Abstract
Omp25 protein, an outer membrane protein of Brucella, can cause damage to the central nervous system. As one type of macrophage, microglial cells play a role in immune surveillance and immune protection in the central nervous system; therefore, they are major targets of bacterial attack. The present study examined BV2 mouse microglial cells that were stimulated with different concentrations of Omp25 recombinant protein, and the secretion of inflammatory cytokines by the BV2 cells as well as their level of apoptosis were observed. The objective of the study was to preliminarily illustrate the possible mechanism that Omp25 uses to damage the central nervous system. Mouse BV2 microglial cells were incubated with different concentrations of Omp25 for 24 h, and an enzyme-linked immunosorbent assay (ELISA) was used to detect the secretion of the inflammatory cytokines interleukin (IL)-6, tumour necrosis factor (TNF)-α and HMGB1 (high mobility group box-1 protein); reverse transcription polymerase chain reaction (RT-PCR) was used to detect the expression of TLR4 (Toll-like receptor 4) mRNA; Annexin V-fluorescein isothiocyanate (FITC) double staining was used to detect apoptosis in the BV2 cells. After the BV2 cells were stimulated with different concentrations of Omp25, the levels of IL-6, TNF-α and HMGB1 was increased, and the difference was statistically significant compared with the control group (P<0.05). The secretion of TNF-α and HMGB1 showed a trend toward an initial increase followed by a decrease. The expression level of TLR4 mRNA was increased. Omp25 protein can inhibit apoptosis in BV2 cells. The outer membrane protein Omp25 of Brucella promotes microglial cells to secrete inflammatory cytokines and inhibit apoptosis. TLR4 may be involved in the immune response of the central nervous system to Brucella infection.
Keywords: Brucella, outer membrane protein, Omp25, TLR4, microglial cells
Introduction
Brucellosis is a seriously harmful zoonotic disease, and it is widespread throughout the world [1]. It has caused large losses to both human health and economic development. The pathogen of this disease, Brucella, is deadly. Only 1000 bovine Brucella are needed to cause the onset of disease in humans, and the Centre for Disease Control and Prevention of the United Nation has listed Brucella as a Category B biological warfare agent [2].
Brucella can cause damage to multiple systems in human and animals, including the central nervous system in approximately 1.7%-10% of cases [3]. The outer membrane of Brucella has many integral proteins, which are called the outer membrane proteins. According to their size and molecular weight, these major outer membrane proteins (OMPs) are divided into three groups. Omp25, which is in the third group, is highly conserved in various types and subtypes of Brucella and can stimulate the body to generate a strong specific immune response [4], which is considered to be closely related to the virulence of Brucella [5]. Therefore, Omp25 has currently become an area of research focus for the damage due to Brucella.
In the present study, after mouse BV2 microglial cells were infected with the Brucella outer membrane protein Omp25, the secretion of the inflammatory cytokines interleukin (IL)-6, tumour necrosis factor (TNF)-α and HMGB1 (high mobility group box-1 protein) and the expression of the Toll-like receptor (TLR) 4 gene were examined. The impact of Omp25 on the apoptosis of BV2 cells was also observed. The goal was to reveal the possible mechanism that Omp25 uses to damage the central nervous system.
Materials and methods
Recombinant Omp25 expression and purification
According to the nucleotide sequences of Brucella outer membrane protein gene in GenBank, a pair of primers of Omp25 gene (sense: 5’-GGAATTCCATATGATGCGCACTCTTAAGTCTC-3’; antisense: 5’-TCCGCTCGAGGAACTTGTAGCCGATGC-3’) were designed and synthesized. The Omp25 gene was amplified by polymerase chain reaction (PCR). The amplified omp25 gene was cloned into the pET-30a vector to generate the recombinant plasmid pET-30a-Omp25. The recombinant plasmid pET-30a-Omp25 was then transformed into cells of Escherichia coli BL21 (DE3). Expression of recombinant protein was induced with 0.1 mM IPTG generating N-terminally His-tagged fusion proteins. The recombinant proteins (25 kDa) expressed from pET-30a-Omp25 were purified by affinity chromatography with Ni (II)-conjugated Sepharose and concentrated by using Centricon 3 concentrators (Amicon). The Omp25 proteins were confirmed by SDS-PAGE. Total protein assays were carried out using a bicinchoninic acid (BCA) protein assay kit (Pierce).
Detection of TLR4 mRNA expression by fluorescence quantitative PCR
TRIzol (Invitrogen, USA) was used to isolate total RNA from cells (5.0 × 105). Moloney murine leukaemia virus reverse transcriptase and the universal primer Oligo (dT) 15 were used to reverse transcribe mRNA into cDNA. Conventional polymerase chain reaction (PCR) was performed on an ABI 9700 instrument under the following conditions: 94°C for 2 min to fully denature the template and activate the polymerase and 35 cycles of 94°C for 30 s and 56°C for 35 s. The PCR reactions used the following primers: TLR4 primers, upstream 5’-ATGAGGACTGGGTGAGAATGA-3’, downstream 5’-ACCAACGGCTCTGAATAAGTG-3’; and β-actin primers, upstream 5’-GAGACCTTCAACACCCAGC-3’, downstream 5’-ATGTCACGCACGATTCCC-3’. β-actin was used as an internal control to determine the relative expression level of the target genes. The reaction conditions followed the instruction manual of the kit. After the reaction had finished, the amplification curve and the melting curve were analysed. The expression level was compared and analysed with a relative quantitation method (2-ΔΔCT method).
Detection of IL-6, TNF-α and HMGB1 expression by ELISA
After incubating the BV2 cells with different concentrations (0.5-40 µg/mL) of recombinant Omp25 protein for 24 h, the supernatants were collected. After centrifugation, the expression levels of IL-6, TNF-α and HMGB1 were detected. The detection was conducted according to the instruction manual of the enzyme-linked immunosorbent assay (ELISA) kit.
Detection of apoptosis by flow cytometry
Cultured cells were harvested in centrifuge tubes, and each tube contained approximately 1-10 × 105 cells. After centrifugation (4°C, 900 g, 3 min), the supernatant was discarded. The cells were washed with incubation buffer and centrifuged to remove the supernatant, and the cells were resuspended in 100 mL of buffer. Five microlitres of fluorescein isothiocyanate (FITC)-labelled Annexin V and 1 mL of 100 µg/L propidium iodide (PI) working solution were added to the cells. After incubation at room temperature for 15 minutes, the sample was analysed by flow cytometry. Cellquest software from Becton Dickinson USA was used for the analysis.
Statistical analysis
Statistical Package for Social Science (SPSS) 17.0 statistical software was used to analyse the experimental data. A normality test was always performed first. Measurement data are presented as the mean ± standard deviation (x̅ ± s). One-way analysis of variance (ANOVA) was used to compare sample means. A P<0.05 was considered statistically significant.
Results
Recombinant Omp25 expression and purification
After induction with IPTG (0.1 mmol/L) at 37 uC for 5 h, E. coli BL21 (DE3) harbouring pET30a-Omp25 or pET30a exhibited a high level of expression (data not shown). The recombinant Omp25 protein was purified using a Ni-NTA affinity column. Clear target bands of approximately 25 kDa were visible (Figure 1).
Figure 1.
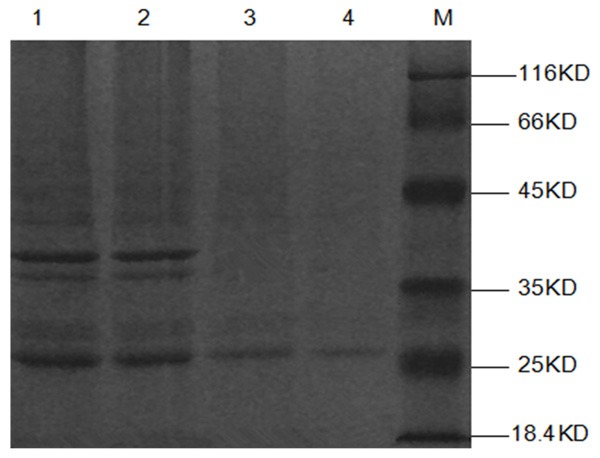
Purification of recombinant Omp25 proteins. Purified recombinant unpurified Omp25 (lanes 1-2) and purified (lanes 3-4) proteins were detected by SDS-PAGE. M, Molecular mass marker.
Omp25 protein induced production of IL-6, TNF-α and HMGB1 in BV2 cells
Different concentrations (0.25-40 µg/mL) of Omp25 protein were used to stimulate BV2 cells for 24 h. Normal cells without the addition of Omp25 were used as controls. ELISA was used to detect IL-6 levels. In Figure 2 (left), the level of IL-6 increased with increasing concentrations of Omp25. At 0.25 µg/mL, it began to increase, and the level was 1.54 ± 0.29 ng/mL. After stimulation at an Omp25 concentration of 40 µg/ml, the IL-6 level peaked at 2.26 ± 0.26 ng/mL. The trend curve of the IL-6 level is shown in Figure 2 (right). These results indicate that Omp25 can promote microglial cells to secrete IL-6.
Figure 2.
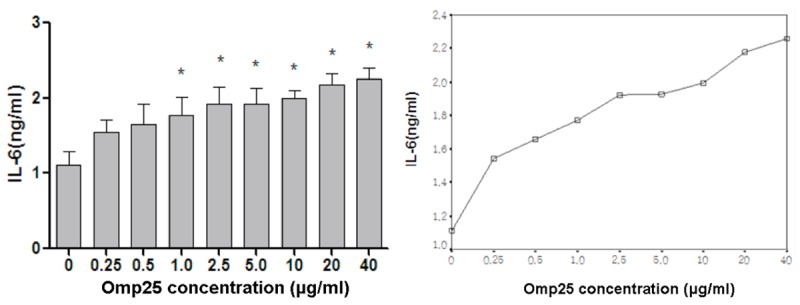
The effect of different concentrations of Omp25 protein on the production of IL-6 by BV2 cells. Gradient concentrations (0.25-40 µg/mL) of Omp25 protein were used to stimulate BV2 cells for 24 h. Normal cells without the addition of Omp25 were used as controls. ELISA was used to detect IL-6 levels. Note: *Represents the comparison of the Omp25 experimental group to the control group, P<0.05.
In Figure 3 (left), the production of TNF-α showed an initial increase followed by a decreasing trend as the Omp25 protein concentration increased, but it was always higher than the TNF-α level in the control group. After stimulation at a concentration of 0.25 µg/mL, the TNF-α level started to increase, and the level was 7.43 ± 0.89 ng/mL. After stimulation with 0.5 µg/mL Omp25, the TNF-α level peaked at 8.58 ± 0.95 ng/mL. After stimulation with 1 µg/mL Omp25, the TNF-α levels started to decrease, and it was 7.66 ± 0.99 ng/mL. The trend curve of the TNF-α level is shown in Figure 3 (right). These results indicate that Omp25 protein can induce microglial cells to secrete TNF-α.
Figure 3.
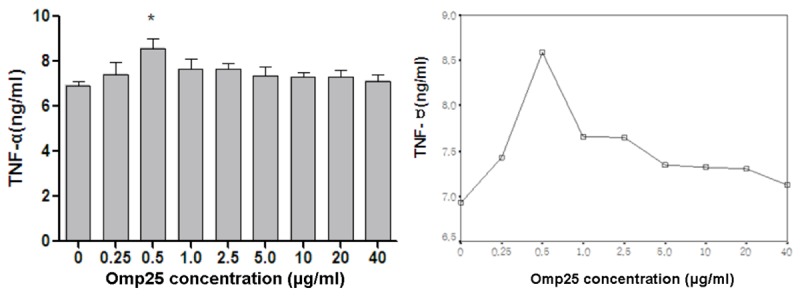
The effect of different concentrations of Omp25 protein on the production of TNF-α in BV2 cells. Gradient concentrations (0.25-40 µg/ml) of Omp25 protein were used to stimulate BV2 cells for 24 h. Normal cells without the addition of Omp25 were used as controls. ELISA was used to detect TNF-α levels. Note: *Represents the comparison of the Omp25 experimental group to the control group, P<0.05.
The level of HMGB1 production also showed an initial increase followed by a decreasing trend with increasing Omp25 protein concentrations (Figure 4), and its trend curve was similar to TNF-α.
Figure 4.
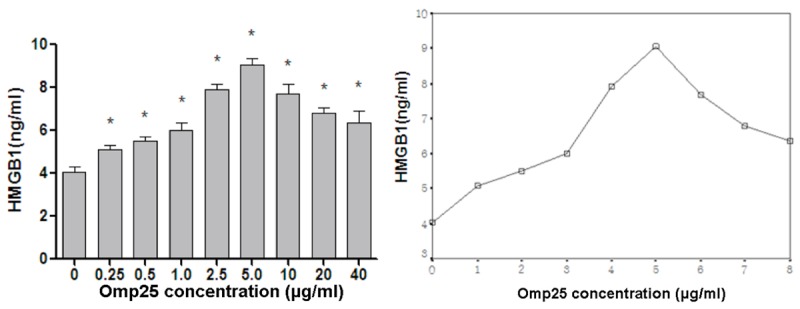
The effect of different concentrations of Omp25 protein on the production of HMGB1 in BV2 cells. Gradient concentrations (0.25-40 µg/mL) of Omp25 protein were used to stimulate BV2 cells for 24 h. Normal cells without the addition of Omp25 were used as controls. ELISA was used to detect HMGB1 levels. Note: *Represents the comparison of the Omp25 experimental group to the control group, P<0.05.
Omp25 protein stimulates TLR4 mRNA expression in BV2 cells
Different concentrations of Omp25 protein were incubated with BV2 cells for 24 h. Normal cells without the addition of protein were used as controls. RT-PCR was used to detect changes in the TLR4 mRNA level inside of the cells. As shown in Figure 5, the relative expression level of TLR4 mRNA showed an initial increase followed by a decreasing trend as the Omp25 protein concentration increased.
Figure 5.
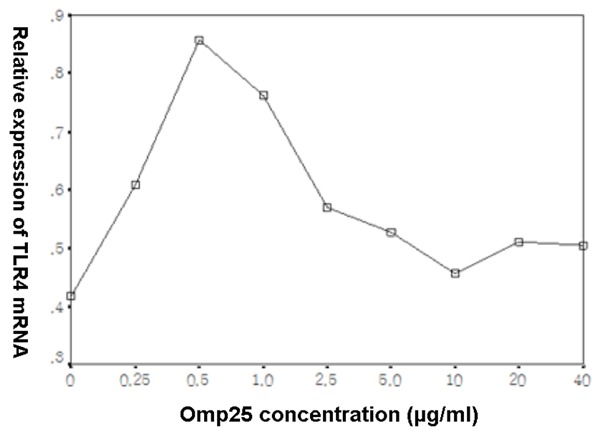
The trend curve of the effect of different concentrations of Omp25 protein on the production of TLR4 mRNA in BV2 cells. Gradient concentrations (0.25-40 µg/mL) of Omp25 protein were incubated with BV2 cells for 24 h. Normal cells without the addition of protein were used as controls. RT-PCR was used to detect changes in the TLR4 mRNA level.
Omp25 protein inhibited apoptosis in BV2 cells
Different concentrations (0.5-40 µg/mL) of recombinant Omp25 protein were incubated with BV cells for 24 h. After Annexin V-FITC double staining, apoptosis was detected with flow cytometry. Figure 6 shows that the percentage apoptotic cells decreased (6.13 ± 0.86, 3.19 ± 0.25, 2.33 ± 0.29 and 2.26 ± 0.37%, respectively) as the Omp25 protein concentration increased. These results indicate that Omp25 protein can inhibit apoptosis in BV2 microglial cells.
Figure 6.
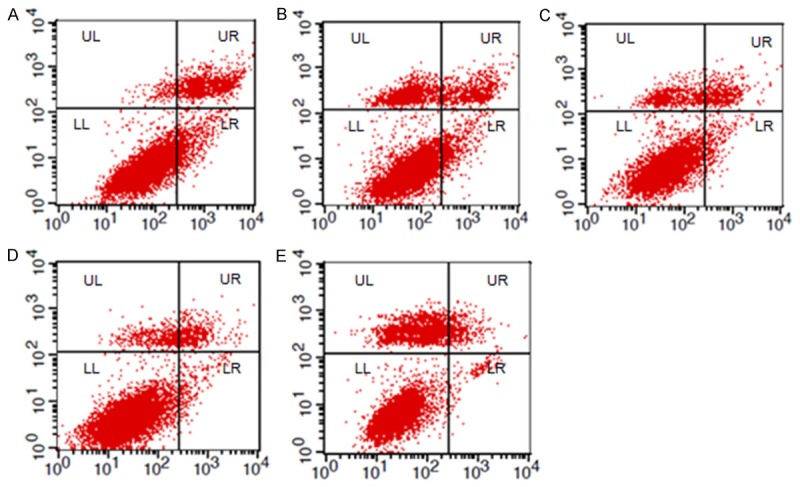
Different concentrations (0.5-40 µg/mL) of recombinant Omp25 protein were incubated with BV cells for 24 h. After Annexin V-FITC double staining, apoptosis was detected with flow cytometry. A: PBS; B: 0.5 µg/mL Omp25; C: 2.5 µg/mL Omp2; D: 10 µg/mL Omp25; E: 40 µg/mL Omp25.
Discussion
Outer membrane proteins play an important role in host interactions. Because they are often located on the surface of bacteria, outer membrane proteins are susceptible to recognition by the immune system, and it is also easier for them to compromise the immune system. Outer membrane proteins play an important role in resisting intracellular bactericidal mechanisms, stabilizing the outer membrane structure of bacteria and adapting to the environment inside and outside the cell, and they are the major bacterial virulence factors. Therefore, the study of outer membrane proteins can facilitate our understanding of the pathogenic mechanisms of bacteria.
Omp25 belongs to the third group of outer membrane proteins of Brucella. Omp25 may be associated with damage to the central nervous system. As a type of macrophage, microglial cells play a role in immune surveillance and immune protection in the central nervous system; therefore, they are major targets of bacterial attack. The present study examined BV2 mouse microglial cells that had been stimulated with different concentrations of Omp25 recombinant protein, and the secretion of various inflammatory cytokines by the BV2 cells as well as their level of apoptosis were observed. The objective was to preliminarily illustrate the possible mechanism that Omp25 uses to damage the central nervous system.
As an important class of inflammatory cytokines, TNF-α is involved in many of the body’s immune and inflammatory responses. As one of the important inflammatory cytokines, IL-6 not only induces T cells to produce IL-2 and IL-2R, but it can also induce cytotoxic T lymphocytes (CTLs) to produce serine lipase and perforin. Therefore, IL-6 plays an important role in bacterial and viral infections and the inflammatory process [6]. In recent years, researchers have found that HMGB1 is an important late inflammatory mediator in the body. Our results showed that the secretion of IL-6, TNF-α and HMGB1 in the supernatant of BV2 cells increased after Omp25 protein stimulation. The expression level of IL-6 increased with increasing concentrations of Omp25 protein. It is noteworthy that the expression of both TNF-α and HMGB1 showed a trend of an initial increase followed by a decrease. The reason for this difference may be that low doses of Omp25 protein have an immuno-protective effect, while high doses of Omp25 have a cytotoxic effect, thereby reducing the secretion of cytokines. But, it is also possible that other factors caused this trend.
In the Toll-like receptor family, TLR4 is mainly expressed on the surface of microglial cells and oligodendrocytes [7]. There is no literature report yet regarding whether TLR4 responds to Omp25 and is thereby involved in the inflammatory response after Omp25 protein enters the host’s central nervous system. Our experiment found that the expression of TLR4 mRNA in BV2 cells first increased and then decreased after Omp25 protein stimulation. This result indicates that Omp25 protein can induce an increase in TLR4 molecules on the surface of BV2 cells. Inside of the body, TLR4 may play an important role in microglial cells in their innate immune response and inflammatory response to Omp25 protein. As one of the important pattern recognition receptors (PRRs), TLR4 can monitor and identify the related structural components of the invading Omp25 and induce auto-activation.
In the present study, we also observed that Omp25 protein could inhibit the apoptosis of BV2 cells, but the detailed mechanism of this inhibition requires further study to be fully illuminated.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81260189).
Disclosure of conflict of interest
None.
References
- 1.Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents. 2010;36(Suppl 1):S8–11. doi: 10.1016/j.ijantimicag.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Gyles CL, Prescott JF, Songer JG, Thoen CO. Pathogenesis of bacterial infections in animals. 4th edition. Wiley-Blackwell; 2011. pp. 429–441. [Google Scholar]
- 3.Türel O, Sanli K, Hatipoğlu N, Aydoğmuş C, Hatipoğlu H, Siraneci R. Acute meningoencephalitis due to Brucella: case report and review of neurobrucellosis in children. Turk J Pediatr. 2010;52:426–429. [PubMed] [Google Scholar]
- 4.Cloeckaert A, Vizcaíno N, Paquet JY, Bowden RA, Elzer PH. Major outer membrane proteins of Brucella spp. : past, present and future. Vet Microbiol. 2002;90:229–247. doi: 10.1016/s0378-1135(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 5.Salhi I, Boigegrain RA, Machold J, Weise C, Cloeckaert A, Rouot B. Characterization of new members of the group 3 outer membrane protein family of Brucella spp. Infect Immun. 2003;71:4326–4332. doi: 10.1128/IAI.71.8.4326-4332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peri F, Piazza M, Calabrese V, Damore G, Cighetti R. Exploring the LPS/TLR4 signal pathway with small molecules. Biochem Soc Trans. 2010;38:1390–1395. doi: 10.1042/BST0381390. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, Li Y, Li S, Shan G, Kristiansen K, Li S, Yang H, Wang J, Wang J. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20:265–272. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


