Abstract
Introduction: With the growing number of immunocompromised patients, the incidence of invasive pulmonary aspergillosis increases. Innate immunity plays a significant role in defensing against fungal infection. Airway epithelial cells induce immune responses like the production of cytokine and chemokine via Dectin-1 signaling pathway in response to Aspergillus fumigatus. Thus, we hypothesized that up-regulation of Dectin-1 on airway epithelium cells would promote the defense against A. fumigatus. Methods: We designed an adenoviral vector encoding full-length Dectin-1, and then transfected it into mice airway epithelial cells via intratracheal injection before the invasion of A. fumigatus. Transfect mice model was verified by using real-time PCR and immunohistochemistry. And also, we studied the effects of up-regulation of Dectin-1 on the production of proinflammatory cytokines, histological changes, fungal burden and survival rate during A. fumigatus infection. Results: The expression level of Dectin-1 in lungs of mice with Dectin-1 recombinant adenoviral vector significantly increased. And also, the mice had higher production of TNF-α, GM-CSF and IL-1β, lower fungal burden, more recruitment of neutrophils into lungs and higher survival rate in response to A. fumigatus infection. Conclusions: The administration of Dectin-1 recombinant adenoviral vector through trachea can elevate the expression of Dectin-1 on airway epithelium, and also, its function during the course of A. fumigatus infection was demonstrated.
Keywords: Dectin-1, lung epithelial cells, Asperguillus fumigatus, adenoviral vector, innate immunity
Introduction
Aspergillus fumigates (A. fumigatus) is a common fungi in our environment. It can be isolated from both indoor and outdoor environment [1,2]. It often can be inhaled and cleared effectively in immunocompetent people [3]. Invasive pulmonary aspergillosis (IPA), characterized by hyphal invasion and necrosis and hemorrhage of lung tissue, is a life-threatening disease among immunocompromised patients [4,5]. Although there are several antifungal agents, it is difficult to carry out the treatment of IPA, and the mortality ranges from 30% to 90% [6]. The defects in immune responses among patients are responsible for bad prognosis.
In the course of defending against A. fumigatus invasion, airway epithelial cells are not just purely physical barrier. They construct the first line defending against Aspergillus, and also, they have the ability to recognized fungal conidia and trigger immune responses similar to those of the innate immune cells. These responses include the endocytosis of conidia and generation of cytokine, reactive oxygen species (ROS) and antimicrobial peptides [7]. Research has already found the up-regulation of Dectin-1 in the human bronchial epithelial cells during A. fumigatus infection [8].
Dectin-1 is a 28-kDa type II transmembrane protein, which mainly expressed on leukocytes surface, such as dendritic cells, neutrophils, and macrophages [9,10]. Dectin-1 is used to recognize several pathogenic fungi. For example, A. fumigatus. And also, it stimulates a variety of cellular responses, including phagocytosis, cytokine production, and the respiratory burst [11]. The mice lacking of Dectin-1 (Dectin-1-/-) gene have a higher mortality rate and a lower cytokine production, resulting in neutrophil accumulation and fungicides impairment [12]. In human bronchial epithelial cells, knockdown of Dectin-1 significantly reduced the A. fumigatus-induced cytokine expression and ROS generation [8].
To our knowledge, investigation into mice model of up-regulation of Dectin-1 on airway epithelial cells has not been reported yet. Both Dectin-1 and epithelial cells play a critical role in defending against A. fumigatus [13]. Adenovirus, a double-stranded DNA virus, which has been widely used as a non-integrating vector in lung, transduces a wide variety of proliferating and non-proliferating cells and shows tropism for airway cells [14]. Thus, we designed an adenoviral encoding full-length Dectin-1, and transfected it to mice airway epithelial cells via intratracheal injection before the invasion of A. fumigatus. Our study aims at exploring whether the up-regulation of Dectin-1 in airway epithelial cells was beneficial during the course of A. fumigatus infection.
Materials and methods
Mice
The BALB/c mice, 8 to 10 weeks of age, 18 to 20 g of weight, were purchased from Yangzhou University, China and were bred with no specific pathogen. All the experiments were approved by the Animal Care and Use Committee of Nanjing University and Jinling Hospital under university-approved standards.
Adenovirus vectors
The Ad-mDectin-1 used in this study was provided by R&S biotechnology Co., Ltd (Shanghai, China) and the final titer is 5×1011 PFU/mL. Mice administrated with PBS or adenoviral encoding green fluorescent protein (Ad-EGFP) were chosen to form the control group. Mice were anesthetized by intraperitoneal injection of 1.8-2 mg of pentobarbital. The trachea was exposed in the standard aseptic environment and 30 ul solution of 3×108 PFU of either Ad-mDectin-1 or Ad-EGFP was delivered via the sterile 26-gauge needle. The skin incision was sutured with surgical staples. Administration of 30 ul solution of PBS was also used the same method.
Aspergillus fumigatus
The strain of A. fumigatus was kindly provided by the Microbiological Laboratory of Jinling Hospital, Nanjing, China. A. fumigatus was cultured on Sabouraud dextrose agar (10 g/L peptone, 40 g/L glucose, and 15 g/L agar) for 7 days at 37°C. Penicillin (12 mg/l) and streptomycin (40 mg/l) were included in the agar to prevent bacterial contamination. Conidia were harvested by washing the surface of the culture plate with sterile PBS supplemented with 0.1% Tween-80. And then, the suspension of conidia was passed through a sterile 40 um nylon membrane, so as to remove the hyphal fragments debris. After that, resuspended it in PBS supplemented with 0.1% Tween-80. The concentration of conidia was enumerated by hemacytometer. Then the suspension was diluted to the concentration we need.
Infection experiment
At first, mice from each group were administrated with PBS, Ad-EGFP, or Ad-mDectin-1, receptively. On the next day, immunocompromised mice were treated with 200 mg/kg of cyclophosphamide (Sigma, St, Louis, MO, USA) by intraperitoneal administration, 4 days before A. fumigatus administration [15]. During the course of infection, the immunocompromised mice were challenged with 3×105 conidia in a volume of 30 ul via intratracheal injection as previously described in adenovirus vector administration.
Lung harvest
The mice were killed at the designated time. Lungs for histologic examination were putted in 4% paraformaldehyde and stained with haematoxylin and eosin (H&E), Gomori methanamine silver (GMS), and immunohistochemistry. Lungs for RNA isolation were stored at -80°C. Lungs for cytokine and myeloperoxidase (MPO) measurements were homogenized and stored at -20°C. Lungs for fungal load were homogenized and inoculated on Sabouraud dextrose agar for 24 hours at 37°C, the results were shown by the numbers of colony forming unit (CFU) per gram of lung tissues.
Immunohistochemistry
After deparaffinization and rehydration, the sections were heated by electromagnetic oven for 10 min in a sodium citrate buffer (0.01 M, pH 6.0) to increase epitope exposure. The slides were treated with 0.3% H2O2 for 10 min to minimize endogenous peroxidase activity, and then blocked with blocking solution (Beyotime, PO102, China) for 30 min at room temperature. The sections were incubated with a multiclonal antibody anti-beta clucan receptor antibody (Dectin-1, Abcam, ab140039). The further operation was performed according to instructions of productions from Ploymer HRP Detection System (ZSGB-BIO, PV-9001, China) and 3,3’-Diaminobenzidine tetrahydrochloride (ZSGB-BIO, ZLI-9032, China). The positive specimen was shown as brown particles. Field of vision was selected randomly and saved under 200 times view, the average optical density of Dectin-1 staining on bronchial wall was analyzed with the help of Image-Pro Plus 6.0.
Cytokine ELISA
The levels of TNF-α, GM-CSF in lung tissues were determined according to the instruction of cytokine specific ELISA kit (R&D Systems, Minneapolis, MN, USA).
Lung MPO assay
Lung MPO activity was measured as a maker of neutrophil sequestration [16]. The MPO Activity Assay kit (Nanjing Jiancheng Bioengineering institute, China) was used for MPO determination. And the activity was measured as it was mentioned previously [17].
RNA isolation, reverse transcription, and real-time PCR
Total RNA was isolated from lung tissues by using RNAiso plus (TaKaRa, 9109, Japan), RNA was reversely transcribed into cDNA by using PrimeScriptTM RT Master Mix (TaKaRa, RR036A, Japan), and the experiment was carried out according to manufacture instructions. Real-time PCR was performed with the help of SYBR Green PCR system (TaKaRa, RR420A, Japan), using β-actin as an internal control for normalization. The forward and reverse primers used for each gene were designed as follows: 5’-TTCAGCACTCAAGACATCCATAA-3’ and 5’-CAGCAACCACTACTACCACAAAG-3’ for Dectin-1; 5’-TCCAGGCGGTGCCTATGT-3’ and 5’-CGATCACCCCGAAGTTCAGTA-3’ for TNF-α; 5’-GGCCTTGGAAGCATGTAGAG-3’ and 5’-GGGGGCAGTATGTCTGGTAG-3’ for GM-CSF; 5’-TGGTGTGTGACGTTCCCATT-3’ and 5’-CAGCACGAGGCTTTTTTGTTG-3’ for IL-1β; 5’-GGTTGCCAAGCCTTATCGGA-3’ and 5’-ACCTGCTCCACTGCCTTGCT-3’ for IL-10; 5’-CTAAGGCCAACCGTGAAAAG-3’ and 5’-TCTCAGCTGTGGTGGTGAAG-3’ for β-actin. Relative quantification was performed by using the formula 2-ΔΔCT.
Survival analysis
For survival studies, we increased the number of conidia used in infection experiment to 5×106. Mice were observed daily so as to get their death rate.
Statistical analyses
SPSS statistical software 13.0 was used for statistical analyses. Data were expressed as mean ± standard deviation. Difference between groups were analyzed by analysis of variance (ANOVA), followed by LSD-t test, Dunnett’s test. Survival analysis was assessed by Kaplan-Meier test. Probability values less than 0.05 were considered to be statistically significant.
Results
The increased expression of Dectin-1 in lung tissue after Ad-mDectin-1 administration
Three days after intratracheal administration of Ad-mDectin-1, we used the lung tissue to determinate the expression of Dectin-1. The Dectin-1 mRNA expression obviously increased in mice treated with Ad-mDectin-1. And its expression level was about 18 times of that of the mice treated with Ad-EGFP or PBS (Figure 1A). And then, we investigated into the distribution of Dectin-1 expression in lungs of each mouse respectively 3 days, 5 days, 7 days and 14 days after administration. In mice treated with Ad-mDectin-1, the immunohistochemistry showed strong immunostaining in the airway epithelium of bronchioles and relatively weak immunostaining in alveolar walls, and the immunostaining of Dectin-1 can last two weeks. However, no immunostaining can be detected from the mice treated with Ad-EGFP or PBS (Figure 1B). The average optical density of Dectin-1 staining on bronchial wall in mice treated with Ad-mDectin-1 showed a time-dependent change. The peak value was reached on the 5th day (Figure 1C).
Figure 1.
The effect of Ad-mDectin-1 on expression of Dectin-1 in the lungs. A. Dectin-1 mRNA expression in the lungs at day 3 after administration of Ad-mDectin-1. Experimental n=5. *P<0.05, compared with mice receiving PBS or Ad-EGFP. B. Immunohistochemical staining of Dectin-1 in lungs at different time points after administration of AdmDectin-1. Original magnification, ×200. C. The results of average optical density of Dectin-1 in mice receiving Ad-mDectin-1 at different time points after administration of Ad-mDectin-1. Experimental n=5. ***P<0.001 compared with data at day 3 and 14 after administration.
Effect of Ad-mDectin-1 on levels of cytokine and chemokine during the course of IPA
The exposure of epithelial cells to A. fumigatus can contribute to the production of inflammatory cytokines and antimicrobial peptides. Dectin-1 was needed for both inflammatory cytokines production and antimicrobial effects of bronchial epithelia against A. fumigatus [8,18,19]. We aimed at figuring out whether up-regulation of Dectin-1 on the airway epithelia cells can protect mice from the invasion of A. fumigatus or not. Although it was already one week after the administration of Ad-mDectin-1, the level of Dectin-1 mRNA was still much higher than that of the control group (Figure 2A). Two days after the administration of conidia, data showed that both the level of TNF-α and GM-CSF in lungs increased significantly in mice treated with Ad-mDectin-1 (Figure 2B-E). We found that the IL-1β mRNA expression level in lungs of mice treated with Ad-mDectin-1 obviously elevated, nearly three times of that of the control group (Figure 2F). No significant differences of IL-10 mRNA were found between Ad-mDectin-1 group and the control group (Figure 2G).
Figure 2.
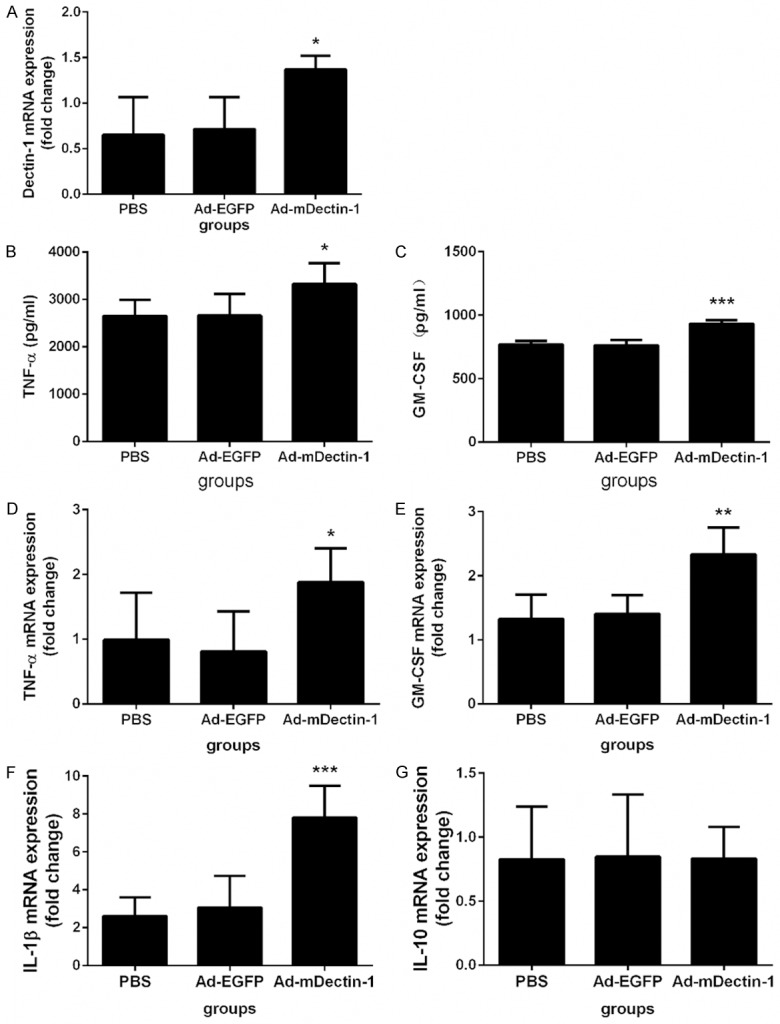
Effect of Ad-mDectin-1 on levels of Dectin-1, TNF-α, GM-CSF, IL-1β, and IL-10 during the course of IPA. A. Depict the levels of Dectin-1 mRNA at day 2 after A. fumigatus challenge (3×105). B, C. Depict the levels of TNF-α and GM-CSF in lung and BAL at day 2 after A. fumigatus challenge (3×105), respectively. D-G. Depict the levels of TNF-α, GM-CSF, IL-1β and IL-10 mRNA at day 2 after A. fumigatus challenge (3×105), respectively. Experimental n=5. *P<0.05, **P<0.01, ***P<0.001 compared with mice receiving PBS or Ad-EGFP.
The increase of the neutrophil accumulation in lungs after the administration of Ad-mDectin-1 in mice administrated with A. fumigatus conidia
Neutrophils play an important role in defending against fungal infection [20]. Therefore, we measured the neutrophil accumulation in lung tissues. Two days after the inoculation of conidia, the MPO level in lung of mice treated with Ad-mDectin-1 was significantly higher than that of the mice treated with PBS or Ad-EGFP, indicating that the Ad-mDectin-1 can stimulate neutrophil accumulation (Figure 3).
Figure 3.
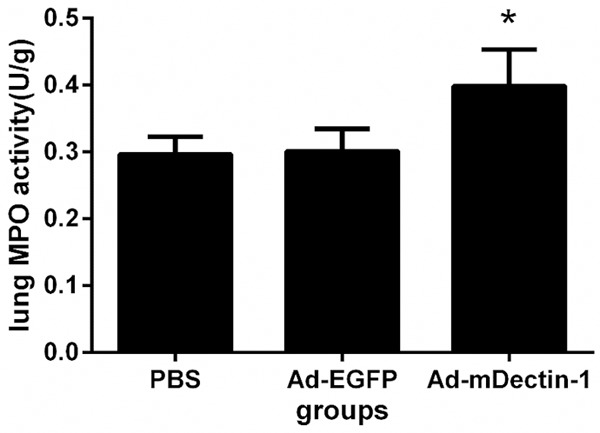
Effect of Ad-mDectin-1 on lung MPO activity at day 2 after A. fumigatus challenge. Experimental n=5. *P<0.05, compared with mice receiving PBS or Ad-EGFP.
Ad-mDectin-1 administration leads to significantly inflammation in lungs and fewer fungal load
Two days after the administration of conidia in immunocompromised mice, we discovered that the lung samples from the control group which were administrated with PBS or Ad-EGFP showed few evidence of inflammation. However, obvious evidence of inflammation was observed in mice treated with Ad-mDectin-1. The Grocott’s methenamine silver (GMS) staining results of lung tissues showed that, when compared with the control group, fewer germinating conidia and relatively fewer hyphae were found in mice administrated with Ad-mDectin-1 (Figure 4). To confirm this histological finding, the fungal load was assessed by plate-count method. Fungal levels in mice administrated with Ad-mDectin-1 were significantly lower than those detected in control group (Figure 5).
Figure 4.

Histological examination results of lung tissue at day 2 after conidia challenge. Representative photomicrographs of hematoxylin and eosin-staining (panels A to C) (magnification, ×100) and Grocott’s methenamine silver staining (panels D to F) (magnification, ×200) of lung tissue sections from treated with PBS (A and D), Ad-EGFP (B and E), Ad-mDectin-1 (C and F) at day 2 after A. fumgatus challenge (3×105 conidia) in immunocompromised mice.
Figure 5.
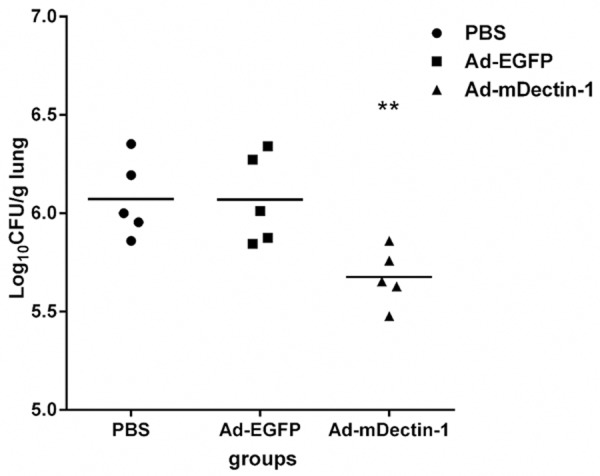
Results of fungal burden in the lungs of mice receiving PBS, Ad-EGFP, and Ad-mDectin-1 at day 2 after conidia challenge. Experimental n=5. **P<0.01, compared with mice receiving PBS or Ad-EGFP.
Effect of Ad-mDectin-1 on survival in immunocompromised mice inoculated with A. fumigatus conidia
The mortality rate in mice administrated with PBS was 50% and that rate in mice administrated with Ad-EGFP was 40%. However, no death existed in mice administrated with Ad-mDectin-1 (Figure 6). Compared with the mice in control group, the survival rate of mice with intratracheal administration of Ad-mDectin-1 was significantly higher.
Figure 6.
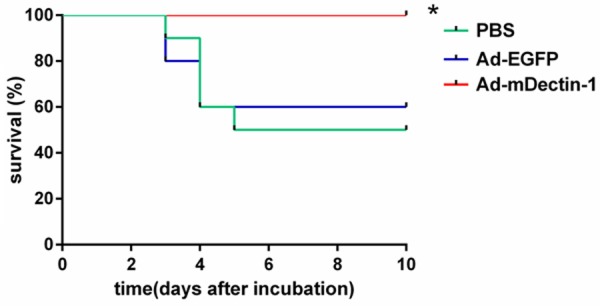
Effect of Ad-mDectin-1 on survival after A. fumigatus challenge (5×106 conidia). Experimental n=10. Data are representative of two independent experiments. *P<0.05, compared with mice receiving PBS or Ad-EGFP.
Discussion
Upon inhalation of conidia, airway epithelial cell is the initial point of confrontation between fungi and host. More and more studies have found that the epithelial cells are capable of triggering an immune response similar to the function of the source bone marrow cells [7,13,21]. For example, corneal epithelium can significantly increase the expression of Dectin-1 in the early stage of A. fumigatus infection [22]; human intestinal epithelial can secrete IL-8 and CCL2 with the present of β-glucan, and after the suppression on Dectin-1 pathway, it can significantly reduce the secretion of chemokine in intestinal epithelial cells [23].
Recognition of pathogen is first step of initiating the immune defense. Dectin-1 expressed on many myeloid cells is capable of identifying β-glucan, a carbohydrate found in fungal walls. After the recognition of fungi, Dectin-1 is responsible for triggering a variety of cellular responses, such as cytokine production, phagocytosis, and ROS generation. Previous studies have shown the critical role of Dectin-1 played in lung when defending against fungal pathogen. Genetic Polymorphism of Dectin-1 links to the susceptibility of IPA [24]. Cyclophosphamide can inhibit the expression of Dectin-1 in lungs, contributing to the susceptibility to A. fumigatus [25]. Up-regulation of Dectin-1 on macrophages has the ability to promote Aspergillus-induced innate immune response [26].
Results from real-time PCR and immunohistochemistry demonstrated that the expression of Dectin-1 obviously increased after the administration of Ad-mDectin-1. Dectin-1 mainly located in bronchioles epithelium. The results of our data are consistent with those of a previous study [27]. The results showed that, after the administration of Ad-GM-CSF through trachea, the GM-CSF mainly expressed in bronchial epithelial cells and didn’t obviously express in alveolar epithelial cells. Maybe, one of explanations for this phenomenon is that the administration method made the adenoviral vector unable to get to the alveoli effectively.
In this study, we figured out whether the increase expression of Dectin-1 could help the immunocompromised mice defend against the invasion of A. fumigatus. In this invasive aspergillosis model, the number of hyphae reached the top on the 2nd day [15]. Our study mainly focused on the changes of innate immunity. The production of cytokines is very important to the host immune response. It can activate the immune system and then affect the development of immune response. During the course of A. fumigatus infection, Dectin-1 is essential for the production of lung cytokine and chemokine, such as TNF-α, IL-1, IL-10, and IL-6 [12,28,29]. We demonstrated that the up-regulation of Dectin-1 on lung epithelial cells played a vital role in the production of inflammatory cytokines. Mice treated with Ad-mDectin-1 mediated a significant increase of the expression of IL-1β, TNF-α, and GM-CSF. No significant differences were found in the expression of IL-10 when compared with that of the control group. The significant higher production of pulmonary cytokine and chemokine may be essential for the increased fungal clearance from the lungs of immunocompromised mice given the Ad-mDectin-1.
Dectin-1 is required for inducing neutrophil-mediated anti-fungal immunity against A. fumigatus [30,31]. Neutrophils play an important role in the host innate immunity against fungi [32]. Long-term neutropenia is a major risk factor for IPA [33]. Our study found more apparent inflammation and fewer fungal loads in mice treated with Ad-mDectin-1 when compared with the control group. In this paper, we did not assess accurate neutrophils accumulation in the lung directly. MPO activity shows a good linear relationship with the level of neutrophils accumulation in lung [15,16,34,35], thus we chosen MPO activity to assess the degree of neutrophils accumulation. By testing the MPO activity, we discovered more neutrophils accumulation in lungs of mice administrated with Ad-mDectin-1. Both the increase of neutrophils accumulation and significant increase of chemokine may account for the reinforcement of fungi clearance capacity in lung tissues of immunocompromised mice treated with Ad-mDectin-1.
In conclusion, we think that our study is the first one to demonstrate that, in immunosuppressed mice, up-regulation of Dectin-1 on airway epithelial cells can increase innate immune responses against A. fumigatus, resulting in higher production of proinflammatory cytokines which were essential for neutrophil mobilization to lungs, more aspergillus clearance and higher survival rate. Therefore, our study may provide new insights to enhance host capacity of defending against IPA.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81270064 and 81200063) and the Nanjing Medical Science and Technology Development Project (No. YKK13089).
Disclosure of conflict of interest
None.
References
- 1.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20:156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurosaki F, Bando M, Nakayama M, Mato N, Nakaya T, Yamasawa H, Yoshimoto T, Fukushima N, Sugiyama Y. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med. 2014;53:1299–1306. doi: 10.2169/internalmedicine.53.1578. [DOI] [PubMed] [Google Scholar]
- 3.Camargo JF, Husain S. Immune correlates of protection in human invasive aspergillosis. Clin Infect Dis. 2014;59:569–577. doi: 10.1093/cid/ciu337. [DOI] [PubMed] [Google Scholar]
- 4.Patterson KC, Strek ME. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest. 2014;146:1358–1368. doi: 10.1378/chest.14-0917. [DOI] [PubMed] [Google Scholar]
- 5.Tochigi N, Okubo Y, Ando T, Wakayama M, Shinozaki M, Gocho K, Hata Y, Ishiwatari T, Nemoto T, Shibuya K. Histopathological implications of Aspergillus infection in lung. Mediators Inflamm. 2013;2013:809798. doi: 10.1155/2013/809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osherov N. Interaction of the pathogenic mold Aspergillus fumigatus with lung epithelial cells. Front Microbiol. 2012;3:346. doi: 10.3389/fmicb.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun WK, Lu X, Li X, Sun QY, Su X, Song Y, Sun HM, Shi Y. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur J Clin Microbiol Infect Dis. 2012;31:2755–2764. doi: 10.1007/s10096-012-1624-8. [DOI] [PubMed] [Google Scholar]
- 9.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 10.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol. 2011;14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2014;32C:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dent Res. 2010;89:666–675. doi: 10.1177/0022034510368784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb M, Inman M, Margetts PJ, Galt T, Gauldie J. Budesonide enhances repeated gene transfer and expression in the lung with adenoviral vectors. Am J Respir Crit Care Med. 2001;164:866–872. doi: 10.1164/ajrccm.164.5.2008066. [DOI] [PubMed] [Google Scholar]
- 15.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162:1633–1640. [PubMed] [Google Scholar]
- 16.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985;59:1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YM, Zhang SK, Cui NQ. Intravenous infusion of mesenteric lymph from severe intraperitoneal infection rats causes lung injury in healthy rats. World J Gastroenterol. 2014;20:4771–4777. doi: 10.3748/wjg.v20.i16.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D’Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Liu R, Noordhoek JA, Kauffman HF. Interaction of airway epithelial cells (A549) with spores and mycelium of Aspergillus fumigatus. J Infect. 2005;51:375–382. doi: 10.1016/j.jinf.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Prufer S, Weber M, Stein P, Bosmann M, Stassen M, Kreft A, Schild H, Radsak MP. Oxidative burst and neutrophil elastase contribute to clearance of Aspergillus fumigatus pneumonia in mice. Immunobiology. 2014;219:87–96. doi: 10.1016/j.imbio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Evans SE, Scott BL, Clement CG, Larson DT, Kontoyiannis D, Lewis RE, Lasala PR, Pawlik J, Peterson JW, Chopra AK, Klimpel G, Bowden G, Hook M, Xu Y, Tuvim MJ, Dickey BF. Stimulated innate resistance of lung epithelium protects mice broadly against bacteria and fungi. Am J Respir Cell Mol Biol. 2010;42:40–50. doi: 10.1165/rcmb.2008-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Che CY, Li C, Gao A, Lin J, Zhang LL, Xu Q, Wang Q, Zhao GQ. Dectin-1 expression at early period of Aspergillus fumigatus infection in rat’s corneal epithelium. Int J Ophthalmol. 2013;6:30–33. doi: 10.3980/j.issn.2222-3959.2013.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen-Kedar S, Baram L, Elad H, Brazowski E, Guzner-Gur H, Dotan I. Human intestinal epithelial cells respond to beta-glucans via Dectin-1 and Syk. Eur J Immunol. 2014;44:3729–3740. doi: 10.1002/eji.201444876. [DOI] [PubMed] [Google Scholar]
- 24.Sainz J, Lupianez CB, Segura-Catena J, Vazquez L, Rios R, Oyonarte S, Hemminki K, Forsti A, Jurado M. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS One. 2012;7:e32273. doi: 10.1371/journal.pone.0032273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Lu Q, Liu W, Wan Z, Wang X, Li R. Cyclophosphamide reduces dectin-1 expression in the lungs of naive and Aspergillus fumigatus-infected mice. Med Mycol. 2010;48:303–309. doi: 10.1080/13693780903136887. [DOI] [PubMed] [Google Scholar]
- 26.Xia D, Sun WK, Tan MM, Ding Y, Liu ZC, Li P, Qian Q, Su X, Shi Y. An Adenoviral Vector Encoding Full-Length Dectin-1 Promotes Aspergillus-Induced Innate Immune Response in Macrophages. Lung. 2015;193:549–57. doi: 10.1007/s00408-015-9740-8. [DOI] [PubMed] [Google Scholar]
- 27.Francisco-Cruz A, Mata-Espinosa D, Estrada-Parra S, Xing Z, Hernandez-Pando R. Immunotherapeutic effects of recombinant adenovirus encoding granulocyte-macrophage colony-stimulating factor in experimental pulmonary tuberculosis. Clin Exp Immunol. 2013;171:283–297. doi: 10.1111/cei.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vautier S, MacCallum DM, Brown GD. C-type lectin receptors and cytokines in fungal immunity. Cytokine. 2012;58:89–99. doi: 10.1016/j.cyto.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Gresnigt MS, van de Veerdonk FL. The Role of Interleukin-1 Family Members in the Host Defence Against Aspergillus fumigatus. Mycopathologia. 2014;178:395–401. doi: 10.1007/s11046-014-9776-y. [DOI] [PubMed] [Google Scholar]
- 31.Hardison SE, Brown GD. C-type Lectin Receptors Orchestrate Anti-Fungal Immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camargo JF, Husain S. Immune correlates of protection in human invasive aspergillosis. Clin Infect Dis. 2014;59:569–577. doi: 10.1093/cid/ciu337. [DOI] [PubMed] [Google Scholar]
- 33.Thompson GR, Patterson TF. Pulmonary aspergillosis. Semin Respir Crit Care Med. 2008;29:103–110. doi: 10.1055/s-2008-1063849. [DOI] [PubMed] [Google Scholar]
- 34.Basher F, Fan H, Zingarelli B, Borg KT, Luttrell LM, Tempel GE, Halushka PV, Cook JA. beta-Arrestin 2: a Negative Regulator of Inflammatory Responses in Polymorphonuclear Leukocytes. Int J Clin Exp Med. 2008;1:32–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Shao C, Qu J, He L, Zhang Y, Wang J, Wang Y, Zhou H, Liu X. Transient overexpression of gamma interferon promotes Aspergillus clearance in invasive pulmonary aspergillosis. Clin Exp Immunol. 2005;142:233–241. doi: 10.1111/j.1365-2249.2005.02828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



