Abstract
In the peroxisome proliferator-activated receptor gamma (PPARG) gene, a polymorphism (rs1801282 C>G), has been shown to change an amino acid residue and then results in alternation of PPARG function. A number of studies have explored the relationship between PPARG rs1801282 C>G variants and polycystic ovary syndrome (PCOS) risk, but yielding inconsistent findings, especially in Asian population. This study aimed to assess the role of PPARG rs1801282 C>G polymorphism in susceptibility to PCOS. Databases of Pubmed, Embase and China National Knowledge Internet (CNKI) were searched until August 2, 2015. The association of PPARG 1801282 C>G polymorphism with PCOS risk was evaluated by crude odds ratios (ORs) with their 95% confidence intervals (CIs). Finally, there were twenty-three studies involving 3,458 PCOS cases and 3,611 controls included in our pooled analysis. Significant associations were identified between PPARG rs1801282 C>G variants and decreased PCOS risk in three genetic comparison models (OR, 0.78; 95% CI, 0.69-0.89; P < 0.001 for G vs. C; OR, 0.77; 95% CI, 0.68-0.89; P < 0.001 for GG+CG vs. CC and OR, 0.79; 95% CI, 0.68-0.91; P = 0.001 for CG vs. CC). In a subgroup analysis by race, significant correlation was also observed between PPARG rs1801282 C>G variants and decreased PCOS risk in three genetic models: G vs. C (OR, 0.83; 95% CI, 0.71-0.97; P = 0.019) and GG+CG vs. CC (OR, 0.83; 95% CI, 0.70-0.99; P = 0.033) among Caucasians and in one genetic models: G vs. C (OR, 0.72; 95% CI, 0.59-0.88; P = 0.001) among Asians. In summary, our results demonstrate that PPARG rs1801282 C>G polymorphism may be a protective factor for PCOS.
Keywords: Polycystic ovary syndrome, polymorphism, peroxisome proliferator-activated receptor gamma, meta-analysis
Introduction
Polycystic ovary syndrome (PCOS) is characterized by exaggerated production of androgens, ovulatory dysfunction and abnormalities in ovarian morphology, which affects about 5%-10% of women of reproductive age and is a leading cause of infertility [1,2]. PCOS is a common disease which is attributed to a handful of genetic and environmental risk factors. Various PCOS phenotypes may probably result from the interaction between a number of predisposing genomic mutations, each exerting only minor functions and strong environmental influences [3]. Of late, many candidate genes, such as insulin receptor substrate-1/2, follicle stimulating hormone receptor and insulin receptor, have been identified to contribute to PCOS risk [4-8].
Accumulating evidences demonstrate that impaired glucose tolerance, insulin resistance (IR) and type 2 diabetes mellitus (T2DM) are correlated with the development of PCOS [9-11]. Peroxisome proliferator-activated receptor gamma (PPARG), a known nuclear hormone receptor, regulates intracellular insulin-signaling events and controls adipocyte differentiation, lipid and glucose homeostasis. Prior studies have therefore explored the hypothesis that the mutation of PPARG gene involves in the development and progression of PCOS. Some single nucleotide polymorphisms (SNPs) of PPARG gene are deemed to influence the activity of PPARG. PPARG gene is polymorphic, and many SNPs have been identified, such as the rs1801282, rs4135247, rs3856806, rs1175543, rs2938395 and rs709158 polymorphisms, etc. Among these SNPs, the PPARG rs1801282 C>G polymorphism are the most widely studied for the relationship with PCOS susceptibility.
Recently, mounting studies have focused on the relationship of PPARG rs1801282 C>G polymorphism with PCOS. In several previous study, PPARG rs1801282 C>G polymorphism was correlated with decreased risk of PCOS [12,13]; however, an association between this SNP and the increased susceptibility of PCOS was found in another study [14]. Several meta-analyses suggested that PPARG rs1801282 C>G polymorphism was correlated with the decreased susceptibility of PCOS, especially in Caucasians [15,16]; however, in these studies, the included studies were seldom conducted in Asians populations. Now, more studies have focused on the association between PPARG rs1801282 C>G polymorphism and the risk of PCOS in Asians; nevertheless, the result remains inconclusive. Therefore, an updated meta-analysis was conducted to further clarify the role of the PPARG polymorphisms in PCOS risk.
Materials and methods
Search strategy
We extensively searched literatures from PubMed, Embase and China National Knowledge Internet (CNKI) databases (published up to August 2, 2015) using the following words ‘Peroxisome proliferator-activated receptor gamma’, ‘PPARG’ ‘PPARγ’, ‘polymorphism’, ‘SNP’, ‘variant’, ‘polycystic ovary syndrome’, ‘PCOS’. The relevant publications in the references were also manually scanned. If there were overlapping data, only the latest investigation with the larger subjects was recruited.
Inclusion and exclusion criteria
In the current analysis, all publications included had to meet the following criteria: (a) designed as a case-control or a cohort study; (b) assessed the relationship of PPARG rs1801282 C>G polymorphism with PCOS risk; (c) the available frequencies of genotypes or alleles must be provided. The major reasons for exclusion were: (a) incomplete data; (b) overlapping data; (c) only relevant to PCOS treatment; (d) review, editorial, comment, meta-analysis or letter.
Data extraction
In a uniform table, three reviewers (S. Zhang, Y. Wang and H. Jiang) independently extracted the relevant data from all included studies. The following characteristics were extracted: (a) first author, (b) year of publication, (c) country of study, (d) ethnicity, (e) the allele and genotype frequencies, (f) genotyping method, (g) sample size and (H) the evidence of HWE in controls. If disputes generated, they were solved by consulting the third author (W. Tang).
Statistical analysis
In our study, the pooled odds ratios (ORs) with their 95% confidence intervals (CIs) were measured for dominant model, recessive model, heterozygote comparison, homozygote comparison and allelic comparison. A stratified analysis was performed by ethnicity. Heterogeneity among the included studies was evaluated by using a χ 2-test-based Q statistic test. The value of P < 0.1 was considered as a substantial heterogeneity across the publications, then the data were pooled by using the random-effects model (DerSimonian and Laird) [17]; otherwise, the fixed-effects model was harnessed (Mantel-Haenszel) [18]. Sensitivity analysis was conducted to check the stability of this meta-analysis. Potential publication bias was assessed by a funnel plots and Egger’s linear regression test [19]. A P < 0.1 was considered as statistical significance. The distribution of the genotypes in controls was tested for Hardy-Weinberg equilibrium (HWE) using a web-based χ 2 test program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Data analysis was conducted with STATA 12.0 software package (Stata Corp LP, College Station, Texas).
Results
Study characteristics
As summarized in Figure 1, a total of eighty-six potentially relevant publications were retrieved based on the search words from PubMed, Embase and CNKI databases. Finally, there were twenty-three publications [12-14,20-39] involving 3,458 PCOS cases and 3,611 controls included in our analysis. In additional, all subjects were female. As for subjects in these studies, sixteen studies focused on Caucasians [13,20-34] and seven studies focused on Asians [12,14,35-39]. As for HWE test, eighteen studies conformed to HWE [13,22-34,37-39], while five studies deviated from the HWE [14,20,21,35,36]. Characteristics of the included studies and distribution of the PPARG rs1801282 C>G variants as well as alleles are listed in Tables 1 and 2, respectively.
Figure 1.
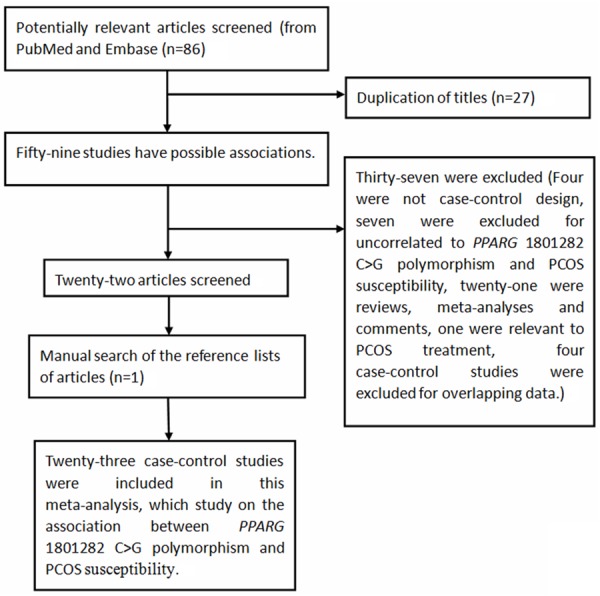
Flow chart shows studies included procedure for meta-analysis.
Table 1.
Characteristics of the included studies
| Study | Year | Country | Ethnicity | Sample size | Genotype method |
|---|---|---|---|---|---|
| Baldani et al. | 2014 | Croatia | Caucasians | 151/179 | TaqMan |
| Yang et al. | 2013 | China | Asians | 120/118 | RFLP |
| Shaikh et al. | 2013 | Indian | Asians | 450/300 | Direct sequencing |
| Hemimi et al. | 2013 | USA | Caucasians | 50/96 | RFLP |
| Dasgupta et al. | 2012 | Indian | Asians | 250/299 | PCR |
| Bidzińska-Speichert et al. | 2011 | Poland | Caucasians | 54/51 | RFLP |
| Christopoulos et al. | 2010 | Greece | Caucasians | 183/148 | RFLP |
| San-Millán et al. | 2010 | Spain | Caucasians | 161/113 | RFLP |
| Chae et al. | 2009 | Korea | Asians | 184/256 | RT-PCR |
| Xita et al. | 2009 | Greece | Caucasians | 180/140 | RFLP |
| Gu et al. | 2009 | Korea | Asians | 238/125 | RFLP |
| Koika et al. | 2009 | Greece | Caucasians | 156/56 | RFLP |
| Zheng et al. | 2008 | China | Asians | 150/135 | RFLP |
| Knebel et al. | 2008 | German | Caucasians | 21/120 | RFLP |
| Antoine et al. | 2007 | USA | Caucasians | 285/187 | TaqMan |
| Guzman et al. | 2007 | Chile | Caucasians | 50/75 | RFLP |
| Yilmaz et al. | 2006 | Turkey | Caucasians | 100/100 | RFLP |
| Wang et al. | 2006 | China | Asians | 201/147 | RFLP |
| Haap et al. | 2005 | German | Caucasians | 57/567 | PCR |
| Hahn et al. | 2005 | German | Caucasians | 102/104 | RFLP |
| Tok et al. | 2005 | Turkey | Caucasians | 60/60 | PCR |
| Oroi et al. | 2004 | Italy | Caucasians | 120/120 | RFLP |
| Korhonen et al. | 2003 | Finland | Caucasians | 135/115 | SSCP |
RT-PCR: real-time PCR; RFLP: restriction fragment length polymorphism; SSCP: single strand conformation polymorphism.
Table 2.
Distribution of PPARG rs1801282 C>G polymorphism genotypes and alleles
| Study | Year | Case | Control | Case | Control | HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| CC | CG | GG | CC | CG | GG | G | C | G | C | |||
| Baldani et al. | 2014 | 106 | 43 | 2 | 129 | 47 | 3 | 47 | 255 | 53 | 305 | Yes |
| Yang et al. | 2013 | 111 | 9 | 0 | 101 | 17 | 0 | 9 | 231 | 17 | 219 | Yes |
| Shaikh et al. | 2013 | 381 | 65 | 4 | 219 | 79 | 2 | 73 | 827 | 83 | 517 | Yes |
| Hemimi et al. | 2013 | 43 | 7 | 0 | 74 | 22 | 0 | 7 | 93 | 22 | 170 | Yes |
| Dasgupta et al. | 2012 | 197 | 36 | 10 | 211 | 53 | 17 | 56 | 430 | 87 | 475 | No |
| Bidzińska-Speichert et al. | 2011 | 35 | 13 | 6 | 30 | 15 | 6 | 25 | 83 | 27 | 75 | Yes |
| Christopoulos et al. | 2010 | 166 | 14 | 3 | 131 | 15 | 2 | 20 | 346 | 19 | 277 | Yes |
| San-Millán et al. | 2010 | 141 | 20 | 0 | 92 | 21 | 0 | 20 | 302 | 21 | 205 | Yes |
| Chae et al. | 2009 | 171 | 11 | 2 | 230 | 23 | 3 | 15 | 353 | 29 | 483 | No |
| Xita et al. | 2009 | 150 | 30 | 0 | 122 | 17 | 1 | 30 | 330 | 19 | 261 | Yes |
| Gu et al. | 2009 | 222 | 16 | 0 | 125 | 0 | 0 | 16 | 460 | 0 | 250 | No |
| Koika et al. | 2009 | 136 | 19 | 1 | 48 | 6 | 2 | 21 | 291 | 10 | 102 | No |
| Zheng et al. | 2008 | 140 | 10 | 0 | 127 | 8 | 0 | 10 | 290 | 8 | 262 | Yes |
| Knebel et al. | 2008 | 17 | 4 | 0 | 99 | 19 | 2 | 4 | 38 | 23 | 217 | Yes |
| Antoine et al. | 2007 | 213 | 52 | 2 | 134 | 29 | 5 | 56 | 478 | 39 | 297 | No |
| Guzman et al. | 2007 | 42 | 7 | 1 | 59 | 14 | 2 | 9 | 91 | 18 | 132 | Yes |
| Yilmaz et al. | 2006 | 85 | 15 | 0 | 78 | 22 | 0 | 15 | 185 | 22 | 178 | Yes |
| Wang et al. | 2006 | 183 | 18 | 0 | 136 | 10 | 1 | 18 | 384 | 12 | 282 | Yes |
| Haap et al. | 2005 | 43 | 9 | 1 | 407 | 133 | 6 | 11 | 95 | 145 | 947 | Yes |
| Hahn et al. | 2005 | 79 | 22 | 1 | 80 | 24 | 0 | 24 | 180 | 24 | 184 | Yes |
| Tok et al. | 2005 | 54 | 6 | 0 | 47 | 13 | 0 | 6 | 114 | 13 | 107 | Yes |
| Oroi et al. | 2004 | 113 | 7 | 0 | 115 | 5 | 0 | 7 | 233 | 5 | 235 | Yes |
| Korhonen et al. | 2003 | 104 | 28 | 3 | 76 | 34 | 5 | 34 | 236 | 44 | 186 | Yes |
HWE: Hardy-Weinberg equilibrium.
Association between PPARG rs1801282 C>G polymorphism and the risk of pcos
A total of 3,458 PCOS cases and 3,611 controls from twenty-three eligible studies were relevant to the relationship between PPARG rs1801282 C>G polymorphism and PCOS risk. In overall meta-analysis, significantly decreased PCOS risk was associated with PPARG rs1801282 C>G variants in three genetic models: G vs. C (OR, 0.78; 95% CI, 0.69-0.89; P < 0.001), GG+CG vs. CC (OR, 0.77; 95% CI, 0.68-0.89; P < 0.001) and CG vs. CC (OR, 0.79; 95% CI, 0.68-0.91; P = 0.001) (Table 3). Additionally, in a subgroup analysis by ethnicity, a significantly decreased PCOS risk was also identified among Caucasians in three genetic models: G vs. C (OR, 0.83; 95% CI, 0.71-0.97; P = 0.019) and GG+CG vs. CC (OR, 0.83; 95% CI, 0.70-0.99; P = 0.033) (Table 3; Figures 2 and 3) and among Asians in one genetic model: G vs. C (OR, 0.72; 95% CI, 0.59-0.88; P = 0.001) (Table 3; Figure 2).
Table 3.
Meta-Analysis of PPARG rs1801282 C>G polymorphism with polycystic ovary syndrome risk
| No. of study | G vs. C | GG vs. CC | GG+CG vs. CC | GG vs. CG+CC | CG vs. CC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||
| OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | ||
| Total | 23 | 0.78 (0.69-0.89) | < 0.001 | 0.458 | 0.66 (0.43-1.02) | 0.060 | 0.962 | 0.77 (0.68-0.89) | < 0.001 | 0.229 | 0.70 (0.46-1.07) | 0.098 | 0.947 | 0.79 (0.68-0.91) | 0.001 | 0.122 |
| Ethnicity | ||||||||||||||||
| Asians | 7 | 0.72 (0.59-0.88) | 0.001 | 0.112 | 0.69 (0.36-1.32) | 0.264 | 0.834 | 0.73 (0.50-1.06) | 0.102 | 0.051 | 0.74 (0.39-1.41) | 0.353 | 0.788 | 0.75 (0.49-1.13) | 0.165 | 0.032 |
| Caucasians | 16 | 0.83 (0.71-0.97) | 0.019 | 0.842 | 0.64 (0.37-1.14) | 0.128 | 0.871 | 0.83 (0.70-0.99) | 0.033 | 0.720 | 0.67 (0.38-1.18) | 0.167 | 0.849 | 0.85 (0.71-1.01) | 0.071 | 0.595 |
| HWE | ||||||||||||||||
| Yes | 18 | 0.76 (0.66-0.88) | < 0.001 | 0.493 | 0.81 (0.46-1.45) | 0.481 | 0.980 | 0.74 (0.63-0.86) | < 0.001 | 0.288 | 0.87 (0.49-1.54) | 0.623 | 0.977 | 0.73 (0.62-0.86) | < 0.001 | 0.206 |
| No | 5 | 0.84 (0.66-1.06) | 0.134 | 0.217 | 0.52 (0.27-0.99) | 0.047 | 0.557 | 0.89 (0.69-1.16) | 0.392 | 0.163 | 0.54 (0.29-1.02) | 0.059 | 0.508 | 0.97 (0.73-1.29) | 0.854 | 0.121 |
HWE: Hardy-Weinberg equilibrium. Bold values are statistically significant (P < 0.05).
Figure 2.
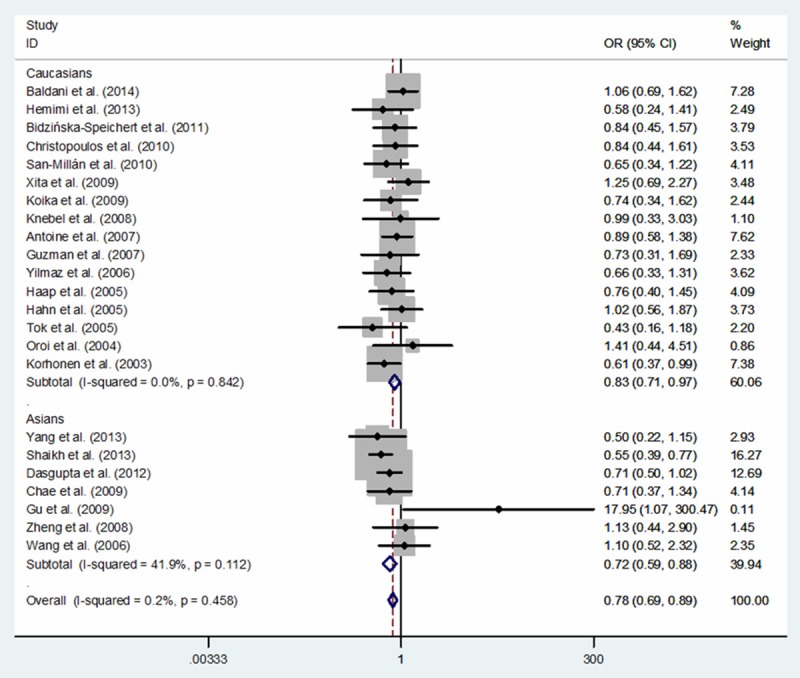
Forest plot of PCOS risk associated with PPARG rs1801282 C>G polymorphism for the G vs. C (fixed effects model).
Figure 3.
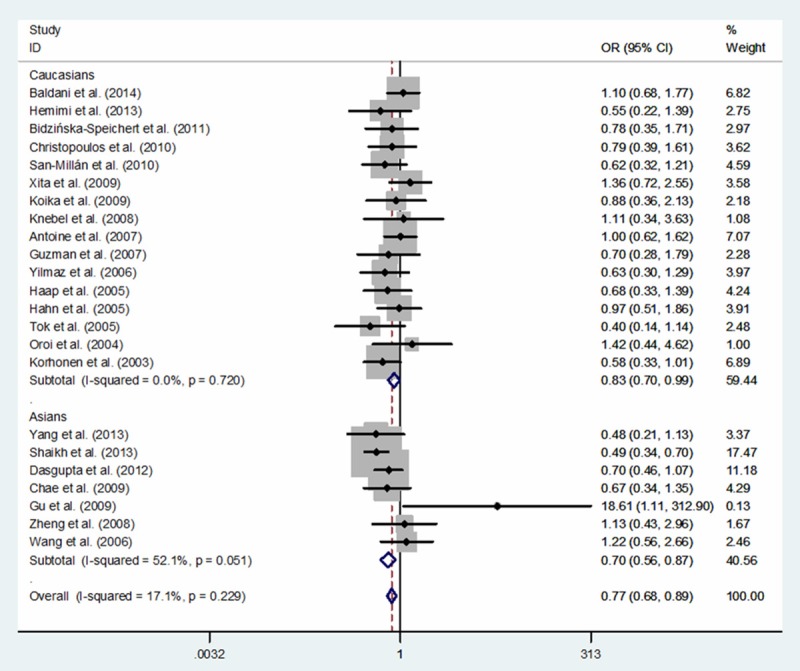
Forest plot of PCOS risk associated with PPARG rs1801282 C>G polymorphism for the GG+CG vs. CC (fixed effects model).
Publication bias and non-parametric ‘trim-and-fill’
Funnel plots and the Egger’s linear regression test were conducted to measure potential publication bias among literatures [19]. A slight publication bias was identified in some genetic comparison models (G vs. C: Begg’s test P = 0.635, Egger’s test P = 0.084; GG vs. CC: Begg’s test P = 0.767, Egger’s test P = 0.925; GG+CG vs. CC: Begg’s test P = 0.492, Egger’s test P = 0.081; GG vs. CG+CC: Begg’s test P = 0.843, Egger’s test P = 0.961; CG vs. CC: Begg’s test P = 0.369, Egger’s test P = 0.090; Figure 4). Thus, non-parametric ‘trim-and-fill’ method was used to calculate the adjusted ORs and CIs. The results also suggested that PPARG rs1801282 C>G variants may be a protective factor for PCOS risk (G vs. C: adjusted pooled OR = 0.76, 95% CI: 0.67-0.86, P < 0.001; GG vs. CC: adjusted pooled OR = 0.67, 95% CI: 0.43-1.04, P = 0.073; GG+CG vs. CC: adjusted pooled OR = 0.74, 95% CI: 0.65-0.85, P < 0.001; GG vs. CG+CC: adjusted pooled OR = 0.71, 95% CI: 0.46-1.09, P = 0.119; CG vs. CC: adjusted pooled OR = 0.713, 95% CI: 0.622-0.818, P < 0.001; Figure 5).
Figure 4.
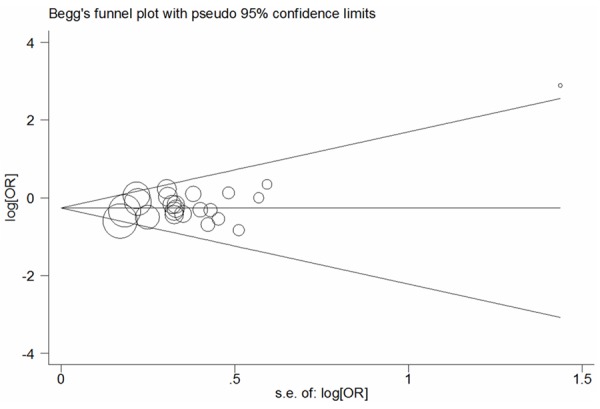
Begg’s funnel plot analysis of PPARG rs1801282 C>G polymorphism with PCOS risk for the G vs. C (fixed-effects model).
Figure 5.
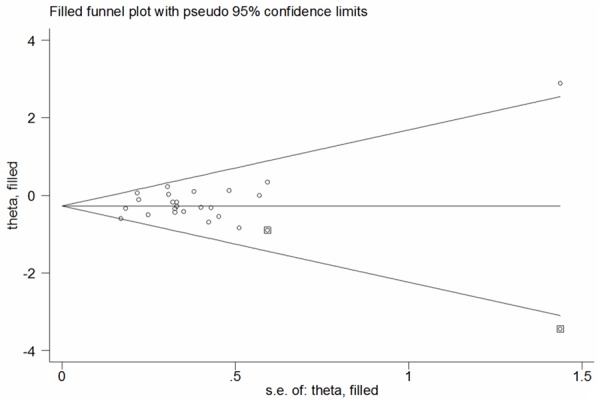
Filled funnel plot of PPARG rs1801282 C>G polymorphism with PCOS risk for the G vs. C (fixed-effects model).
Sensitivity analyses
One-way sensitivity analysis was harnessed to check the stability of our findings. The results demonstrated that there were not significantly altered when anyone was omitted at a time, attesting the robustness of our findings (Figure 6).
Figure 6.
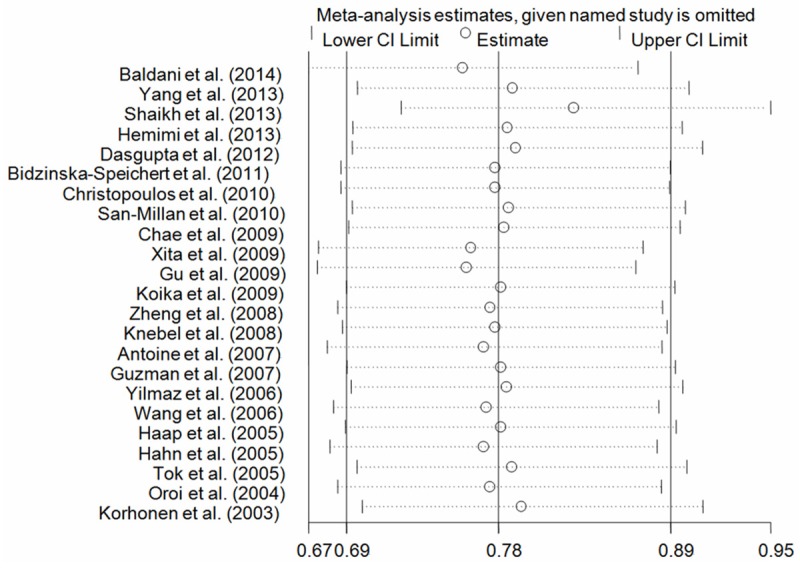
One-way sensitivity analysis of PPARG rs1801282 C>G polymorphism with PCOS risk for the G vs. C (fixed-effects model).
Tests for heterogeneity
Heterogeneity across studies was listed in Table 3. There was no significant heterogeneity in five genetic models, suggesting the robustness of our findings.
Discussion
PCOS is a common cause of infertility, which affects 6-10% of reproductive-aged women [40]. The etiology of PCOS is very complex and was not completely understood. PCOS is characterized by hyperandrogenism, peripheral IR, glucose-stimulated hyperinsulinemia, abnormalities of energy expenditure and dyslipidemia and chronic anovulation [41-43]. Additionally, a higher risk of developing impaired glucose tolerance at a relatively younger age and probably T2DM later in life is found in PCOS cases [44,45]. It is also reported that PCOS patients accompanied with significant IR in a few classic insulin target tissues, including adipocytes and skeletal muscle [46]. In view of these primary findings, the PPARG rs1801282 C>G polymorphism have been extensively studied for the relationship with PCOS susceptibility. Results of previous meta-analyses demonstrated that the PPARG rs1801282 G allele modified the risk of PCOS in Caucasians [15,16]. Recently, more case-control studies were conducted on the association of PPARG rs1801282 C>G polymorphism with PCOS susceptibility; however, the results were inconclusive, especially in Asian population. In this study, we summarized data for 3,458 PCOS cases and 3,611 controls from twenty-three included studies and attempted to examine the risk of PPARG rs1801282 C>G variants to PCOS by an updated meta-analysis. Our findings suggested that PPARG rs1801282 G allele might significantly decrease the risk of PCOS in both Caucasians and Asians.
PPARG, a nuclear hormone receptor, recognizes and binds to the certain PPARG response elements, then regulates the target genes in their promoter region. PPARG plays a vital role in insulin sensitization, lipogenesis, glucose homeostasis, inflammatory cytokine production and cell differentiation [47]. The PPARG rs1801282 C>G variant, a common polymorphism in exon 2 of PPARG gene, encodes a Pro→Ala substitution (Pro12Ala) [48]. As demonstrated in a previous study, the missense substitution of PPARG Pro12Ala could cause less transcriptional activation of target genes in vitro [49], it may presumably affect the risk of PCOS. In combination with this pooled analysis, our findings suggested that PPARG rs1801282 C>G polymorphism may decreased the risk of PCOS, probably through changing binding capacity for PPARG response elements, and then promoting anti-proliferative, pro-differentiation and pro-apoptotic properties. In our study, five studies deviated from the HWE, which showed the presence of population stratification and/or genotyping errors [14,20,21,35,36]. When we omitted these studies, the relationship between PPARG rs1801282 C>G polymorphism and PCOS was also significant with respect to the three genetic models (OR, 0.76; 95% CI, 0.66-0.88; P < 0.001 for G vs. C; OR, 0.74; 95% CI, 0.63-0.86; P < 0.001 for GG+CG vs. CC and OR, 0.73; 95% CI, 0.62-0.86; P < 0.001 for CG vs. CC; Table 3), attesting the robustness of our findings.
Some merits should be mentioned in our study. First of all, the sample sizes of our study were larger than several previous studies [15,16,50]. Secondly, for the first time, we highlighted PPARG rs1801282 G allele decreased the susceptibility of PCOS in Asians. Finally, in current study, there was no significant heterogeneity in all genetic models, suggesting the robustness of our findings. But, some limitations need to be acknowledged. Only published studies were included in our pooled analysis, certain bias may inevitably exist. Moreover, in this study, most of publications were conducted mainly in Caucasians. Only seven studies focused on Asians, which limited the power to measure a real influence. Hence, large-scale studies in Asian populations are needed. Furthermore, due to lack of sufficient information, further evaluation of potential interactions, such as age, family history, and body mass index, was not carried out. In consideration of the complex etiology of PCOS, these factors should not be ignored. Finally, the correlation of other important SNPs of PPARG gene (e.g., rs4135247, rs1175543, rs709158, rs3856806 and rs2938395) with PCOS was seldom studied, these SNPs were not considered in current study.
In summary, our findings highlight that PPARG rs1801282 C>G variants are correlated with a decreased risk of PCOS in both Caucasians and Asians. In the future, larger and well-designed epidemiological studies are definitely demanded to further confirm our findings.
Acknowledgements
This study was supported in part by Jiangsu University Clinical Medicine Science and Technology Development Fund (JLY20140012), National Natural Science Foundation of China (81472332, 81341006), Fujian Province Natural Science Foundation (2013J01126, 2013J05116), Fujian Medical University professor fund (JS12008), The Fund of Union Hospital (2015TC-1-048 and 2015TC-2-004) and Fujian Province Science and Technology Programmed Fund (2012Y0030).
Disclosure of conflict of interest
None.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Pasquali R, Gambineri A. Polycystic ovary syndrome: a multifaceted disease from adolescence to adult age. Ann N Y Acad Sci. 2006;1092:158–174. doi: 10.1196/annals.1365.014. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Piperi C. Genetics of polycystic ovary syndrome: searching for the way out of the labyrinth. Hum Reprod Update. 2005;11:631–643. doi: 10.1093/humupd/dmi025. [DOI] [PubMed] [Google Scholar]
- 4.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villuendas G, Botella-Carretero JI, Roldan B, Sancho J, Escobar-Morreale HF, San Millan JL. Polymorphisms in the insulin receptor substrate-1 (IRS-1) gene and the insulin receptor substrate-2 (IRS-2) gene influence glucose homeostasis and body mass index in women with polycystic ovary syndrome and non-hyperandrogenic controls. Hum Reprod. 2005;20:3184–3191. doi: 10.1093/humrep/dei205. [DOI] [PubMed] [Google Scholar]
- 6.Saxena R, Georgopoulos NA, Braaten TJ, Bjonnes AC, Koika V, Panidis D, Welt CK. Han Chinese polycystic ovary syndrome risk variants in women of European ancestry: relationship to FSH levels and glucose tolerance. Hum Reprod. 2015;30:1454–1459. doi: 10.1093/humrep/dev085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, Wolfe A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63:1270–1282. doi: 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Youngren JF, Dunaif A, Goldfine ID, Maddux BA, Zhang BB, Evans JL. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 2002;87:4088–4093. doi: 10.1210/jc.2002-020363. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood EA, Pasch LA, Shinkai K, Cedars MI, Huddleston HG. Putative role for insulin resistance in depression risk in polycystic ovary syndrome. Fertil Steril. 2015;104:707–714.e1. doi: 10.1016/j.fertnstert.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014;102:1167–1174.e4. doi: 10.1016/j.fertnstert.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Mukherjee A, Shah N, Meherji P, Mukherjee S. Peroxisome proliferator activated receptor gamma gene variants influence susceptibility and insulin related traits in Indian women with polycystic ovary syndrome. J Assist Reprod Genet. 2013;30:913–921. doi: 10.1007/s10815-013-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korhonen S, Heinonen S, Hiltunen M, Helisalmi S, Hippelainen M, Koivunen R, Tapanainen JS, Laakso M. Polymorphism in the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Hum Reprod. 2003;18:540–543. doi: 10.1093/humrep/deg128. [DOI] [PubMed] [Google Scholar]
- 14.Gu BH, Baek KH. Pro12Ala and His447His polymorphisms of PPAR-gamma are associated with polycystic ovary syndrome. Reprod Biomed Online. 2009;18:644–650. doi: 10.1016/s1472-6483(10)60008-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Bi Y, Hu C, Lu W, Zhu D. Association between the Pro12Ala polymorphism of PPAR-gamma gene and the polycystic ovary syndrome: a meta-analysis of case-control studies. Gene. 2012;503:12–17. doi: 10.1016/j.gene.2012.04.083. [DOI] [PubMed] [Google Scholar]
- 16.He J, Wang L, Liu J, Liu F, Li X. A meta-analysis on the association between PPAR-gamma Pro12Ala polymorphism and polycystic ovary syndrome. J Assist Reprod Genet. 2012;29:669–677. doi: 10.1007/s10815-012-9772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua Z, Li D, Xiang G, Xu F, Jie G, Fu Z, Jie Z, Da P, Li D. PD-1 polymorphisms are associated with sporadic breast cancer in Chinese Han population of Northeast China. Breast Cancer Res Treat. 2011;129:195–201. doi: 10.1007/s10549-011-1440-3. [DOI] [PubMed] [Google Scholar]
- 18.Bayram S, Akkiz H, Ulger Y, Bekar A, Akgollu E, Yildirim S. Lack of an association of programmed cell death-1 PD1.3 polymorphism with risk of hepatocellular carcinoma susceptibility in Turkish population: a case-control study. Gene. 2012;511:308–313. doi: 10.1016/j.gene.2012.09.119. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoine HJ, Pall M, Trader BC, Chen YD, Azziz R, Goodarzi MO. Genetic variants in peroxisome proliferator-activated receptor gamma influence insulin resistance and testosterone levels in normal women, but not those with polycystic ovary syndrome. Fertil Steril. 2007;87:862–869. doi: 10.1016/j.fertnstert.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koika V, Marioli DJ, Saltamavros AD, Vervita V, Koufogiannis KD, Adonakis G, Decavalas G, Georgopoulos NA. Association of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma2 with decreased basic metabolic rate in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;161:317–322. doi: 10.1530/EJE-08-1014. [DOI] [PubMed] [Google Scholar]
- 22.Baldani DP, Skrgatic L, Cerne JZ, Ferk P, Simunic V, Gersak K. Association of Pro12Ala polymorphism with insulin sensitivity and body mass index in patients with polycystic ovary syndrome. Biomed Rep. 2014;2:199–206. doi: 10.3892/br.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bidzinska-Speichert B, Lenarcik A, Tworowska-Bardzinska U, Slezak R, Bednarek-Tupikowska G, Milewicz A, Krepula K. Pro12Ala PPAR gamma2 gene polymorphism in women with polycystic ovary syndrome. Ginekol Pol. 2011;82:426–429. [PubMed] [Google Scholar]
- 24.Hemimi NSE, Shaafie IA, Alshawa HHA. Association of genetic polymorphism of peroxisome proliferator-activated receptor-gamma gene and polycystic ovary syndrome. FASEB J. 2013:27. [Google Scholar]
- 25.Christopoulos P, Mastorakos G, Gazouli M, Deligeoroglou E, Katsikis I, Diamanti-Kandarakis E, Panidis D, Creatsas G. Peroxisome proliferator-activated receptor-gamma and -delta polymorphisms in women with polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:185–191. doi: 10.1111/j.1749-6632.2010.05647.x. [DOI] [PubMed] [Google Scholar]
- 26.San-Millan JL, Escobar-Morreale HF. The role of genetic variation in peroxisome proliferator-activated receptors in the polycystic ovary syndrome (PCOS): an original case-control study followed by systematic review and meta-analysis of existing evidence. Clin Endocrinol (Oxf) 2010;72:383–392. doi: 10.1111/j.1365-2265.2009.03679.x. [DOI] [PubMed] [Google Scholar]
- 27.Xita N, Lazaros L, Georgiou I, Tsatsoulis A. The Pro12Ala polymorphism of the PPAR-gamma gene is not associated with the polycystic ovary syndrome. Hormones (Athens) 2009;8:267–272. doi: 10.1007/BF03401274. [DOI] [PubMed] [Google Scholar]
- 28.Knebel B, Janssen OE, Hahn S, Jacob S, Gleich J, Kotzka J, Muller-Wieland D. Increased low grade inflammatory serum markers in patients with polycystic ovary syndrome (PCOS) and their relationship to PPARγ gene variants. Exp Clin Endocrinol Diabetes. 2008;116:481–6. doi: 10.1055/s-2008-1058085. [DOI] [PubMed] [Google Scholar]
- 29.Guzman N, Erices L, Valdes P, Salazar L. A common 34C>G variant at the peroxisome proliferator-activated receptor-2 gene in Chilean women with polycystic ovary syndrome and controls. International Journal of Morphology. 2007;25:7. [Google Scholar]
- 30.Yilmaz M, Ergun MA, Karakoc A, Yurtcu E, Cakir N, Arslan M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma gene in women with polycystic ovary syndrome. Gynecol Endocrinol. 2006;22:336–342. doi: 10.1080/09513590600733357. [DOI] [PubMed] [Google Scholar]
- 31.Haap M, Machicao F, Stefan N, Thamer C, Tschritter O, Schnuck F, Wallwiener D, Stumvoll M, Haring HU, Fritsche A. Genetic determinants of insulin action in polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2005;113:275–281. doi: 10.1055/s-2005-837665. [DOI] [PubMed] [Google Scholar]
- 32.Hahn S, Fingerhut A, Khomtsiv U, Khomtsiv L, Tan S, Quadbeck B, Herrmann BL, Knebel B, Muller-Wieland D, Mann K, Janssen OE. The peroxisome proliferator activated receptor gamma Pro12Ala polymorphism is associated with a lower hirsutism score and increased insulin sensitivity in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2005;62:573–579. doi: 10.1111/j.1365-2265.2005.02261.x. [DOI] [PubMed] [Google Scholar]
- 33.Tok EC, Aktas A, Ertunc D, Erdal EM, Dilek S. Evaluation of glucose metabolism and reproductive hormones in polycystic ovary syndrome on the basis of peroxisome proliferator-activated receptor (PPAR)-gamma2 Pro12Ala genotype. Hum Reprod. 2005;20:1590–1595. doi: 10.1093/humrep/deh769. [DOI] [PubMed] [Google Scholar]
- 34.Orio F Jr, Palomba S, Cascella T, Di Biase S, Labella D, Russo T, Savastano S, Zullo F, Colao A, Vettor R, Lombardi G. Lack of an association between peroxisome proliferator-activated receptor-gamma gene Pro12Ala polymorphism and adiponectin levels in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5110–5115. doi: 10.1210/jc.2004-0109. [DOI] [PubMed] [Google Scholar]
- 35.Chae SJ, Kim JJ, Choi YM, Kim JM, Cho YM, Moon SY. Peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha gene polymorphisms in Korean women with polycystic ovary syndrome. Gynecol Obstet Invest. 2010;70:1–7. doi: 10.1159/000279309. [DOI] [PubMed] [Google Scholar]
- 36.Dasgupta S, Sirisha P, Neelaveni K, Anuradha K, Sudhakar G, Reddy BM. Polymorphisms in the IRS-1 and PPAR-gamma genes and their association with polycystic ovary syndrome among South Indian women. Gene. 2012;503:140–146. doi: 10.1016/j.gene.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Gong H, Liu W, Tao T. The association of Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma2 gene with the metabolic characteristics in Chinese women with polycystic ovary syndrome. Int J Clin Exp Pathol. 2013;6:1894–1902. [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J BH. Peroxisome proliferator-activated receptor-γ2 gene pro12Ala polymorphisms cannot predict ovarian reproductive function through rosiglitazone in women with polycystic ovarian syndrome. Journal of Xi’an Jiaotong University (Medical Sciences) 2008;29:4. [Google Scholar]
- 39.Wang Y, Wu X, Cao Y, Yi L, Fan H, Chen J. Polymorphisms of the peroxisome proliferator-activated receptor-gamma and its coactivator-1alpha genes in Chinese women with polycystic ovary syndrome. Fertil Steril. 2006;85:1536–1540. doi: 10.1016/j.fertnstert.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 40.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, Zapanti ED, Bartzis MI. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 41.Franks S, Gharani N, Waterworth D, Batty S, White D, Williamson R, McCarthy M. The genetic basis of polycystic ovary syndrome. Hum Reprod. 1997;12:2641–2648. doi: 10.1093/humrep/12.12.2641. [DOI] [PubMed] [Google Scholar]
- 42.Tan BK, Heutling D, Chen J, Farhatullah S, Adya R, Keay SD, Kennedy CR, Lehnert H, Randeva HS. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes. 2008;57:1501–1507. doi: 10.2337/db08-0127. [DOI] [PubMed] [Google Scholar]
- 43.Vigil P, Contreras P, Alvarado JL, Godoy A, Salgado AM, Cortes ME. Evidence of subpopulations with different levels of insulin resistance in women with polycystic ovary syndrome. Hum Reprod. 2007;22:2974–2980. doi: 10.1093/humrep/dem302. [DOI] [PubMed] [Google Scholar]
- 44.Celik C, Abali R, Bastu E, Tasdemir N, Tasdemir UG, Gul A. Assessment of impaired glucose tolerance prevalence with hemoglobin A(1)cand oral glucose tolerance test in 252 Turkish women with polycystic ovary syndrome: a prospective, controlled study. Hum Reprod. 2013;28:1062–1068. doi: 10.1093/humrep/det002. [DOI] [PubMed] [Google Scholar]
- 45.Trakakis E, Basios G, Peppa M, Simeonidis G, Labos G, Creatsa M, Misailidou M, Boutati E, Vaggopoulos V, Panagopoulos P, Dimitriades G, Kassanos D. The prevalence of glucose metabolism abnormalities in Greek women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:867–870. doi: 10.3109/09513590.2012.683058. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001;56:295–308. doi: 10.1210/rp.56.1.295. [DOI] [PubMed] [Google Scholar]
- 47.He W. PPARgamma2 polymorphism and human health. PPAR Res. 2009;2009:849538. doi: 10.1155/2009/849538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, Yarnall DP, Burns DK, Roth J, Shuldiner AR. Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun. 1997;241:270–274. doi: 10.1006/bbrc.1997.7798. [DOI] [PubMed] [Google Scholar]
- 49.Masugi J, Tamori Y, Mori H, Koike T, Kasuga M. Inhibitory effect of a proline-to-alanine substitution at codon 12 of peroxisome proliferator-activated receptor-gamma 2 on thiazolidinedione-induced adipogenesis. Biochem Biophys Res Commun. 2000;268:178–182. doi: 10.1006/bbrc.2000.2096. [DOI] [PubMed] [Google Scholar]
- 50.Tang ST, Wang CJ, Tang HQ, Peng WJ, Wang YM, Zhang Q. Association of Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma with polycystic ovary syndrome: a meta-analysis. Mol Biol Rep. 2012;39:9649–9660. doi: 10.1007/s11033-012-1830-6. [DOI] [PubMed] [Google Scholar]


