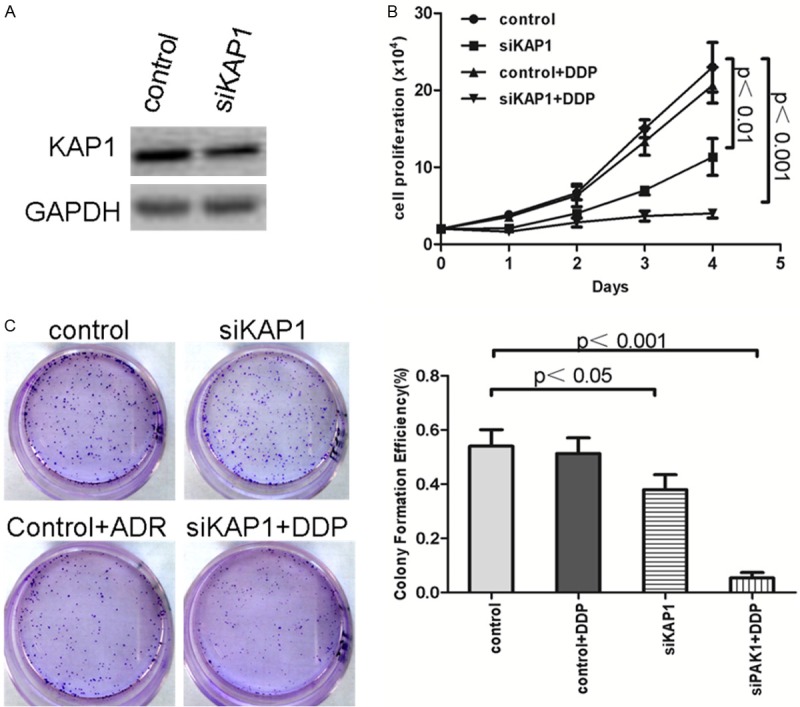
The down-regulated expression of KAP1 could increase the sensibility of chemotherapeutic drug DDP. A: Western blot showed that KAP1 down-regulated in SKOV3/DDP cells; B: MTT cell proliferation assay demonstrated that down-regulated KAP1 expression could significantly increase the sensitivity of SKOV3/DDP; C: Clonogenic survival assay showed 6-7-fold decrease in cell growth in SKOV3/DDP cell line of stable KAP1 knockdown cells than control cells.
In order to verify our results deeply, alternative drug resistance cell line SKOV3/paclitaxel, which was resistant to paclitaxel, was utilized. Using the same research methods, SKOV3/paclitaxel was interfered with siKAP1 (Figure 7A), meanwhile detected the different drug sensibility between SKOV3/paclitaxel-siKAP1 and control cells using MTT and colony formation assay. The results of MTT cell proliferation assay showed that down-regulated KAP1 expression also significantly increased the sensitivity of SKOV3/paclitaxel for paclitaxel (Figure 7B, P<0.001). After treatment of paclitaxel, SKOV3/paclitaxel-siKAP1 cell line exhibited a decreasing in cell growth based on the focus formation assay (P<0.01) (Figure 7C). These results suggested that down-regulated expression of KAP1 in SKOV3 drug resistance cell line could increase the sensitivity of chemotherapeutic drugs.
Figure 7.
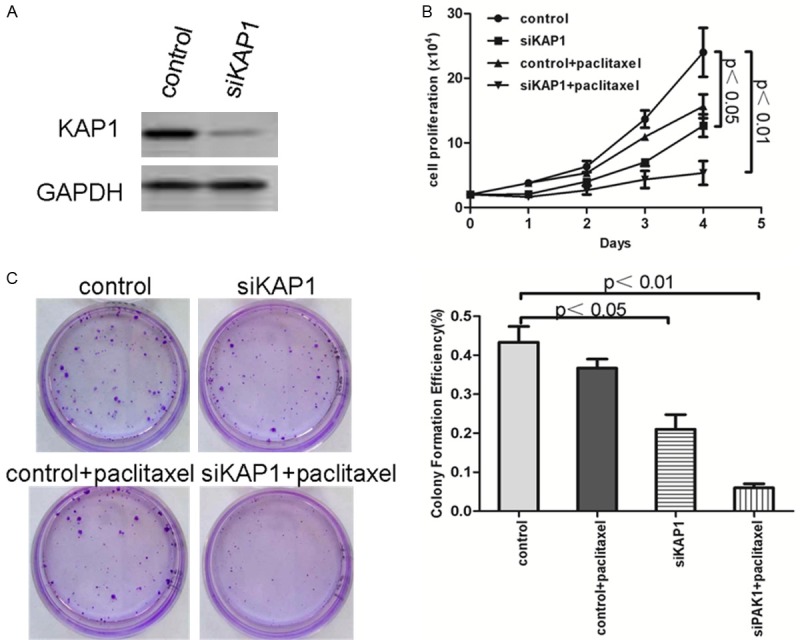
The down-regulated expression of KAP1 could increase the sensibility of chemotherapeutic drug paclitaxel. A: Western blot showed that KAP1 down-regulated in SKOV3/paclitaxel cells; B: MTT cell proliferation assay demonstrated that down-regulated KAP1 expression could significantly increase the sensitivity of SKOV3/paclitaxel; C: Clonogenic survival assay showed 4-5-fold decrease in cell growth in SKOV3/pacliaxel cell line of stable KAP1 knockdown cells than control cells.
Discussion
In our study, we detected KAP1 expression levels by immunohistological stain and investigate the correlation with the expression of BCRP and P-gp. The results showed that the expression levels of KAP1 in ovarian cancer tissues were higher than matched non-tumor ovarian tissues. High levels of KAP1 expression were associated with the expression of BCRP and P-gp.
Because ovarian cancer is located deep within the pelvis and lack of typical early symptoms, many patients are diagnosed at advanced stage, with peritoneal cavity or distant metastasis. So the 5-year survival in patients with advanced ovarian cancer is less than 30% [19]. Although surgical treatment and chemotherapy of ovarian cancer have improved in recent years, the prognosis of ovarian cancer remains poor. The current standard of treatment for primary ovarian cancer is the combination of optimal cytoreductive surgery and platinum-based chemotherapy. Despite state of the art treatment, more than 50% of the patients will relapse and consequently die from this disease [20]. The high recurrence rates suggested development of chemoresistance.
Chemotherapy is one of the principal modes of treatment for ovarian cancer, but the effectiveness of chemotherapy is limited by drug resistance [21,22]. Resistance to chemotherapy and molecularly targeted therapies is a major problem facing current cancer research. A diverse range of molecular mechanisms, one of which was increased rates of drug efflux, have been implicated in drug resistance [23]. Several cell membrane transporter proteins have been linked to resistance to commonly used chemotherapeutics by promoting drug efflux. Most notably, the ATP-binding cassette transporter family of transmembrane proteins, BCRP and P-gp, regulate the flux across the plasma membrane of multiple structurally and mechanistically unrelated chemotherapeutic agents [24]. BCRP lends resistance to many cytostatics, and its role in the resistance of ovarian cancer to topotecan is well described [25]. P-gp overexpression has been associated with chemotherapy failure in many cancers, including ovarian cancer [26].
Chemotherapeutic resistance, particularly in the recurrent setting, plagues the disease. Targeting the pathways and mechanisms behind the development of chemoresistance in ovarian cancer could lead to significant improvement in patient outcomes. In our paper, we found positive correlations between the level of KAP1 expression and the expression of P-glycoprotein, between the level of KAP1 expression and BCRP in ovarian cancer tissues. When knockdown the expression of KAP1 in SKOV3/DDP and SKOV3/paclitaxel cell line, the levels of BCRP and P-gp were also down-regulated, and the cell’s sensitivity for DDP and paclitaxel was recovered. We speculated that KAP1 maybe an important factor that regulates the expression of BCRP and P-gp.
KAP1 is an essential partner in several multiple-protein complexes and involves in a wide range of biological processes [12]. KAP1, a nuclear protein, was thought to function as a co-repressor of the zinc-finger proteins associated with the Kruppel-associated box (KRAB) domain [27]. In mammals, KRAB functions as a transcriptional regulation domain, exhibiting the capacity for long-range transcriptional regulation across much of the genome [28]. In mouse, KAP1 is essential for post-implantation development, normal gene expression, and epigenetic stability during the zygotic transition [29]. KAP1 can also promote ubiquitination and degradation of TP53, a tumor suppressor. If KAP1 could regulate the expression of BCRP and P-gp directly, or regulate their expression indirectly through other molecular mechanism need more experiments to confirm.
In this study, we found that the level of KAP1 expression in ovarian cancer tissues was higher than matched non-tumor tissues, which was also found in other kinds of cancer samples. The upregulation of the KAP1 gene in cancer tissues has been shown in gastric cancer and is associated with poor prognosis [30], and its upregulation was also detected in peripheral blood of gastric cancer patients [31]. Previous studies showed that KAP1 was overexpressed in both liver and peritoneal metastases from patients with colorectal adenocarcinoma, melanoma and malignant thyroid neoplasms [32]. KAP1 showed significant increase in the colorectal carcinomatous epithelium compared with the adenomatous epithelium [33]. The level of serum KAP1 in patients with melanoma has previously been shown to be significantly higher than in controls, leading to the speculation that serum level of KAP1 may be a new useful marker for melanoma progression [33]. The expression of KAP1 gene was significantly higher in cancerous tissues than in noncancerous tissues and was a new marker to predict metastasis and prognosis in early stage non-small cell lung cancer patients [34]. The level of KAP1 mRNA expression was found to be an independent diagnostic marker of malignant thyroid neoplasms [35].
There have been a number of indirect evidences seen in recent studies that suggest KAP1’s role to promote tumorigenesis. KAP1 knockdown resulted in an impaired proliferation rate and increased G1-phase cell cycle fraction [34]. Furthermore, KAP1 was observed to inhibit P53 acetylation and to promote P53 ubiquitination by interacting with MDM2 [36], suggesting KAP1 also plays a role in cancer by inhibiting p53. KAP1 also suppressed the cyclin-dependent kinase inhibitor P21 [37,38]. The results of the present study were consistent with the disruption of the G1/S checkpoint via inhibition of P53 and P21 function. Furthermore, KAP1 may play a role in inducing EMT [15], which is considered to be involved in migration, tumor invasion, and dissemination. Lifang Lin et al. showed the first direct evidence demonstrating that KAP1 has the ability to promote tumor metastasis both in vitro and in vivo [25]. These results indicated that KAP1was a universal corepressor.
In our study, the expression level of KAP1 was higher in ovarian cancer samples than matched non-tumor ovarian tissues, which suggested that KAP1 may play an important role in ovarian cancer genesis. Furthermore, the results in our paper had showed that KAP1 expression correlated with aggressive clinical features and promoted the development of ovarian cancer. Furthermore, KAP1 high expression was an independent predictor for ovarian cancer patients [18]. In this study, the level of KAP1 expression was positively correlated with the expression of P-glycoprotein and BCRP (P = 0.07 and P<0.001 respectively), which suggested that KAP1 could regulate the expression of drug resistance proteins. Additional studies are needed to more clearly and comprehensively articulate the molecular mechanisms of both the cause and the effects of altered expression of KAP1 in the development and/or progression of ovarian cancer.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 81301895), and the Scientific Research Fund Project of Binhai New Area Health Bureau, Tianjin (No. 2011BHKL004).
Disclosure of conflict of interest
None.
References
- 1.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, McGahan CE, Turner D, Marrett L, Gjerstorff ML, Johannesen TB, Adolfsson J, Lambe M, Lawrence G, Meechan D, Morris EJ, Middleton R, Steward J, Richards MA ICBP Module 1 Working Group. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–38. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Cai J, Yang Q, Ding H, Wu L, Li T, Wang Z. Prognostic significance of several biomarkers in epithelial ovarian cancer: a meta-analysis of published studies. J Cancer Res Clin Oncol. 2013;139:1257–77. doi: 10.1007/s00432-013-1435-z. [DOI] [PubMed] [Google Scholar]
- 5.Reade CJ, Riva JJ, Busse JW, Goldsmith CH, Elit L. Risks and benefits of screening asymptomatic women for ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;130:674–81. doi: 10.1016/j.ygyno.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Gómez García I, Kortázar D, Oyenarte I, Mato JM, Martínez-Chantar ML, Martínez-Cruz LA. Purification, crystallization and preliminary crystallographic analysis of protein MJ1225 from Methanocaldococcus jannaschii, a putative archaeal homologue of gamma-AMPK. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:813–7. doi: 10.1107/S1744309109026475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 8.Palmeira A, Sousa E, Vasconcelos MH, Pinto MM. Three decades of P-gp inhibitors: skimming through several generations and scaffolds. Curr Med Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 9.Usuda J, Tsunoda Y, Ichinose S, Ishizumi T, Ohtani K, Maehara S, Ono S, Tsutsui H, Ohira T, Okunaka T, Furukawa K, Sugimoto Y, Kato H, Ikeda N. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer. 2010;67:198–204. doi: 10.1016/j.lungcan.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Goda K, Bacsó Z, Szabó G. Szabo, Multidrug resistance through the spectacle of P-glycoprotein. Curr Cancer Drug Targets. 2009;9:281–97. doi: 10.2174/156800909788166493. [DOI] [PubMed] [Google Scholar]
- 11.Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science. 2012;335:1499–502. doi: 10.1126/science.1216154. [DOI] [PubMed] [Google Scholar]
- 12.Shibata M, Blauvelt KE, Liem KF Jr, García-García MJ. TRIM28 is required by the mouse KRAB domain protein ZFP568 to control convergent extension and morphogenesis of extraembryonic tissues. Development. 2011;138:5333–43. doi: 10.1242/dev.072546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science. 2012;335:1499–502. doi: 10.1126/science.1216154. [DOI] [PubMed] [Google Scholar]
- 14.Shibata M, Blauvelt KE, Liem KF Jr, García-García MJ. TRIM28 is required by the mouse KRAB domain protein ZFP568 to control convergent extension and morphogenesis of extraembryonic tissues. Development. 2011;138:5333–43. doi: 10.1242/dev.072546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, Neilson EG. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007;117:482–91. doi: 10.1172/JCI29544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White DE, Negorev D, Peng H, Ivanov AV, Maul GG, Rauscher FJ 3rd. KAP1, a novel substrate for PIKK family members, colocalizes with numerous damage response factors at DNA lesions. Cancer Res. 2006;66:11594–9. doi: 10.1158/0008-5472.CAN-06-4138. [DOI] [PubMed] [Google Scholar]
- 17.White D, Rafalska-Metcalf IU, Ivanov AV, Corsinotti A, Peng H, Lee SC, Trono D, Janicki SM, Rauscher FJ 3rd. The ATM substrate KAP1 controls DNA repair in heterochromatin: regulation by HP1 proteins and serine 473/824 phosphorylation. Mol Cancer Res. 2012;10:401–14. doi: 10.1158/1541-7786.MCR-11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Y, Yang S, Fu X, Feng J, Xu S, Ying G. High Levels of KAP1 expression are associated with aggressive clinical features in ovarian cancer. Int J Mol Sci. 2014;16:363–77. doi: 10.3390/ijms16010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Liu JY, Song FJ, Chen KX. Advances in circulating microRNAs as diagnostic and prognostic markers for ovarian cancer. Cancer Biol Med. 2013;10:123–30. doi: 10.7497/j.issn.2095-3941.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40:1213–23. doi: 10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Bonneau C, Rouzier R, Geyl C, Cortez A, Castela M, Lis R, Daraï E, Touboul C. Predictive markers of chemoresistance in advanced stages epithelial ovarian carcinoma. Gynecol Oncol. 2015;136:112–20. doi: 10.1016/j.ygyno.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Nuti SV, Mor G, Li P, Yin G. TWIST and ovarian cancer stem cells: implications for chemoresistance and metastasis. Oncotarget. 2014;5:7260–71. doi: 10.18632/oncotarget.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 25.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Helmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–63. [PubMed] [Google Scholar]
- 26.Odening KE, Li W, Rutz R, Laufs S, Fruehauf S, Fishelson Z, Kirschfink M. Enhanced complement resistance in drug-selected P-glycoprotein expressing multi-drug-resistant ovarian carcinoma cells. Clin Exp Immunol. 2009;155:239–48. doi: 10.1111/j.1365-2249.2008.03817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, Rauscher FJ 3rd. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–78. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 28.Cammas F, Mark M, Dollé P, Dierich A, Chambon P, Losson R. Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development. 2000;127:2955–63. doi: 10.1242/dev.127.13.2955. [DOI] [PubMed] [Google Scholar]
- 29.Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science. 2012;335:1499–502. doi: 10.1126/science.1216154. [DOI] [PubMed] [Google Scholar]
- 30.Yokoe T, Toiyama Y, Okugawa Y, Tanaka K, Ohi M, Inoue Y, Mohri Y, Miki C, Kusunoki M. KAP1 is associated with peritoneal carcinomatosis in gastric cancer. Ann Surg Oncol. 2010;17:821–8. doi: 10.1245/s10434-009-0795-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang YY, Li L, Zhao ZS, Wang HJ. Clinical utility of measuring expression levels of KAP1, TIMP1 and STC2 in peripheral blood of patients with gastric cancer. World J Surg Oncol. 2013;11:81. doi: 10.1186/1477-7819-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varghese S, Burness M, Xu H, Beresnev T, Pingpank J, Alexander HR. Site-specific gene expression profiles and novel molecular prognostic factors in patients with lower gastrointestinal adenocarcinoma diffusely metastatic to liver or peritoneum. Ann Surg Oncol. 2007;14:3460–71. doi: 10.1245/s10434-007-9557-7. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery N, McLean MH, El-Omar EM, Murray GI. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology. 2009;54:820–8. doi: 10.1111/j.1365-2559.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Zhao E, Li C, Huang L, Xiao L, Cheng L, Huang X, Song Y, Xu D. TRIM28, a new molecular marker predicting metastasis and survival in early-stage non-small cell lung cancer. Cancer Epidemiol. 2013;37:71–8. doi: 10.1016/j.canep.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kebebew E, Peng M, Reiff E, McMillan A. Diagnostic and extent of disease multigene assay for malignant thyroid neoplasms. Cancer. 2006;106:2592–7. doi: 10.1002/cncr.21922. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Welm B, Boucher KM, Ebbert MT, Bernard PS. TRIM29 functions as a tumor suppressor in nontumorigenic breast cells and invasive ER+ breast cancer. Am J Pathol. 2012;180:839–47. doi: 10.1016/j.ajpath.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YK, Thomas SN, Yang AJ, Ann DK. Doxorubicin down-regulates Kruppel-associated box domain-associated protein 1 sumoylation that relieves its transcription repression on p21WAF1/CIP1 in breast cancer MCF-7 cells. J Biol Chem. 2007;282:1595–606. doi: 10.1074/jbc.M606306200. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto K, Kitabayashi I, Taya Y. KAP1 dictates p53 response induced by chemotherapeutic agents via Mdm2 interaction. Biochem Biophys Res Commun. 2006;351:216–22. doi: 10.1016/j.bbrc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Mo W, Zhao J, Zheng J, Ding W, Zhou J. Clinicopathological features associated with EGFR gene mutation in non-small cell lung cancer patients. Zhonghua Yi Xue Za Zhi. 2014;94:2332–6. [PubMed] [Google Scholar]