Abstract
Purpose: A meta-analysis was undertaken to examine the correlation between ankylosing spondylitis (AS) progression and serum levels of pro-inflammatory cytokines, Interleukin-6 (IL-6) and Interleukin-17 (IL-17) in AS patients. Methods: PubMed, EBSCO, Cochrane Library database, Ovid, Springer link, WANFANG, China national knowledge infrastructure (CNKI) and VIP databases(last updated search in October, 2014) were exhaustively searched for published case-control studies using keywords related to IL-6, IL-17 and AS. The search results were screened using stringent inclusion and exclusion criteria, and the data from selected high-quality studies was analyzed with Comprehensive Meta-analysis 2.0 software. Results: Thirteen case-control studies were selected for this meta-analysis and contained a pooled total of 514 AS patients and 358 healthy controls. Our main result revealed strikingly higher serum levels of IL-6 and IL-17 in AS patients, compared to healthy controls (IL-6: SMD = 2.51, 95% CI = 1.33~3.70, P = 0.01; IL-17: SMD = 3.05, 95% CI = 2.09~4.02, P < 0.001). Ethnicity-based subgroup analysis showed a statistically correlation of high IL-6 and IL-17 serum levels with AS both in Asian (IL-6: SMD = 3.15, 95% CI = 0.75~5.55, P < 0.001; IL-17: SMD = 3.30, 95% CI = 1.93~4.66, P < 0.001) and Caucasian populations (IL-6: SMD = 1.34, 95% CI = 0.33~2.35, P = 0.009; IL-17: SMD = 2.52, 95% CI = 1.06~3.98, P = 0.001). Conclusion: Meta-analysis of pooled data from thirteen high-quality studies revealed a strong correlation between elevated IL-6 and IL-17 serum levels and the development of AS. Therefore, IL-6 and IL-17 could be used as markers for diagnosis and assessment of treatment outcomes in AS patients.
Keywords: Ankylosing spondylitis, interleukin-6, interleukin-17, rheumatism, spondyloarthropathies, meta-analysis
Introduction
Spondyloarthritis (SpA) refers to a group of interrelated inflammatory rheumatic disorders that include ankylosing spondylitis (AS), arthritis associated inflammatory bowel disease (IBD), psoriatic arthritis, reactive arthritis, non-radiographic axial SpA and undifferentiated SpA [1,2]. AS is the most common and a severe form of spondyloarthropathies, and its estimated prevalence is 0.2~0.5% worldwide with a higher prevalence among young adult males [3-5]. The underlying disease mechanisms in AS are related to inflammation, therefore, AS symptoms may also occur outside the spine and joints [6]. Typically, AS affects joints and ligaments of the spine, shoulders, hips and knees [7]. AS is characterized by sacroiliitis and spondylitis, with intense joint pain, stiffness and functional disability in the affected joints [8]. The chronic spinal and extra-spinal infiammation, and progressive irreversible structural damage caused by new bone formation contribute to the morbidity, functional degeneration and socioeconomic burden associated with AS significantly [5,9]. Although the cause of AS is still unknown, a number of studies have clearly demonstrated the correlation of cytokine with disease activity in AS [10,11]. Indeed, pathways involving interleukin-6 (IL-6), interleukin-17 (IL-17), transforming growth factor beta-1, macrophage colony-stimulating factor, vascular endothelial growth factor and soluble Interleukin-2 (IL-2) receptor are suspected to contribute majorly to AS progression [11,12].
Cytokines are proteins that play important roles in pro- and anti-inflammatory pathways, and function as soluble mediators between immune cells to elicit specific immune responses [13]. IL-6 is a pleiotropic cytokine involved in both pro- and anti-inflammatory pathways, metabolic control, bone metabolism, regeneration and neural processes, with prominent role in modulation and growth of various malignancies [14]. Beside these biological activities, IL-6 also displays homeostatic and anti-inflammatory properties in obesity-associated inflammation and exercise [15]. IL-6 is secreted by many cell types, with monocytes, fibroblasts and endothelial cells constituting the main source of IL-6, and cell types such as T-cells, B-cells, osteoblasts and adipocytes producing significant amounts of IL-6 upon stimulation [16]. Importantly, overexpression and abnormal activation of IL-6 signaling pathways is an indicator of aggressive disease course in autoimmunity and cancer [17], and elevated levels of IL-6 is implicated in the pathogenesis of several autoimmune diseases, including AS [18]. IL-17 is important for host defense against bacterial and fungal pathogens, and IL-17 induces the production of pro-inflammatory cytokines, attracting neutrophils and macrophages to inflammation sites [12,19,20]. IL-17 is mainly produced by mast cells, neutrophils and lymphoid-tissue inducer-like cells [21]. AS infiltrating neutrophils express IL-17 and AS patients often exhibit increased IL-17 levels in the synovial fluid [12]. Although IL6 and IL-17 role is suspected in the pathogenesis of AS, the clinical relevance of their serum levels and its implications to AS pathology continues to be hotly debated [10,18,22]. In this context, we undertook a meta-analysis based approach to investigate the relationship between IL-6 and IL-17 serum levels with AS development.
Materials and methods
Literature retrieval and data collection
PubMed, EBSCO, Cochrane Library database, Ovid, Springer link, WANFANG, China national knowledge infrastructure (CNKI) and VIP databases were exhaustively searched to identify published studies relevant to IL-6 and IL-17 levels in AS patients. The database search retrieved studies published prior to October 2014 and the language of publication was restricted to Chinese and English. The search terms were: (“ankylosing spondylitis” or “bechterew’s disease” or “ankylosing spondyloarthritis” or “rheumatoid spondylitis” or “marie-Struempell disease” or “ankylosis spondylitis” or “von bechterew’s disease”), (“Interleukin-6” or “receptors, interleukin-6” or “IL-6” or “IL-6 Receptors”) and (“interleukin-17” or “receptors, interleukin-17” or “IL-17” or “interleukin-17” or “cytokine synthesis inhibitory factor”). We also manually examined the bibliographies of selected studies to identify additional relevant articles.
Inclusion and exclusion criteria
Study selection for meta-analysis was based on the following inclusion criteria: (1), the published study must be a case-control study; (2), study must report the correlation between serum IL-6 and IL-17 levels and AS; (3), study must include AS patients as case group and healthy controls as control group; (4), sufficient information must be available on country, ethnicity, publication year, sample size, gender, IL-6 and IL-17 detection methods, serum IL-6 level sand serum IL-17 levels. The exclusion criteria were: (1), inconsistent diagnostic criteria for AS; (2), studies that are not case-control studies; (3), incomplete original data.
Data collection and quality assessment
The study screening and selection procedures were undertaken independently by two reviewers. The relevant information extracted from finally selected studies included: authors’ information, year, country, ethnicity, sample size, number of patients and controls, gender, age, detection method, serum IL-6 and IL-17 levels. In case of any disagreements during study selection or data extraction, a third investigator was consulted for resolution after careful re-examination of the data. The quality assessment of each eligible study was conducted using Critical Appraisal Skill Program (CASP) criteria.
Statistical analysis
Statistical analysis was performed with Comprehensive Meta-analysis version 2.0 software (Biostat Inc., Englewood, New Jersey, USA). The difference in serum IL-6 and IL-17 levels between the case and control groups was compared by Standardized mean difference (SMD) with 95% confidence intervals (95% CI) [23]. Cochran’s Q-statistic (P < 0.05 was considered significant) and I2 tests were applied to determine heterogeneity [23,24]. In order to evaluate the pooled SMDs, either flxed effect model or random effect model was used based on the presence of heterogeneity [25]. Meta-regression analysis of single-factor and multi-factor were utilized to identify potential sources of heterogeneity and confirmed by Monte Carlo simulation [26,27]. Sensitivity analysis was performed to calculate the influence of one single study in the overall outcomes. Publication bias was evaluated by funnel plot and Egger’s linear regression test to ensure reliability of the results [28]. A bilateral test was conducted, with P ≤ 0.05 considered as being significant.
Results
The database and manual searches retrieved a relatively small total of 286 relevant articles. After eliminating duplicates (n = 20), non-human studies (n = 22), reviews, meta-analyses, letters (n = 28) and studies on unrelated topic (n = 69), 147 full-text articles remained. Thirteen studies [3,10,18,29-38] ultimately satisfied the inclusion criteria after excluding non case-control studies (n = 14), studies that were not relevant to AS (n = 46) or not relevant to IL-6 or IL-17 (n = 69) and studies that contained insufficient information (n = 5). The thirteen selected studies for meta-analysis contained a combined total of 514 AS patients and 358 healthy controls. Sample sizes in the studies varied from 34 to 108 and the studies were published during 1999~2013. Seven studies were performed in Chinese population and the other six studies were performed in Caucasian populations, with one study each from Turkey, Columbia, Spain, Mexico, New Zealand and France. CASP scores for all the eligible studies are shown in Figure 1. Baseline characteristics for the thirteen included studies are presented in Table 1.
Figure 1.
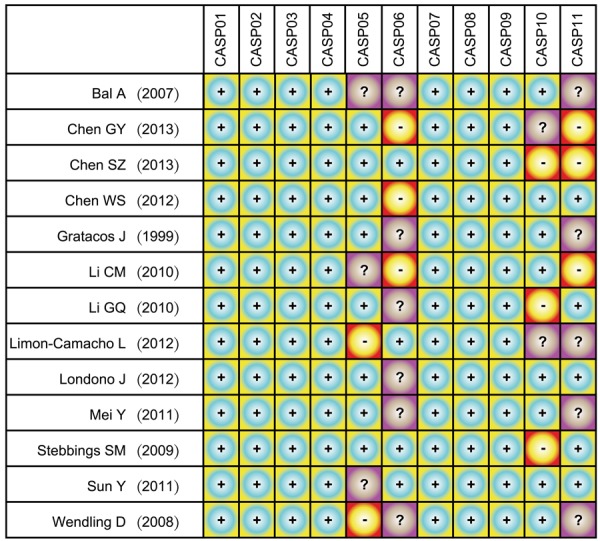
Critical Appraisal Skill Program score for the thirteen eligible studies investigating the correlations of Interleukin-6 (IL-6) and Interleukin-17 (IL-17) serum levels with ankylosing spondylitis.
Table 1.
Baseline characteristics for included studies in present meta-analysis investigating the correlation of Interleukin-6 and Interleukin-17serum levels with ankylosing spondylitis
| First author | Year | Country | Ethnicity | Protein | Sample size | Gender (M/F) | Age (years) | Method | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Case | Control | Case | Control | |||||||
| Chen SZ [31] | 2013 | China | Asians | IL-6 | Large | 52/9 | 30/6 | 25.0 ± 8.2 | 25.0 ± 7.0 | ELISA |
| Chen GY [30] | 2013 | China | Asians | IL-6 | Small | 29/12 | - | 29.5 (15-59) | - | ELISA |
| Londono J [18] | 2012 | Colombia | Caucasians | IL-6 | Large | 43/19 | - | - | - | ELISA |
| Limon-Camacho L [10] | 2012 | Mexico | Caucasians | IL-6 | Small | 38/10 | - | - | - | ELISA |
| Mei Y [38] | 2011 | China | Asians | IL-6 | Large | 41/9 | 35/8 | 28.1 ± 8.9 | 25.3 ± 6.7 | ELISA |
| Li GQ [34] | 2010 | China | Asians | IL-6 | Small | 25/5 | 25/5 | 22.4 ± 5.2 | 23.6 ± 5.9 | ELISA |
| Bal A [29] | 2007 | Turkey | Caucasians | IL-6 | Large | - | - | - | - | ELISA |
| Gratacos J [32] | 1999 | Spain | Caucasians | IL-6 | Small | 12/2 | 15/5 | 33 ± 1 | 31 ± 1 | ELISA |
| Chen SZ [31] | 2013 | China | Asians | IL-17 | Large | 52/9 | 30/6 | 25.0 ± 8.2 | 25.0 ± 7.0 | ELISA |
| Chen GY [30] | 2013 | China | Asians | IL-17 | Small | 29/12 | - | 29.5 (15-59) | - | ELISA |
| Londono J [18] | 2012 | Colombia | Caucasians | IL-17 | Large | 43/19 | - | - | - | ELISA |
| Limon-Camacho L [10] | 2012 | Mexico | Caucasians | IL-17 | Small | 38/10 | - | - | - | ELISA |
| Chen WS [3] | 2012 | China | Asians | IL-17 | Small | - | - | - | - | ELISA |
| Sun Y [36] | 2011 | China | Asians | IL-17 | Small | 15/5 | 6/14 | 24.4 (17-38) | - | ELISA |
| Mei Y [38] | 2011 | China | Asians | IL-17 | Large | 41/9 | 35/8 | 28.1 ± 8.9 | 25.3 ± 6.7 | ELISA |
| Li GQ [34] | 2010 | China | Asians | IL-17 | Small | 25/5 | 25/5 | 22.4 ± 5.2 | 23.6 ± 5.9 | ELISA |
| Li CM [33] | 2010 | China | Asians | IL-17 | Large | 45/17 | 35/10 | 28.6 | 39.1 | ELISA |
| Stebbings SM [35] | 2009 | New Zealand | Caucasians | IL-17 | Small | 14/7 | 14/7 | 44.9 ± 15.1 | 43.4 ± 16.9 | ELISA |
| Wendling D [37] | 2008 | France | Caucasians | IL-17 | Small | - | - | 39.9 | 41.2 | ELISA |
M: male, F: female, ELISA: enzyme-linked immunosorbent assay.
Meta-analysis results for serum level of IL-6
A total of 8 studies reported the correlation between IL-6 serum level and AS. Our meta-analysis observed the existence of heterogeneity in the 8 published studies, thus random effect model was utilized (I2 = 96.5%, P < 0.001). The main result of this meta-analysis showed that serum levels of IL-6 in AS patients were strikingly higher than healthy controls (SMD = 2.51, 95% CI = 1.33~3.70, P < 0.001) (Figure 2B). Subgroup analysis based on ethnicity demonstrated that serum IL-6 level was markedly higher in AS patients, compared with healthy controls, in both Asians (Asians: SMD = 3.15, 95% CI = 0.75~5.55, P = 0.01) and Caucasians (Caucasians: SMD = 1.34, 95% CI = 0.33~2.35, P = 0.009) (Figure 3C). The subgroup analysis based on sample size also suggested that IL-6 serum levels were higher in AS patients, in comparison with healthy controls, whether a large sample size was considered (large sample size: SMD = 2.02, 95% CI = 0.44~3.60, P = 0.012) or a small sample size was used (small sample size: SMD = 3.23, 95% CI = 1.06~5.40, P = 0.003) (Figure 3D).
Figure 2.
Forest plots for correlation of Interleukin-6 (IL-6) and Interleukin-17 (IL-17) serum levels with ankylosing spondylitis (A: Comparison of IL-17 between case and control groups; B: Comparison of IL-6 between case and control groups).
Figure 3.
Forest plots for subgroup analysis on the correlation of Interleukin-6 (IL-6) and Interleukin-17 (IL-17) serum levels with ankylosing spondylitis (A: Subgroup analysis of ethnicity on IL-17; B: Subgroup analysis of sample size on IL-17; C: Subgroup analysis of ethnicity on IL-6; D: Subgroup analysis of sample size on IL-6).
Meta-analysis results for serum level of IL-17
A total of 11 studies examined the association between serum IL-17 level and AS. Heterogeneity was observed among these studies (I2 = 95.5%, P < 0.001), therefore random effects model was employed. The main result of this meta-analysis showed that AS patients exhibited remarkably elevated serum IL-17 levels, compared to healthy controls (SMD = 3.05, 95% CI = 2.09~4.02, P < 0.001) (Figure 2A). Subgroup analysis based on ethnicity revealed that both Asian and Caucasian AS patients showed increased serum IL-17 levels, compared to their healthy counterparts (Asians: SMD = 3.30, 95% CI = 1.93~4.66, P < 0.001 and in Caucasians (Caucasians: SMD = 2.52, 95% CI = 1.06~3.98, P = 0.001) (Figure 3A). The subgroup analysis based on sample size suggested significantly higher serum IL-17 levels in AS patients in both large sample size (large sample size: SMD = 2.69, 95% CI = 1.01~4.37, P = 0.002) and in small sample size (small sample size: SMD = 3.32, 95% CI = 2.02~4.62, P < 0.001) (Figure 3B).
Regression analysis
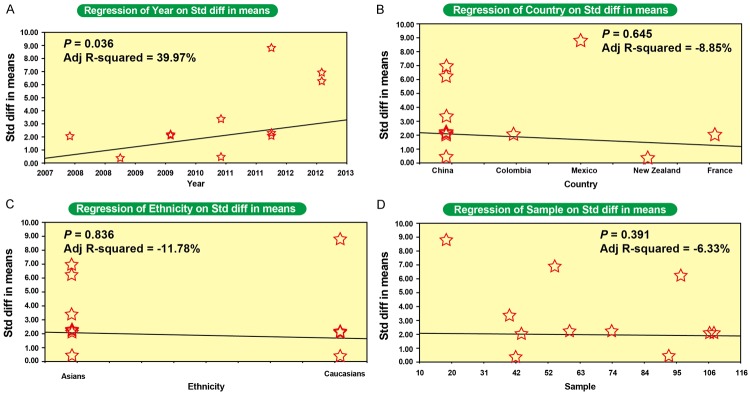
Univariate meta-regression analysis demonstrated that publication year, country, ethnicity and sample size were not the key factors influencing the overall effect size for studies investigating the correlations of serum IL-17 level or serum IL-6 level with AS (both P > 0.05) (Figures 4A-D, 5A-D). As shown in Tables 2 and 3, multivariate meta-regression analysis revealed that publication year, sample size, country, and ethnicity were not the sources of heterogeneity among the studies that investigated the correlations between serum IL-6 and IL-17 levels with AS.
Figure 4.
Meta-regression analysis on the correlation betweenInterleukin-17 (IL-17) serum level and ankylosing spondylitis (A: Meta-regression analysis on year; B: Meta-regression analysis on country; C: Meta-regression analysis on ethnicity; D: Meta-regression analysis on sample).
Figure 5.
Meta-regression analysis on the correlation betweenInterleukin-6 (IL-6) serum level and ankylosing spondylitis (A: Meta-regression analysis on year; B: Meta-regression analysis on country; C: Meta-regression analysis on ethnicity; D: Meta-regression analysis on sample).
Table 2.
Meta-regression analyses on IL-17 of potential sources of heterogeneity investigating the correlation of Interleukin-6 and Interleukin-17serum levels with ankylosing spondylitis
| Heterogeneity factors | Coefficient | SE | t | P (Adjusted) | 95% CI | |
|---|---|---|---|---|---|---|
|
| ||||||
| LL | UL | |||||
| Year | 1.637 | 0.573 | 2.86 | 0.079 | 0.236 | 3.038 |
| Country | 1.183 | 1.315 | 0.90 | 0.741 | -2.035 | 4.401 |
| Ethnicity | -1.986 | 2.916 | -0.68 | 0.852 | -9.121 | 5.148 |
| Sample | -0.031 | 0.025 | -1.26 | 0.513 | -0.091 | 0.029 |
SE: Standard Error, LL: Lower Limit, UL: Upper Limit.
Table 3.
Meta-regression analyses on IL-6 of potential sources of heterogeneity investigating the correlation of Interleukin-6 and Interleukin-17 serum levels with ankylosing spondylitis
| Heterogeneity factors | Coefficient | SE | t | P (Adjusted) | 95% CI | |
|---|---|---|---|---|---|---|
|
| ||||||
| LL | UL | |||||
| Year | 0.647 | 0.736 | 0.88 | 0.684 | -1.695 | 2.99 |
| Country | 1.535 | 3.693 | 0.42 | 0.989 | -10.218 | 13.288 |
| Ethnicity | -2.301 | 6.888 | -0.33 | 0.996 | -24.22 | 19.619 |
| Sample | -0.046 | 0.044 | -1.04 | 0.624 | -0.188 | 0.095 |
SE: Standard Error, LL: Lower Limit, UL: Upper Limit.
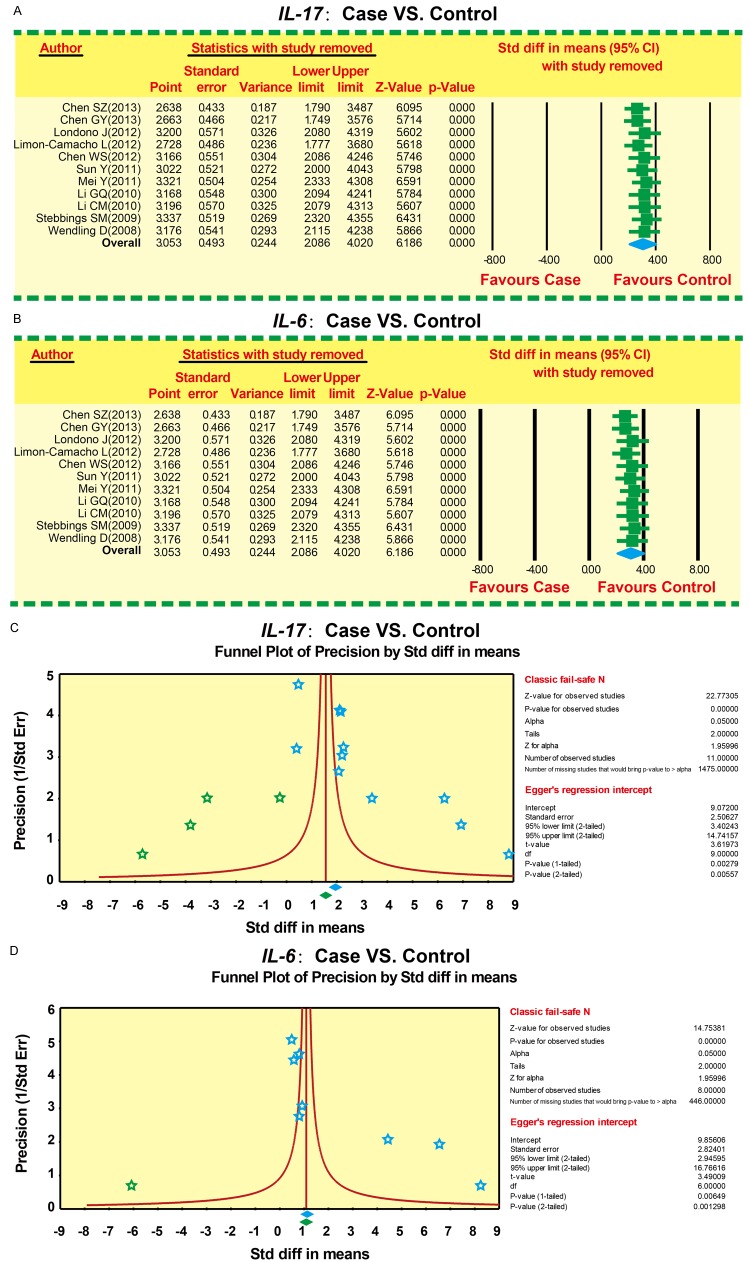
Sensitivity analysis and risk of publication bias
No single study had the weight to significantly impact the pooled SMDs (Figure 6A, 6B). Funnel plots were symmetrically distributed, indicating no publication bias. Egger’s test further confirmed the lack of publication bias (P < 0.05) (Figure 6C, 6D).
Figure 6.
Publication bias and sensitivity analysis on the correlation of Interleukin-6 (IL-6) and Interleukin-17 (IL-17) serum levels with ankylosing spondylitis (A: Sensitivity analysis of IL-17 between case and control groups; B: Sensitivity analysis of IL-6 between case and control groups; C: Publication bias of IL-17 between case and control groups; D: Publication bias of IL-6 between case and control groups).
Discussion
In this study, we undertook a meta-analysis based approach to investigate the significance of elevated serum levels of IL-6 and IL-17 in AS development. Our main results provide convincing evidence that serum levels of IL-6 and IL-17 are substantially higher in AS patients, compared with healthy controls. Recent studies have also examined the impact of cytokines on the pathogenesis of inflammatory arthritis by using detailed measurements of cytokine levels in synovial fluids and serum [39-41]. However, the underlying mechanisms of disease progression remain unresolved. IL-17 is secreted by specialized Th17 subset of CD4+ T cells and is involved in host defense mechanisms against pathogens by inducing synthesis and secretion of pro-infiammatory molecules from flbroblasts, endothelial cells and epithelial cells, including chemokines, antimicrobial peptides and matrix metalloproteinases [12,21,42]. Interestingly, the percentage of Th17 subset was significantly higher in the peripheral blood mononuclear cells (PBMC) of AS patients, compared to healthy controls, and TNF-inhibitors efficiently lowered the Th17 cell numbers to control levels, suggesting that the ongoing inflammatory cascade sustains high Th17 cell numbers in AS patients [10]. Furthermore, in recent years, CD4 + Th17 T cells and interleukin-23 (IL-23)/IL-17 axis has also gained significant attention as therapeutic target in AS and other inflammatory diseases because IL-23 inhibitors dramatically reduce IL-17 serum levels [3]. On the other hand, direct targeting of IL-6 activity is used in treatment strategies against several autoimmune diseases, partly due to the pleotropic functions of IL-6 and its different cognate receptors in various cell types [43,44]. IL-6 also regulates the balance between IL-17-induced Th17 cells and regulatory Tregs [15], therefore therapeutic approaches to neutralize IL-6 activity could potentially benefit AS patients by targeting multiple pathways to halt AS progression. Evidence from animal models also supports this argument since anti-IL-6 based therapeutic strategies in these models indeed show the expected beneficial changes, i.e., increase in Th17 cell differentiation and decrease in Tregs [45]. AS is a chronic inflammatory disease, with a persistent pro-inflammatory environment suitable for differentiation of Th17 and Th1 cells. It is possible that the observed increase in Treg cell numbers is caused by peripheral tolerance mechanisms in response to inflammation, but the suppressive capacity of Treg cells is overwhelmed by the ongoing pro-inflammatory state. Alternately, effect or cells may be resistant to immune regulation, promoting the inflammatory state, as seen in other diseases [46,47]. Our results are consistent with previous studies that showed strikingly elevated serum levels of IL-17 and IL-6 in AS patients, compared to healthy controls, indicating that the two cytokines play a significant role in AS pathogenesis [10,12]. The critical roles played by IL-17 and IL-17 receptor (IL-17RA) regulated pathway in AS progression was further validated in studies that used anti-IL-17RA monoclonal antibodies to inhibit the production of pro-infiammatory cytokines and metalloproteinases, dramatically relieving AS symptoms [48].
To investigate the contribution of other potential factors influencing the correlations of serum level of IL-6 and IL-17 with AS development, subgroup analyses were conducted. Ethnicity-stratified analysis showed a significant association of elevated serum IL-6 and IL-17 levels with AS development in both Asian and Caucasian populations. The subgroup analysis based on sample size also demonstrated that serum IL-6 and IL-17 levels were significantly elevated in AS patients both when the sample size was small and when large sample sizes were considered. Our overall results are consistent with several previous studies, indicating a prominent role for IL-6 and IL-17 in AS progression. Limitations of the present meta-analysis must be acknowledged. First, only studies published in English and Chinese languages were included in this meta-analysis, which may have excluded potentially important studies published in other languages. Second, included studies did not contain information on Th17 cells or other related cytokines, which may have led to an overestimation of the contribution of IL-6 and IL-17 to AS progression. Third, follow-up of treatment response was not performed in the selected studies and the serum IL-17 and IL-6 levels were not recorded after treatment. Finally, an accurate definition or cut-off point for ‘high’ or ‘low’ serum level for IL-6 and IL-17 was not provided in the included studies.
In conclusion, the current study revealed that AS patients exhibited strikingly higher serum levels of IL-17 and IL-6, compared to healthy controls, suggesting that IL-17 and IL-6 may be important players in AS pathogenesis. Therefore, serum IL-17 and IL-6 levels could be important markers for the diagnosis and evaluation of treatment outcomes in AS patients.
Acknowledgements
This study was supported by a grant from National Natural Science Foundation of China (No. 81273709, 81473635) and Doctoral Fund of Ministry of Education of China (No. 20131210110003). We acknowledge the helpful comments on this paper received from our reviewers.
Disclosure of conflict of interest
None.
Abbreviations
- SpA
Spondyloarthritis
- AS
ankylosing spondylitis
- IBD
inflammatory bowel disease
- CNKI
China national knowledge infrastructure
- CASP
Critical Appraisal Skill Program
- SMD
Standardized mean difference
- IL-17RA
IL-17 receptor
References
- 1.Ozgocmen S, Khan MA. Current concept of spondyloarthritis: special emphasis on early referral and diagnosis. Curr Rheumatol Rep. 2012;14:409–14. doi: 10.1007/s11926-012-0274-2. [DOI] [PubMed] [Google Scholar]
- 2.Burgos-Vargas R. The assessment of the spondyloarthritis international society concept and criteria for the classification of axial spondyloarthritis and peripheral spondyloarthritis: A critical appraisal for the pediatric rheumatologist. Pediatr Rheumatol Online J. 2012;10:14. doi: 10.1186/1546-0096-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen WS, Chang YS, Lin KC, Lai CC, Wang SH, Hsiao KH, Lee HT, Chen MH, Tsai CY, Chou CT. Association of serum interleukin-17 and interleukin-23 levels with disease activity in Chinese patients with ankylosing spondylitis. J Chin Med Assoc. 2012;75:303–8. doi: 10.1016/j.jcma.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 4.El Maghraoui A. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. 2011;22:554–60. doi: 10.1016/j.ejim.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, McInnes I, van Laar JM, Landewe R, Wordsworth P, Wollenhaupt J, Kellner H, Paramarta J, Wei J, Brachat A, Bek S, Laurent D, Li Y, Wang YA, Bertolino AP, Gsteiger S, Wright AM, Hueber W. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382:1705–13. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 6.Feng X, Li Y, Gao W. A case report on nephrotic syndrome associated with ankylosing spondylitis effectively treated with infliximab. Int J Clin Exp Med. 2014;7:2936–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Veras MM, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev. 2013;2:CD004524. doi: 10.1002/14651858.CD004524.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun J, van der Horst-Bruinsma IE, Huang F, Burgos-Vargas R, Vlahos B, Koenig AS, Freundlich B. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum. 2011;63:1543–51. doi: 10.1002/art.30223. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Wang P, Li H. The association between TNF-alpha promoter polymorphisms and ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2010;29:983–90. doi: 10.1007/s10067-010-1499-y. [DOI] [PubMed] [Google Scholar]
- 10.Limon-Camacho L, Vargas-Rojas MI, Vazquez-Mellado J, Casasola-Vargas J, Moctezuma JF, Burgos-Vargas R, Llorente L. In vivo peripheral blood proinflammatory T cells in patients with ankylosing spondylitis. J Rheumatol. 2012;39:830–5. doi: 10.3899/jrheum.110862. [DOI] [PubMed] [Google Scholar]
- 11.Mattey DL, Packham JC, Nixon NB, Coates L, Creamer P, Hailwood S, Taylor GJ, Bhalla AK. Association of cytokine and matrix metalloproteinase profiles with disease activity and function in ankylosing spondylitis. Arthritis Res Ther. 2012;14:R127. doi: 10.1186/ar3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, Liao F, Xia G, Wang S, Xu S, Xu J, Zhang L, Ye D. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–73. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 13.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–7. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263–71. doi: 10.1074/jbc.M111.238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 16.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90:484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Londono J, Romero-Sanchez MC, Torres VG, Bautista WA, Fernandez DJ, Quiroga Jde A, Valle-Onate R, Santos AM, Medina JF. The association between serum levels of potential biomarkers with the presence of factors related to the clinical activity and poor prognosis in spondyloarthritis. Rev Bras Reumatol. 2012;52:536–44. [PubMed] [Google Scholar]
- 19.Ji W, Li H, Gao F, Chen Y, Zhong L, Wang D. Effects of glycosides on interleukin-17 and CD4CD25CD127 regulatory T-cell expression in the peripheral blood of patients with ankylosing spondylitis. Biomed Rep. 2014;2:517–20. doi: 10.3892/br.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–21. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebihara S, Date F, Dong Y, Ono M. Interleukin-17 is a critical target for the treatment of ankylosing enthesitis and psoriasis-like dermatitis in mice. Autoimmunity. 2014:1–8. doi: 10.3109/08916934.2014.976630. [DOI] [PubMed] [Google Scholar]
- 22.Ciccia F, Bombardieri M, Principato A, Giardina A, Tripodo C, Porcasi R, Peralta S, Franco V, Giardina E, Craxi A, Pitzalis C, Triolo G. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–65. doi: 10.1002/art.24389. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Manning AK, Dupuis J. A method of moments estimator for random effect multivariate meta-analysis. Biometrics. 2012;68:1278–84. doi: 10.1111/j.1541-0420.2012.01761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 25.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 26.Huizenga HM, Visser I, Dolan CV. Testing overall and moderator effects in random effects meta-regression. Br J Math Stat Psychol. 2011;64:1–19. doi: 10.1348/000711010X522687. [DOI] [PubMed] [Google Scholar]
- 27.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 29.Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R. Comparison of serum IL-1β, sIL-2R, IL-6, and TNF-α levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26:211–5. doi: 10.1007/s10067-006-0283-5. [DOI] [PubMed] [Google Scholar]
- 30.Chen GY, Chen SZ, Su ML, Xie YH. Preliminary study on serum cytokines in patients with ankylosing spondylitis. Chinese Community Doctors. 2013:285–6. [Google Scholar]
- 31.Chen SZ, Bai JP, Xie YH, You YQ, Su ML, Xu XX, Li ZX. Expression of transcription factor Th17, Treg and Th1 inperipheral blood from patients with ankylosing spondylitis and its correlation with disease activity. Chinese Journal of Immunology. 2013;29:834–847. [Google Scholar]
- 32.Gratacos J, Collado A, Pons F, Osaba M, Sanmarti R, Roque M, Larrosa M, Múñoz-Gómez J. Significant loss of bone mass in patients with early, active ankylosing spondylitis: a followup study. Arthritis Rheum. 1999;42:2319–24. doi: 10.1002/1529-0131(199911)42:11<2319::AID-ANR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 33.Li CM, Chen JG, Wang J. Study on Serum IL-17 Levels in Patients with Ankylosing Spondylitis. J Radioimmunol. 2010;23:432–3. [Google Scholar]
- 34.Li GQ, Liu YQ, Zhang Y, Guo SY, Jiu ZG. The effect of leptin on the Th 17 correlated cytokine in ankylosing spondylitis. Journal of Yangzhou University (Agricultural and Life Science Edition) 2010;31:45–9. [Google Scholar]
- 35.Stebbings SM, Taylor C, Tannock GW, Baird MA, Highton J. The immune response to autologous bacteroides in ankylosing spondylitis is characterized by reduced interleukin 10 production. J Rheumatol. 2009;36:797–800. doi: 10.3899/jrheum.080964. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Jiang L, Sun DD, Cui HD, Zhang YL, Zhang YL, Ding N, Wang XF. Study on four kinds of serum IL-17 levels in patients with rheumatic diseases. Chinese Journal of Cellular and Molecular Immunology. 2011;27:85–6+9. [Google Scholar]
- 37.Wendling D, Cedoz JP, Racadot E. Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist therapy. Joint Bone Spine. 2008;75:559–62. doi: 10.1016/j.jbspin.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Gao J, Pang FM, Gr Y, Shen PP, Duan ZH, Wang S, Zeng Z, Li GX, Xu SQ, Zhang L. Clinical features and the mutations screening of a Chinese family with open angle glaucoma. Acta Universitatis Medicinalis Anhui. 2011;46:269–72. [Google Scholar]
- 39.Rosu A, Margaritescu C, Stepan A, Musetescu A, Ene M. IL-17 patterns in synovium, serum and synovial fluid from treatment-naive, early rheumatoid arthritis patients. Rom J Morphol Embryol. 2012;53:73–80. [PubMed] [Google Scholar]
- 40.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 5):v3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 42.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 44.Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, Iwata N, Umebayashi H, Murata T, Miyoshi M, Tomiita M, Nishimoto N, Kishimoto T. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 45.Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 2009;48:602–6. doi: 10.1093/rheumatology/kep028. [DOI] [PubMed] [Google Scholar]
- 46.Lohr J, Knoechel B, Caretto D, Abbas AK. Balance of Th1 and Th17 effector and peripheral regulatory T cells. Microbes Infect. 2009;11:589–93. doi: 10.1016/j.micinf.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012;64:3129–38. doi: 10.1002/art.34565. [DOI] [PubMed] [Google Scholar]
- 48.Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359:419–29. doi: 10.1007/s11010-011-1036-6. [DOI] [PubMed] [Google Scholar]