Abstract
The aim of this study is to systematically evaluate the safety and efficacy of rituximab plus CHOP (R-CHOP combined regimen) in patients with previously untreated diffuse large B cell lymphoma (DLBCL). Electronic database were searched for randomized-controlled studies only comparing R-CHOP to CHOP standard alone in patients with untreated DLBCL were included. The risk ratios (RRs) with their 95% corresponding intervals (95% CI) were employed to estimate the efficacy of overall response (OR), complete response (CR), risk of dying and relapse rate in followed-up period. Total ten case-control studies containing 2941 patients met the inclusion criteria. The addition of R to standard CHOP were showed to increase the proportion of CR (RR=1.23, 95% CI=1.13-1.35, P<0.00001) and OR (RR=1.39, 95% CI=1.24-1.55, P<0.00001) in a fixed-effect model, indicating that rituximab combined with CHOP regimen is efficacy than CHOP alone. It did not increase the overall risk of dying as a consequence of infection (RR=0.79, 95% CI=0.55-1.13, P=0.20). Furthermore, the relapse rates is significantly lower in R-CHOP (RR=0.52, 95% CI=0.38-0.71, P<0.0001). The adverse effects were also not significant (P>0.05). In summary, R-CHOP regimen is superior to standard CHOP in terms of overall response and complete response. It does not increase the incidence of adverse effects. However, more studies concerning different age groups and special patients are needed to discuss the potential role of R in DLBCL.
Keywords: Diffuse large B-cell lymphoma, rituximab, CHOP, meta-analysis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent lymphoid malignancy, comprising 30%-40% of all new diagnoses of non-Hodgkin lymphoma (NHL) [1]. It is characterized by heterogeneity involving clinical presentation, morphology, and molecular pathogenesis [2]. DLBCL is consisted of molecularly distinct subtypes that differ in gene expression, oncogenic aberrations and clinical outcome [3]. The 4th edition of the WHO Classification of Tumors of Haematopoietic and lymphoid tissues creates that DLBCL includes the categories of “diffuse large cell” and “immunoblastic” lymphoma from the Working Formulation and “centroblastic” and “immunoblastic” lymphoma in the Kiel classification [4]. DLBCLs are aggressive but potentially curable malignancies. Management of elderly patients is challenging as critical co-morbidities often account for increased number of treatment-related complications.
The combination of cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) has been the standard therapy for DLBCL since 1970s with the 5-year survival of 30-35% [5]. However, CHOP is associated with a high risk of developing febrile neutropenia, which is an important cause of treatment failure in DLBCL patients for the occurrence of treatment-related morbidities. Rituximab is a chimeric monoclonal antibody that targets the CD20 molecule on the surfaces of normal and malignant pre-B and mature B lymphocytes [6]. It depletes B lymphocytes through a variety of mechanisms. Rituximab alone has a reaction rate of 30-40% in relapsed DLBCL [7]. In 2002, the addition of the chimeric anti-CD20 monoclonal antibody rituximab to CHOP (R-CHOP) was shown to significantly improve the prognosis of patients with DLBCL. In patients with relapsed/resistant DLBCL, R-CHOP increased the 5-year overall survival of 58% compared with 45% for CHOP alone [8]. R-CHOP has modified the prognostic factors and biological prognostic factors must be re-evaluated in the era of rituximab especially with young patients with poor prognosis.
The combination of rituximab and CHOP chemotherapy (every 14 or 21 days) has since become the standard treatment for DLBCL patients. However, over 30% of patients will not respond to currently available regimens or will relapse with resistant disease. The aim of this study is to systematic assess the efficacy and safety of rituximab combined with chemotherapy in the treatment of DLBCL.
Materials and methods
Identification and selection of relevant studies
The electronic database of PubMed, Medline, Embase and CNKI (China National Knowledge Infrastructure) were employed for searching relevant articles published between January 2002 and March 2014 (Any new reports need to be updated?). The following terms: “diffuse large B-cell lymphoma or DLBCL”, “rituximab”, “combined”, “CHOP”, and “versus or compare” as well as their combinations were used. References of retrieved articles were searched with no language restrictions. Only full-text articles and the most recent studies were included in this meta-analysis.
Criteria for inclusion
The inclusion criteria were as follows: 1) the paper should be randomized-controlled studies; 2) all patients were diagnosed with untreated DLBCL; 3) the R-CHOP group and CHOP group were matched in age, sex, ECOG scores and serum LDH level; 4) evaluation of efficacy and disease recurrence according to the international curative effect evaluation standard [9]; and 5) the results were expressed in complete response (CR), overall response (OR), overall survival (OS) and adverse effect.
Exclusion criteria: 1) non-randomized studies; 2) without data about clinical outcome; 3) including non-lymphoma patients; 4) without identical CHOP in both arms or an R-free arm; and 5) studies about maintenance purging and sequential treatment.
Data extraction
Two investigators (only one author?!) independently assessed the quality of the included studies. Any disagreement was subsequently resolved by discussion with a third author. The following information was extracted from each article: first author, year of publication, country, ethnicity, sample size, clinical stage and duration of follow-up.
Statistical analyses
The overall result was measured by risk ratios (RRs) and 95% confidence interval (CI) [10]. The significance of the pooled ORs was determined by the Z test with a P value less than 0.05. The Q-statistic test and the I2 test were used to assess the heterogeneity among induced studies. The fixed-effect model was employed when the P-value more than 0.10 for the Q-test and I2 less than 50%, assuming homogenous among studies; Otherwise, the random-effect model was used. The evidence of publication bias was assessed by visual funnel plot inspection. Statistical analyses were conducted in Review Manager (version 5.2, The Cochrane Collaboration). All the tests were two-sided.
Results
Characteristics of studies included
The electronic database search identified 118 references. After applying the inclusion criteria, 10 articles (3 in English and 7 in Chinese) including 2941 patients were ultimately included in the systematic review and meta-analysis. The study selection process is shown in Figure 1.
Figure 1.
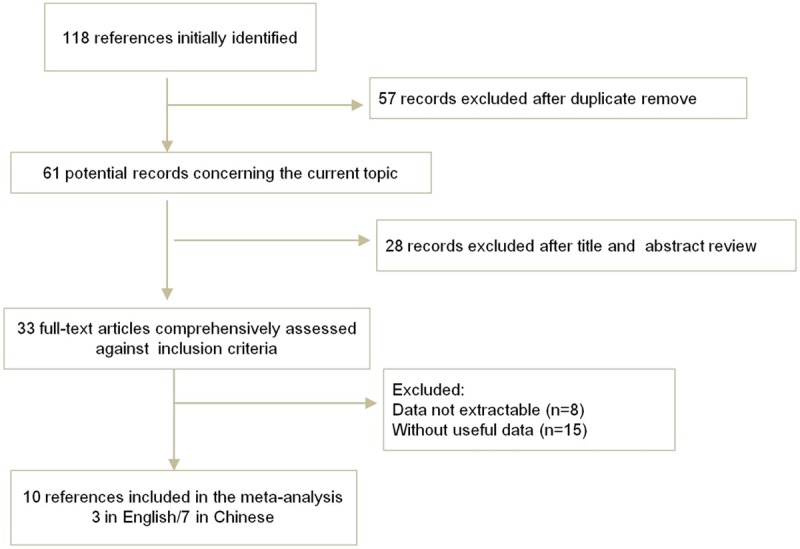
Flow chart demonstrating studies that were processed for inclusion in the meta-analysis.
Of them, seven was from China, one from Belgium [8], and two from Mexico [11,12]. The number of patients range from 36 to 399. The median age was more than 50 year old. The clinical stage is from I to IV, and the follow-up period is from 1 to 66 months. Characteristics of the studies included in this analysis were presented in Table 1. Table 2 summarized the main outcomes of included articles.
Table 1.
Main characteristic of the included studies
| First author | Year | Country | Age | Patients | Clinical stage | Duration of follow-up | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total | Cases | Controls | ||||||
| Feugier | 2005 | Belgium | 60-75 | 399 | 202 | 197 | I-IV | 60 |
| Wang YH | 2006 | China | 24-78 | 48 | 26 | 22 | I-IV | 24 |
| Aviles-a | 2007 | Mexico | 65-85 | 204 | 101 | 103 | IV | 60 |
| Aviles-b | 2007 | Mexico | 25-65 | 196 | 98 | 98 | III-IV | 53.4 |
| Jia XL | 2011 | China | 23-76 | 36 | 18 | 18 | II-IV | 26 (12-48) |
| Yang SL | 2011 | China | 18-78 | 49 | 23 | 26 | I-IV | 35 (4-66) |
| Jin H | 2012 | China | 19-78 | 43 | 25 | 18 | I-IV | 12-36 |
| Liu J | 2012 | China | 19-82 | 116 | 52 | 64 | I-IV | 12-36 |
| Xu SF | 2013 | China | 14-74 | 73 | 39 | 34 | I-IV | 28 (6-59) |
| Zeng AP | 2013 | China | 29-69 | 48 | 24 | 24 | I-IV | 36 |
Table 2.
Outcomes of the retrieved articles during follow-up period
| R-CHOP | CHOP group | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CR | OR | Death | Relapse | CR | OR | Death | Relapse | |
| Feugier | 106 | 121 | 17 | 40 | 72 | 83 | 5 | 67 |
| Wang YH | 20 | 22 | 3 | 8 | 13 | 14 | 5 | 13 |
| Aviles-a | 75 | 33 | 20 | 73 | 30 | 20 | ||
| Aviles-b | 79 | 20 | 77 | 19 | ||||
| Jia XL | 10 | 16 | 5 | 7 | 11 | 11 | ||
| Yang SL | 19 | 7 | 3 | 17 | 14 | 6 | ||
| Jin H | 21 | 22 | 6 | 1 | 12 | 14 | 10 | 6 |
| Liu J | 18 | 39 | 28 | 10 | 34 | 30 | ||
| Xu SF | 16 | 38 | 3 | 10 | 12 | 23 | 5 | 12 |
| Zeng AP | 20 | 22 | 4 | 9 | 13 | 16 | 12 | 15 |
CR, complete response; OR, overall response.
Clinical outcomes
All the ten articles reported the outcome of CR. The rate of CR was higher with patients in R-CHOP group than that in CHOP group (63.2% versus 50.7%). As shown in Figure 2, the pooled RRs of risk for CR has a significantly difference between these two groups (RR=1.23, 95% CI=1.13-1.35, P<0.00001), which suggested that patients receiving R-CHOP had a better end of treatment outcome, than patients in CHOP arm.
Figure 2.
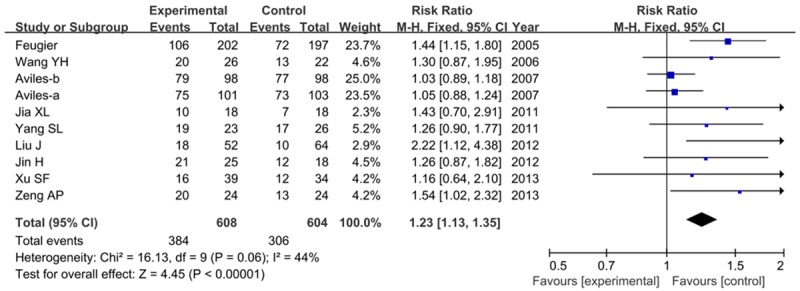
Clinical outcome of complete response with R-CHOP compared with CHOP alone.
Severn studies involved the OR (compete response and partial response). As shown in Figure 3, we found a significantly difference between these two groups (RR=1.39, 95% CI=1.24-1.55, P<0.00001), indicating that rituximab combined with CHOP regimen is efficacy than CHOP alone. No significantly heterogeneity was found between studies (I2=0, P=0.90).
Figure 3.
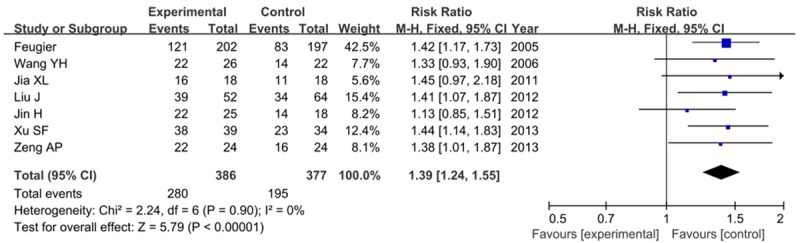
Forest plot for risk ratios of overall response with R-CHOP compared with CHOP alone.
Overall survival and relapse during follow-up period
All ten studies reported mortality during follow-up period. The mortality rate was lower in R-CHOP group. As shown in Figure 4, no significant difference was noted between these two groups (RR=0.79, 95% CI=0.55-1.13, P=0.20) in a random-effect model.
Figure 4.
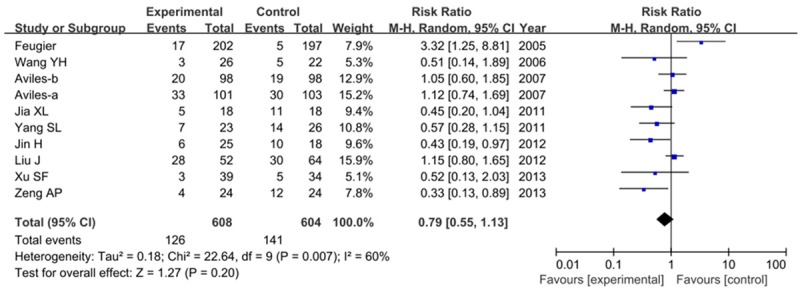
Risk ratios of mortality with R-CHOP compared with CHOP alone.
Seven articles considered the relapse cases. The frequency of relapse rate is lower with patients in R-CHOP group than that in CHOP group (20.7 vs. 32.8). Figure 5 showed the forest plots with results of relapse rates (RR=0.52, 95% CI=0.38-0.71, P<0.0001), indicating that patients with R-CHOP has a good prognosis than that with CHOP alone.
Figure 5.
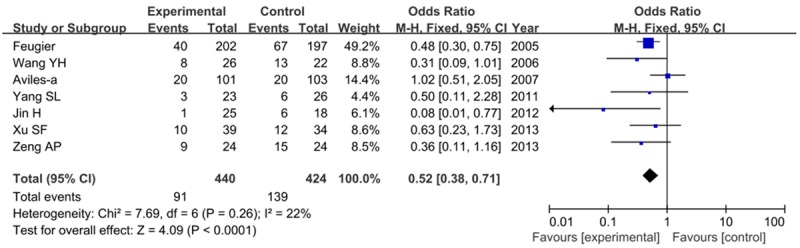
Risk of relapse rate when comparing R-CHOP with CHOP alone.
Adverse effect
The adverse events were not consistent with the expected toxic effects of CHOP chemotherapy and occurred with similar frequency in both groups. So we can’t perform statistical analysis. No differences were observed in relation to toxicity and other adverse effect when comparing these two groups.
Publication bias
Begger’s funnel plot was conducted to assess the publication bias of the literature. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry. As shown in Figure 6.
Figure 6.
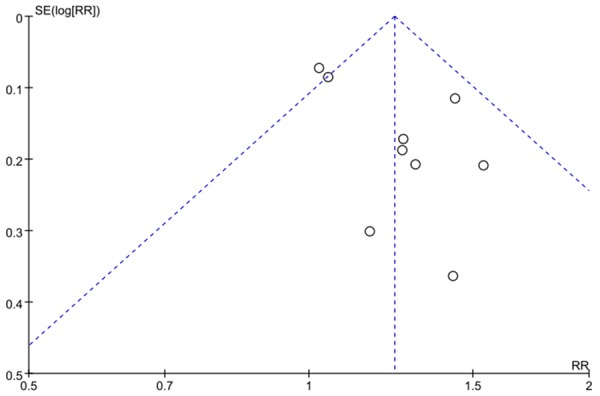
Funnel plot analysis on the detection of publication bias in the meta-analysis.
Discussion
DLBCL, a heterogeneous group of lymphomas, is considered an aggressive lymphoma [13]. Although new developments in chemotherapy have improved the survival of patients with DLBCL, to date, there is still no absolute consensus of the efficacy and safety of rituximab in DLBCL. Our data demonstrate that R-CHOP is safe and highly effective for the management of DLBCL, with less relapse rate and morality during follow-up period.
Rituximab is a chimeric monoclonal antibody against CD20 and is the first monoclonal antibody approved for the treatment of low-grade or follicular B-cell non-Hodgkin’s lymphoma in relapse or refractory stage [14]. It can induce a high level of complete response when used as a first-line treatment in follicular lymphoma [15]. The addition of rituximab to chemotherapy has improved the overall survival of the patients to 50-80% [16]. Researchers have showed that rituximab can improve the efficacy of chemotherapy after relapse [17]. However, the rationale for including an independently active agent with non-overlapping toxicity is strong.
Rituximab added to six cycles of CHOP-like chemotherapy improved long-term outcomes of young patients with good-prognosis DLBCL [18]. Long-term outcome of patients in the LNH-98.5 trial conducted by Coiffier et al. found that the end points of survival were improved in patients treated with R-CHOP: the 10-year progression-free survival and overall survival was 36.5% and 43.5%, respectively, compared with 20% and 27.6% with CHOP alone [19]. Rituximab is partially antagonistic with inhibitors of the B-cell receptor pathway in DLBCL [20], which regulates signaling pathways and alters gene expression associated with cell death and survival in DLBCL, and may provide new targets in future treatment protocols [21].
Rituximab can also combine with other monoclonal antibodies or chemotherapies to improve the results. Fu et al. proved that bevacizumab combined with R-CHOP regimen is effective for untreated DLBCL [22]. Ruan et al. showed that bortezomib with R-CHOP-21 can be safely administered and may enhance outcomes, particularly in non-germinal center B cell DLBCL, justifying randomized studies [23]. Recher et al. identified that intensified immunochemotherapy with R-ACVBP significantly improves survival of patients aged 18-59 years with DLBCL with low-intermediate risk according to the International Prognostic Index when compared with standard R-CHOP [24]. Katterer et al. found that rituximab combined with three cycles of ACVBP plus consolidation is significantly superior to ACVBP plus consolidation alone in young patients with low-risk localized DLBCL [25]. Phan’s study showed significant improvements in OS and PFS among patients who received consolidation RT after R-CHOP chemotherapy for DLBCL [26].
Several limitations are presented in this meta-analysis. First, real associations between R-CHOP and countable outcomes may be misinterpreted. Second, several risk factors such as the age group should be considered [27]. Third, the method of rituximab administration should be considered. Studies have shown that R-CHOP-21 remains the standard first-line treatment in patients with this haematological malignancy [28]; In elderly patients with untreated DLBCL, a 2-week dose-dense R-CHOP regimen did not improve efficacy compared with the 3-week standard schedule [29].
Conclusions
In conclusion, our results indicate that R-CHOP is an effective regimen for management of untreated DLBCL. However, the value of adding rituximab to standard CHOP remains to be determined in more well-designed, large-scale randomized trials.
Disclosure of conflict of interest
None.
References
- 1.Pasqualucci L, Dalla-Favera R. SnapShot: Diffuse Large B Cell Lymphoma. Cancer Cell. 2014;25:132. doi: 10.1016/j.ccr.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Staudt LM, Dave S. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol. 2005;87:163–208. doi: 10.1016/S0065-2776(05)87005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coiffier B. Diffuse large cell lymphoma. Curr Opin Oncol. 2001;13:325–334. doi: 10.1097/00001622-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gatter K, Pezzella F. Diffuse large B-cell lymphoma. Diagnostic Histopathology. 2010;16:69–81. [Google Scholar]
- 5.Jones SE, Grozea PN, Miller TP, Van Slyck EJ, Balcerzak SP, Costanzi JJ, Morrison FS, Eyre HJ, Fabian CJ, Dabich L. Chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone alone or with levamisole or with levamisole plus BCG for malignant lymphoma: a Southwest Oncology Group Study. J. Clin. Oncol. 1985;3:1318–1324. doi: 10.1200/JCO.1985.3.10.1318. [DOI] [PubMed] [Google Scholar]
- 6.Plosker GL, Figgitt DP. Rituximab. Drugs. 2003;63:803–843. doi: 10.2165/00003495-200363080-00005. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, Johnson P, Lister A, Feuring-Buske M, Radford JA, Capdeville R, Diehl V, Reyes F. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 8.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E, Tilly H, Morschhauser F. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J. Clin. Oncol. 1999;17:1244–1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 10.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 11.Avilés A, Nambo MJ, Neri N, Cleto S, Castañeda C, Huerta-Guzmàn J, Murillo E, Contreras M, Talavera A, González M. Dose dense (CEOP-14) vs dose dense and rituximab (CEOP-14+ R) in high-risk diffuse large cell lymphoma. Med Oncol. 2007;24:85–89. doi: 10.1007/BF02685907. [DOI] [PubMed] [Google Scholar]
- 12.Avilés A, Nambo MJ, Castañeda C, Cleto S, Neri N, Murillo E, Huerta-Guzmán J, Contreras M. Rituximab and escalated chemotherapy in elderly patients with aggressive diffuse large-cell lymphoma: a controlled clinical trial. Cancer Biother Radiopharm. 2007;22:194–199. doi: 10.1089/cbr.2006.360. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009;2009:523–531. doi: 10.1182/asheducation-2009.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 15.Colombat P, Salles G, Brousse N, Eftekhari P, Soubeyran P, Delwail V, Deconinck E, Haïoun C, Foussard C, Sebban C. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 16.Ivano V, Coso D, Chetaille B, Esterni B, Olive D, Aurran-Schleinitz T, Schiano JM, Stoppa AM, Broussais-Guillaumot F, Blaise D. Efficacy and safety of lenalinomide combined with rituximab in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2014;55:1–6. doi: 10.3109/10428194.2014.889822. [DOI] [PubMed] [Google Scholar]
- 17.Fields PA, Townsend W, Webb A, Counsell N, Pocock C, Smith P, Jack A, El-Mehidi N, Johnson PW, Radford J. De Novo Treatment of Diffuse Large B-Cell Lymphoma With Rituximab, Cyclophosphamide, Vincristine, Gemcitabine, and Prednisolone in Patients With Cardiac Comorbidity: A United Kingdom National Cancer Research Institute Trial. J. Clin. Oncol. 2014;32:282–287. doi: 10.1200/JCO.2013.49.7586. [DOI] [PubMed] [Google Scholar]
- 18.Pfreundschuh M, Kuhnt E, Trümper L, Österborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 19.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter N, Hutter G, Zimmermann Y, Hiddemann W, Dreyling M. Rituximab But Not GA101 Is Partially Antagonistic With Inhibitors Of The B-Cell Receptor Pathway In Diffuse Large B-Cell Lymphoma. Blood. 2013;122:4420–4420. [Google Scholar]
- 21.Koivula S, Valo E, Raunio A, Hautaniemi S, Leppä S. Rituximab regulates signaling pathways and alters gene expression associated with cell death and survival in diffuse large B-cell lymphoma. Oncol Rep. 2011;25:1183–1190. doi: 10.3892/or.2011.1179. [DOI] [PubMed] [Google Scholar]
- 22.Fu Z, Zhu J, Zheng W, Liu W, Ying Z, Xie Y, Wang X, Lin N, Tu M, Ping L. Safety and efficacy of bevacizumab combined with R-CHOP regimen in seven Chinese patients with untreated diffuse large B-cell lymphoma. Cancer Cell Int. 2014;14:5. doi: 10.1186/1475-2867-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan J, Martin P, Furman RR, Lee SM, Cheung K, Vose JM, LaCasce A, Morrison J, Elstrom R, Ely S. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 24.Récher C, Coiffier B, Haioun C, Molina TJ, Fermé C, Casasnovas O, Thiéblemont C, Bosly A, Laurent G, Morschhauser F. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): an open-label randomised phase 3 trial. Lancet. 2011;378:1858–1867. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 25.Ketterer N, Coiffier B, Thieblemont C, Fermé C, Brière J, Casasnovas O, Bologna S, Christian B, Connerotte T, Récher C. Phase III study of ACVBP versus ACVBP plus rituximab for patients with localized low-risk diffuse large B-cell lymphoma (LNH03-1B) Ann Oncol. 2013;24:1032–1037. doi: 10.1093/annonc/mds600. [DOI] [PubMed] [Google Scholar]
- 26.Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J. Clin. Oncol. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 27.Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146–171. doi: 10.1016/j.critrevonc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, Pocock C, Ardeshna KM, Radford JA, McMillan A. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 29.Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, Bologna S, Ghesquières H, Hacini M, Fruchart C. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14:525–533. doi: 10.1016/S1470-2045(13)70122-0. [DOI] [PubMed] [Google Scholar]


