Abstract
Background: Anti-CD11c antibodies target to the CD11c receptor that mediates antigen presentation to T cells by dendritic cells (DCs). To exploit these properties for immunization purposes, we obtained DC-targeting DNA vaccines by fusing tumor-associated antigen HER2/neu ectodomain to single chain antibody fragment (scFv) from N418 (scFvN418), a monoclonal antibody binding the mouse DC-restricted surface molecule CD11c, and explored its antitumoral efficacy and underlying mechanisms in mouse breast cancer models. Methods: Induction of humoral and cellular immune responses and antitumoral activity of the DNA vaccines were tested in transplantable HER2/neu-expressing murine tumor models and in transgenic BALB-neuT mice developing spontaneous Neu-driven mammary carcinomas. Results: Upon injection of the breast tumor cell line D2F2/E2 (stably expressing human wild-type HER2), scFvN418-HER2 immunized mice were protected against tumor growth. Even more important for clinical applications, we were able to substantially slow the growth of implanted D2F2/E2 cells by injection of scFvN418-HER2 conjugates into tumor bearing hosts. The existing tumors were eradicated by treatment with scFvN418-HER2 combined with low-dose cyclophosphamide (CTX), which can make a temporary regulatory T cells (Treg) depletion. What’s more, in combination with the low-dose CTX, vaccination with scFvN418-neu significantly retarded the development of spontaneous mammary carcinomas in transgenic BALB-neuT mice. Conclusion: Our results show that DNA vaccine which targeting of dendritic cells in situ by the means of antibody-antigen conjugates may be a novel way to induce long-lasting antitumor immunity.
Keywords: DNA vaccine, dendritic cell-targeted, HER2/neu, breast cancer, cyclophosphamide
Introduction
Activation of both specific immune effector responses and innate immunity are fundamental for a powerful DNA vaccine. Multiple approaches have considerable effective to induce strong immune responses following DNA vaccination. As usual, they are the DNA fusion gene vaccines encode different types of immunostimulatory molecules, fusion proteins which can target to specific immune molecules, or types to target pattern recognition and “alarmin” receptors to improve triggering of the innate immune system [1-4]. Although these extensive literatures have repeatedly shown that the immune response to DNA vaccines can be increased, the findings have yet to prove that enhanced presentation of vaccine antigens by dendritic cells (DCs) is being achieved [5,6].
Dendritic cells have been characterized by their outstanding ability to be taken up, processed and presented antigens, to present antigen-derived peptides in the context of MHC molecules to naive T cells. The induction of robust CD4+ and CD8+ T cell responses can be achieved because of DC’s so exceptional T-lymphocyte stimulatory capacity [7]. CD11c is a component of complement receptor 4 in the mouse, it is expressed predominantly on DC together with some NK and CD8 cells [8,9], and is present at high levels on all conventional DC subsets, including both CD8+ and CD8- subtypes [10]. Some studies have certified that CD11c is a terrifically effective immunotarget for the generation of antibody responses in vivo, and it has also been used as an immunotarget for CTL responses [11-13]. Due to its expression pattern, we hypothesized that CD11c may provide an effective target for the delivery of HER2/neu (its rat homologue neu) to DC for the generation of both CD4 and CD8 T cell-mediated immunity.
Overexpression of the HER-2 receptor tyrosine kinase has been found in various human malignancies, including breast, ovarian and gastric carcinomas, non-small cell lung cancer, and salivary gland cancers, and has been associated with poor prognosis [14-16]. Because of this enhanced expression on tumor cells and its involvement in essential signaling processes, HER2 constitutes an important target for directed cancer therapy with monoclonal antibodies or small molecular weight tyrosine kinase inhibitors. Due to HER2 expression in tumor cells is usually retained after development of trastuzumab resistance, active vaccination aiming at the initiation or enhancement of endogenous HER2-specific immune responses may offer a valuable treatment alternative. Unlike passive immunotherapy with antibodies, antigen-specific vaccination has the potential to induce a broad spectrum of immune effector mechanisms, which includes CD4+ and CD8+ T-cell responses [17,18]. Accordingly, the HER2/neu oncogenic protein is advised to be a tumor-associated antigen.
In the present study, we have derived single chain antibody fragment (scFv) from the monoclonal antibody N418, which are directed to CD11c mouse DC receptors. To investigate whether specific targeting of tumor antigens (HER2/neu) to activated DCs via CD11c can induce potent antigen-specific immune responses, we generated scFvN418-HER2/neu fusion protein consisting of scFvN418 fused to the extracellular domain of human HER2 or rat homologue neu. As what we expected, the results show that immunization with DNA vectors encoding antigens fused to a CD11c binding scFv is a powerful mean for eliciting stronger specific immune responses in vivo.
Materials and methods
Mice and cell lines
6- to 8-week-old female BALB/c (H-2d) mice were purchased from the Shanghai laboratory Animal Center (Shanghai, China). BALB-neuT mice (H-2d) expressing a transforming neu under the control of mouse mammary tumor virus promoter were obtained from Charles River.
Mouse breast tumor cell line D2F2, 4T1 and 293T cell lines were maintained in DMEM supplemented with 10% (v/v) FCS. D2F2/E2 stably expressing human wild-type HER2 were maintained in medium containing 0.4 mg/mL G418 (Sigma-Aldrich). TUBO cells are neu-expressing breast carcinoma cells established from a lobular carcinoma of a female BALB-neuT mouse, and maintained in DMEM containing 20% FCS. All tissue culture reagents were purchased from Life Technologies unless described otherwise.
Reagents
Peptides used in this study were obtained from Sigma-Aldrich. All peptides were > 95% pure as indicated by analytical HPLC. Lyophilized peptides were diluted in DMSO and stored at -20°C until use. Recombinant HER2 and TRP2 proteins were purchased from R&D Systems. Cyclophosphamide (CTX) were obtained from Sigma-Aldrich and reconstituted in sterile PBS for in vivo injections. Monoclonal antibodies (Mabs) to the following antigens were purchased from eBiosciences (San Diego, CA): CD4 (GK 1.5) and CD8 (53-6.7) conjugated to fluorescein isothiocyanate (FITC); FoxP3 (FJK-16 s) conjugated to PE. Immunoglobulins with isotypes corresponding to the above Mabs and conjugated to the appropriate fluorochromes, were used as control for nonspecific binding.
Construction of DNA vaccines
The backbone for the construction of DNA vaccines was the mammalian expression vector pcDNA3.1 (Invitrogen). In this vector encoding vaccine proteins are expressed under the control of the CMV promoter as an in-frame fusion with a vector-encoded signal peptide (SP) leader sequence for secretion and are followed by C-terminal Myc tag for detection. The genes encoding the variable regions of the heavy (VH) and light (VL) chains of scFvN418 were synthesized according to the published sequences [19]. Each VH fragment was bound to its VL partner by use of a spacer encoding a 15 amino-acid flexible linker (Gly4Ser)3, yielding scFv constructs scFvN418. The sequence encoding for the extracellular domain of human HER2 or its rat homologue neu was amplified from cDNA of SK-BR-3 and TUBO cell lines using the following primers HER2-HindIII-s 5’-TTA AGC TTG AGC TGG CGG CCT TGT GCC-3’, HER2-XbaI-as 5’-TT T CTA GAC AAA CAG TGC CTG GCA TTC ACA TAC-3’ and neu-HindIII-s 5’-TT A AGC TTA TCA TCA TGG AGC TGG CGG-3’, neu-XbaI-as 5’-TTT CTA GAT CCA AAG CAG GTC TCT GAG CTG TTT TGA-3’. The resultant encoding sequences were then cloned in-frame downstream of the scFvN418.
Expression of protein encoded by DNA vaccines
The different pcDNA3.1 constructs were transiently transfected in 293T cells using Lipofectamine 2000 according to the manual instruction (Invitrogen). The resultant supernatants were harvested at 72 hours post-transfection and concentrated and dialyzed using centrifugal filter devices (Amicon Ultra, 10 kDa, Millipore). Protein expression was analyzed by Western blotting. Recombinant proteins were detected with Myc-tag-specific monoclonal antibody (mAb) 9E10 followed by horseradish peroxidase (HRP)-conjugated secondary antibody.
Binding assays
Binding of scFvN418-HER2 fusion proteins from supernatants of transfected 293T cells to mouse DCs was determined by fluorescence-activated cell sorting analysis. DCs (5 × 105) were incubated with 100 μl cleared culture supernatant taken 5 days after transfection for 45 min on ice followed by incubation with 2 μg mAb 9E10 and PE-labeled goat anti-mouse IgG for 30 min. Then, cells were washed and bound proteins were detected using a FACSCalibur (Becton Dickinson) flow cytometer. Data were analyzed with CellQuest (Becton Dickinson) software.
Protective and therapeutic vaccination
For protective vaccination, female BALB/c mice or BALB-neuT mice were vaccinated on days -21 and -7 by intramuscular injections of 50 µg plasmid DNA in 50 µL PBS into the upper leg muscle of the left hind limb followed by in vivo electroporation as described previously [19]. On day 0, animals were inoculated subcutaneously (s.c.) with 2 × 105 D2F2/E2, D2F2 or TUBO tumor cells in the opposite flank. Then tumor growth was monitored with a caliper by measuring two perpendicular tumor diameters every week, and tumor volumes were calculated according to the formula: length × (width)2 × 0.5. For therapeutic vaccination, when the tumors were 2-3 mm in diameter (day 8), mice were injected i.p. with cyclophosphamide (100 mg/kg). Four days later (day 12), animals were vaccinated as described above. Treatment was repeated 14 days (day 26) after the first treatment, and tumor growth was followed. If animals appeared moribund or the diameter of the tumors reached 15 mm, the mice were sacrificed and this was recorded as the date of death for survival studies. For rechallenging experiments, the long-term surviving mice were injected s.c. either with 2 × 105 D2F2/E2, D2F2, or 4T1 tumor cells. All animal experiments had been reviewed and approved by the appropriate government committee and were done in accordance with the relevant guidelines and regulations.
Prevention of spontaneous tumors
Preventive effects of the DNA vaccines were investigated in virgin female BALB-neuT mice that endogenously expresses neu in their mammary glands and develops neu-driven mammary carcinomas. Animals were immunized twice at 8 and 10 weeks ages with scFvN418-neu, scFvN418-HER2, neu, HER2 or pcDNA3.1. Mammary glands were inspected every week to monitor the appearance of tumors. And mice were injected i.p. with CTX (100 mg/kg), four days before the first immunization. Measurable/palpable masses > 2 mm in diameter were regarded as tumors. Data are reported as tumor multiplicity (cumulative number of tumors per number of mice in each group) and shown as mean ± SE.
Cytometric identification of Treg cells
For detection of regulatory T cells, splenocytes and tumor-infiltrating lymphoid cells (TIL) from immunized mice were surface stained with the indicated monoclonal antibodies. After that, cells were washed with fluorescence-activated cell sorting buffer (PBS with 1% fetal bovine serum and 0.09% sodium azide), fixed and permeabilized with the Cytofix/Cytoperm reagent (BD Bioscience) for 20 minutes at 4°C, after which they were washed in Perm/Wash buffer (BD Bioscience), and stained with PE anti-mouse FoxP3 (FJK-16 s; eBioscience) at 4°C for 45 minutes. Immunoglobulin G-PE and immunoglobulin G-FITC (mouse) were used as negative controls. All analysis was performed on the FACSCalibur (Becton Dickinson) flow cytometer.
Evaluation of T-cell responses
For detection of HER2-specific CD4+ T cells, T cells from vaccinated mice were isolated with anti-CD4 beads on MACS columns according to the manufacturer’s protocol (Miltenyi). CD4+ T cells were then restimulated with bone marrow-derived DCs pulsed with recombinant HER2 or TRP2 protein in vitro for 3 d, and supernatants were collected and analyzed for production of IFN-γ, TNF-α, IL-4 and IL-10 by ELISA kits (R&D Systems).
For detection of IFN-γ-producing CD8 T cells, intracellular cytokine staining assays were performed. Briefly, splenocytes harvested from vaccinated mice were cultured in the presence of HER263-71 peptide (TYLPTNASL) or TRP2180-188 peptide (SVYDFFVWL) (10 µg/mL) for 6 h. During the final 4 h of incubation, 10 µg/ml brefeldin A (Sigma) were added. After surface staining with FITC-CD8, cells were permeabilized and stained with PE-IFN-γ prior to analysis by flow cytometry as described above. For CTL measurements, 51Cr-release assays were performed as described previously [20].
Analysis of antibody responses
Peripheral blood was collected from the tail vein, and 1:100 dilutions of sera were analyzed by ELISA with recombinant HER2 protein. Normal mouse serum served as negative control.
Statistical analysis
Differences in tumor growth kinetics, tumor multiplicity, and specific cytotoxicity were evaluated by ANOVA or the Student’s test. Values of P < 0.05 were considered significant. For survival studies, Kaplan-Meier survival curves were plotted and analyzed using Prism 5.00 software (GraphPad Software).
Results
Construction and expression of DNA vaccines
We obtained the genes encoding scFvN418 by whole gene synthesis according to the published sequences [21]. The COOH terminus of the scFvN418 was directly fused in-frame to the sequences encoding the extracellular domain of HER2 (amino acids 1-222) or neu (amino acids 1-224) amplified from SK-BR-3 or TUBO breast cell lines, followed by Myc tag (Figure 1A). To confirm the expression of these constructs, 293T cells were transiently transfected with these plasmids, and then supernatants were harvested 72 hours later and tested for protein secretion by Western blotting. As shown in Figure 1B, we can detect the production of scFvN418-HER2 and scFvN418-neu proteins (lane 1, 2) or HER2 and neu fragments (lane 3, 4) in the supernatants by anti-Myc tag antibody respectively. Specific binding of scFvN418-HER2 fusion protein from culture supernatant to mouse dendrtic cells could be shown by fluorescence-activated cell sorting analysis (Figure 1C).
Figure 1.

Plasmid DNA vaccines encoding secreted scFvN418-HER2/neu fusion proteins. A. Schematic representation of expression vectors. scFvN418-HER2, scFvN418-neu, pcDNA3.1-HER2, or pcDNA3.1-neu encode under the control of a CMV promoter, all the fusion proteins consisting of an signal peptide, amino acid residues 1 to 222 of human HER2 or amino acid residues 1 to 224 of rat neu, and COOH-terminal Myc tag. The control plasmids pcDNA3.1-HER2 and pcDNA3.1-neu lack the scFvN418 domain. B. 293T cells grown in 100-mm dishes were transfected with various expression vectors using Lipofectamine 2000 (invitrogen). Immunoblot analysis of supernatants from 293T cells transfected with scFvN418-HER2, scFvN418-neu, pcDNA3.1-HER2 and pcDNA3.1-neu (lane 1, 2, 3 and 4). Vaccine proteins were probed with mouse anti-Myc tag mAb followed by HRP-conjugated secondary anti-mouse antibody. C. Binding of scFvN418-HER2 fusion protein from culture supernatant of transfected 293T cells to mouse dendrtic cells was investigated by fluorescence-activated cell sorting analysis with mAb 9E10 followed by PE-conjugated secondary antibody. Control cells were treated with supernatant from mock-transfected cells.
Protection of mice from challenge with HER2-expressing tumor cells
To investigate whether immunization with DC-targeted vaccines induce antitumoral immunity and protect animals from subsequent tumor challenge, BALB/c mice were i.m. vaccinated twice at two week interval with various vaccines. Seven days after last immunization, the mice were subcutaneously challenged with HER2-positive D2F2/E2 tumor cells, and tumor development was monitored. All animals vaccinated with scFvN418-HER2 remained tumor free upon challenge with D2F2/E2 cells (Figure 2A). In contrast, after vaccination with scFvN418-neu, HER2, neu or pcDNA3.1, no protection was apparent. In these instances, all animals developed continuously growing tumors and died by 60 days. Interestingly, vaccination with scFvN418-neu alone also moderately delayed tumor growth, although this preventive effect was not statistically significant. To examine whether HER2-specific responses induced by the vaccines were responsible for protection, a similar experiment was done using parental, HER2-negative D2F2 cells for tumor challenge. Rapid tumor growth was observed in all animals regardless of either treatment (Figure 2B), strongly suggesting that the observed rejection of HER2-expressing D2F2/E2 cells was due to HER2-specific immune responses induced by scFvN418-HER2.
Figure 2.

Vaccination with scFvN418-HER2 protects mice from challenge with HER2-expressing tumor cells and induces memory immune responses. A. Animals (10 mice per group) were vaccinated with HER2, neu, scFvN418-HER2 or scFvN418-neu on days -21 and -7. Control animals received pcDNA3.1. On day 0, mice were inoculated s.c. with D2F2/E2 tumor cells. Tumor developments were monitored, and animal survival was calculated. Left panel, kinetics of tumor growth; Right panel, survival curve. B. The vaccinated animals were challenged with parental HER2-negative D2F2 cells on day 0, Left panel, kinetics of tumor growth; Right panel, survival curve. C. The long-term surviving mice from scFvN418-HER2 group were rechallenged with D2F2/E2, D2F2, or syngeneic unrelated 4T1 tumor cells 3 months after initial tumor challenge. D. Naive mice injected s.c. with D2F2/E2, D2F2, or 4T1 tumor cells served as a control. Tumor growth was followed by caliper measurements, and results are represented as the mean tumor volume (mm3). The data were representative of two experiments with comparable results. Bars, SE. *P < 0.001, scFvN418-HER2 compared with other groups.
To test whether immunologic memory was developed, long-term surviving mice initially vaccinated with scFvN418-HER2 were rechallenged with D2F2/E2 tumor cells. The parental D2F2 cells or unrelated syngeneic 4T1 cells were used as controls. As shown in Figure 2C, the mice rejected subsequent rechallenges with the D2F2/E2 tumor cells and remained tumor-free, however, the mice could not reject unrelated syngeneic 4T1 tumor. Interestingly, about 60% of tumor surviving mice completely protected against the rechallenge with the parental D2F2 tumor cells, and the remaining mice displaying drastically slow tumor growth (Figure 2D), suggesting that vaccination and initial tumor challenge can result in determinant spreading and subsequent immunity to an otherwise parental HER2-negative tumor variants. In summary, these data indicates that scFvN418-HER2 vaccination induced long-lasting HER2-specific antitumor immunity, which can protect mice from HER2-expressing tumor challenge.
Induction of HER2-specific T cells
To analyze the nature of the immune responses induced by scFvN418-HER2, CD4+ T cells were isolated from the vaccinated mice and restimulated with HER2- or TRP2-pulsed bone marrow-derived DCs in vitro. As shown in Figure 3A, CD4+ T cells obtained from scFvN418-HER2-vaccinated mice showed vigorous proliferation upon restimulation with HER2-pulsed, but not TRP2-pulsed, bone marrow-derived DCs. A slightly increased proliferation was also detected from scFvN418-neu-vaccinated mice. In contrast, no evident T-cell proliferation could be observed when mice were vaccinated with untargeted HER2 or neu.
Figure 3.
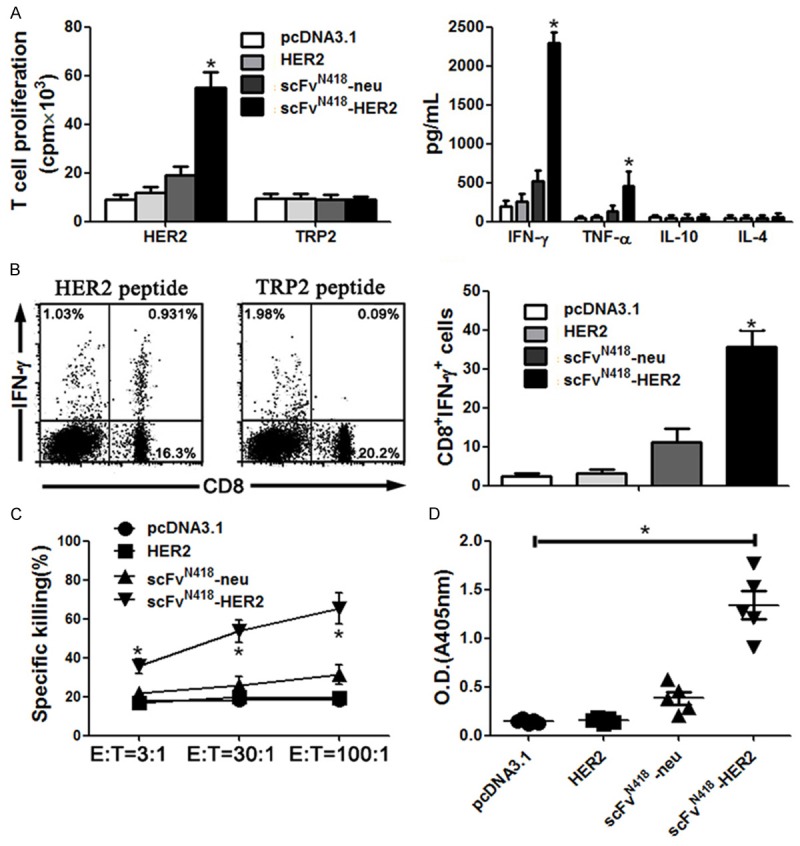
Vaccination with scFvN418-HER2 induced HER2-specific cellular and antibody immune response. BALB/c mice were vaccinated with HER2, scFvN418-neu or scFvN418-HER2. Control animals received pcDNA3.1. The draining lymph nodes and spleens were harvested from the vaccinated animals after two vaccinations. A. CD4+ T cells isolated from the draining lymph were cultured in the presence of 10 µg/ml recombinant HER2 or TRP2 protein for 4 d with the addition of [3H] thymidine in the last 16 h. T-cell proliferation was determined by [3H] thymidine incorporation (left panel). Right panel, the supernatant recovered from the assay in left was tested for cytokine production by ELISA. B. Splenocytes isolated from the vaccinated animals were stimulated for 6 h with H-2Kd-restricted HER263-71 peptide TYLPTNASL before flow cytometric analysis with anti-CD8 and anti-IFN-γ antibodies. Left, increase in CD8+ IFN-γ+ cells upon peptide stimulation of splenocytes. Representative results from one animal of scFvN418-HER2 group upon restimulation in the presence of HER2 or TRP2 peptide. Right, absolute numbers of CD8+ IFN-γ+ splenocytes (mean values from five mice per group). C. Splenocytes were cocultured with D2F2/E2 cells for 5 d. The resultant splenocytes (E) were cocultured for 4 h with the 51Cr-labeled target cells (T). Percentages of target cells killing by the splenocytes from the vaccinated mice are shown. Data represent the means of triplicate cultures and are representative of two independent experiments. D. HER2-specific total IgG and IgG subclass (IgG1 and IgG2a) antibodies in sera from the vaccinated animals after 1:100 dilution were determined by ELISA. The mean OD405 values of pooled sera from each group (5 mice per group) were presented. The background OD405 of normal mouse sera was < 0.04. Bars, SE. *P < 0.01, scFvN418-HER2 compared with other groups.
The supernatants of stimulated T cells were tested for the presence of cytokines by ELISA. Splenocytes obtained from scFvN418-HER2-vaccinated mice produced substantial amounts of TNF-α and IFN-γ (Figure 3A on right); similarly, a mildly higher level of IFN-γ and TNF-α cytokine was also detected in the supernatant from scFvN418-neu-vaccinated mice. We did not detect the secretion of IL-4 and IL-10 cytokines with immunosuppressive activity in any group.
Next, we checked for the induction of HER2-specific CD8+ T cells and CTLs. As shown in Figure 3B, splenocytes from scFvN418-HER2-vaccinated mice contained populations of activated CD8+ T cells that produced IFN-γ upon in vitro restimulation with HER2-derived synthetic p63-71 peptide TYLPTNASL whereas splenocytes from other group mice did not display an increase in CD8+ IFN-γ+ cells. These T cells were HER2-specific since no cells produced IFN-γ upon restimulation with TRP2180-188 peptide SVYDFFVWL, and a representative dot plot was shown in Figure 3B left. In addition, splenocytes from scFvN418-HER2-vaccinated mice exhibited significantly higher target cell killing than did those from other group mice (Figure 3C). The cytotoxic effect was mediated by CD8+ CTLs, because the killing was inhibited by the anti-CD8, but not anti-CD4, antibody (data not shown). Taken together, the results indicate the superiority after initial tumor cell inoculation.
Induction of HER2-specific antibody
We also evaluated the induction of HER2-specific antibody in these mice. As shown in Figure 3D, vaccination with scFvN418-HER2 induced a high titer of HER2-specific antibody specifically binding to recombinant HER2 protein in ELISA experiments. Detailed analysis of antibody isotype demonstrated that antibody induced by scFvN418-HER2 vaccine was mainly IgG2a, which is consistent with the cytokine profile of splenocytes.
scFvN418-HER2 immunization combination with Treg depletion eradicated the established tumors
We next evaluated the therapeutic effect of scFvN418-HER2 vaccination on established tumors in D2F2/E2 breast tumor model. BALB/c mice were subcutaneously inoculated with D2F2/E2 tumor cells. On day 10, animals with tumors sizing ~40 mm3 were randomized into groups treated with scFvN418-HER2, HER2 or pcDNA3.1. Treatment was repeated once 2 weeks later. As shown in Figure 4A, scFvN418-HER2 vaccination substantially slowed tumor development and protected up to 20% only of the mice from tumor growth at the end of experiment (120 days after tumor inoculation).
Figure 4.
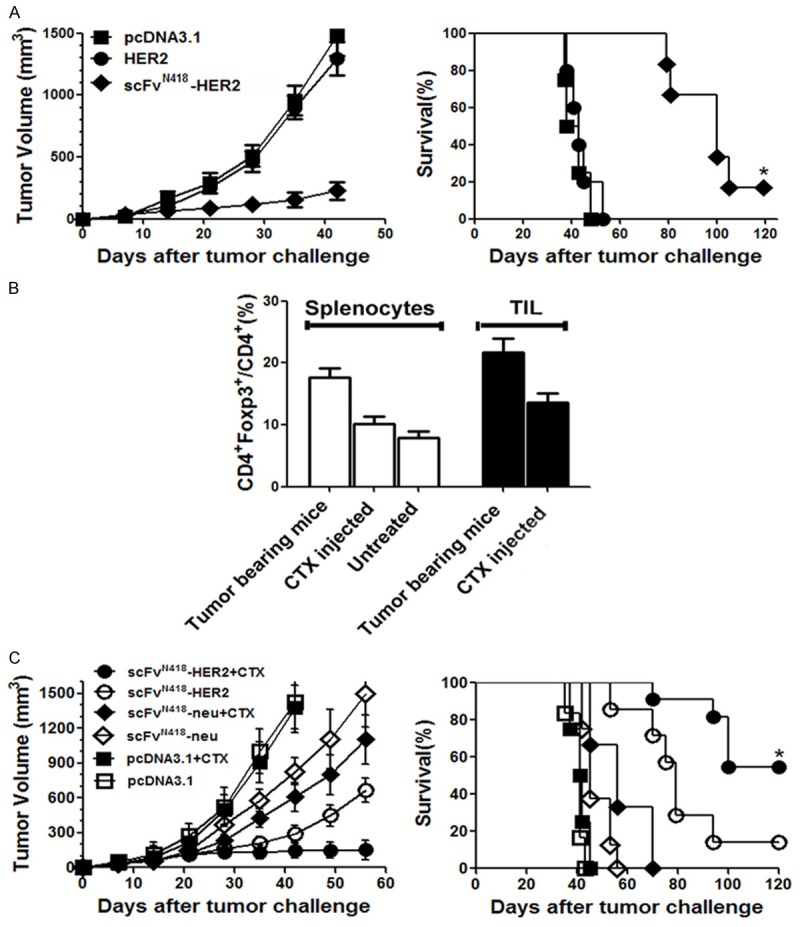
Therapeutic efficacy of scFvN418-HER2 vaccine. A. BALB/c mice (10 mice per group) were inoculated s.c. with D2F2/E2 tumor cells. On day 10, animals with tumors sizing ~40 mm3 were immunized with scFvN418-HER2 or respective controls. Treatment was repeated on day 24. Tumor developments were monitored, and animal survival was calculated. Left panel, kinetics of tumor growth; Right panel, survival curve. The data were represented as the mean tumor volume (mm3) and representative of two experiments with comparable results. *P < 0.01, scFvN418-HER2 compared with other groups. B. Temporary depletion of CD4+ Foxp3+ regulatory T cells by a single injection of low-dose CTX. BALB/c mice (3 mice per group) were inoculated s.c. with D2F2/E2 tumor cells. When the tumors were 2-3 mm in diameter (day 8), mice were injected i.p. with CTX or PBS. Naive mice were used as control. The spleen or tumor-infiltrating lymphoid cells (TIL) were harvested and analyzed for the regulatory T cells 4 days later. Percentage of CD4+ Foxp3+ in total CD4+ cells (mean values from three mice per group). Bars, SE. C. BALB/c mice (10 mice per group) were inoculated s.c. with D2F2/E2 tumor cells. When the tumors were 2-3 mm in diameter (day 8), mice received CTX injection. Four days later (day 12), animals were vaccinated with various DNA vaccines. Treatment was repeated after two weeks. Left panel, kinetics of tumor growth; Right panel, survival curve. The data were represented as the mean tumor volume (mm3) and representative of two experiments with comparable results. *P < 0.01, scFvN418-HER2/CTX compared with other groups.
Since regulatory T cells (Treg) have been shown to mediate immune-tolerance towards tumor-antigens in various tumor models, we further tested whether systemic depletion of regulatory T cells would increase the therapeutic efficacy of DC-targeting vaccines. An approach that we and others successfully applied in various models utilized intraperitoneal injection of low-dose (100 mg/kg) cyclophosphamide (CTX). As shown in Figure 4B, low-dose CTX injection in the D2F2/E2-bearing mice spleen or tumor-infiltrating lymphoid cells (TIL) efficiently depleted Treg 4 days post-injection, however, no evidently direct killing effect on tumor cells were observed (data not shown). We therefore tested DC-targeted vaccines in combination with Treg depletion by low-dose CTX. As shown in Figure 4C, this combination significantly improved the therapeutic effects of scFvN418-HER2 vaccine; at the end of experiment, 75% (15/20) mice rejected the established tumor and remaining 5 (25%) mice had stably small tumors (~40 mm3). These tumor-free mice also rejected the rechallenge with the same tumor cells (date not shown). Untargeted DC vaccines failed to exert therapeutic effects although this vaccine in combination with CTX mildly delayed tumor growth. The experiment was repeated with similar results. The data indicate that DC-targeting vaccines are able to induce strong antitumoral activity, when in combination with systemic Treg depletion, mount impressive tumor-rejecting effects.
Antitumoral activity of the scFvN418-neu DNA vaccine in BALB-neuT mice
Although tumor models based on human HER2-expressing D2F2/E2 cells are useful to assess the basic functionality of cancer vaccines, such models do not fully reflect the situation of human cancer usually characterized by immunologic tolerance toward HER2. Hence, we further tested fusion protein vaccines in female BALB-neuT mice that represent an immunotolerant model of spontaneous cancer [22,23]. Because human HER2 and rat neu proteins are not fully identical in their amino acid sequences [24], we used scFvN418-neu, which is similar to scFvN418-HER2 but fuses scFvN418 with the corresponding rat neu fragment.
We first evaluated the preventive efficacy of scFvN418-neu vaccine using transplantable neu-expressing TUBO tumor model in BALB-neuT mice. BALB-neuT mice received twice scFvN418-neu or control vaccination at two weeks interval. One week after last vaccination, the animals were challenged with TUBO tumor cells. As shown in Figure 5A, the animals receiving scFvN418-neu vaccination were significantly protected against a subsequent challenge with TUBO cells. Sixty days after tumor challenge, 80% (8/10) mice in this group remained tumor free and 2 mice had small tumors (~40 mm3). The experiment was repeated with similar results.
Figure 5.
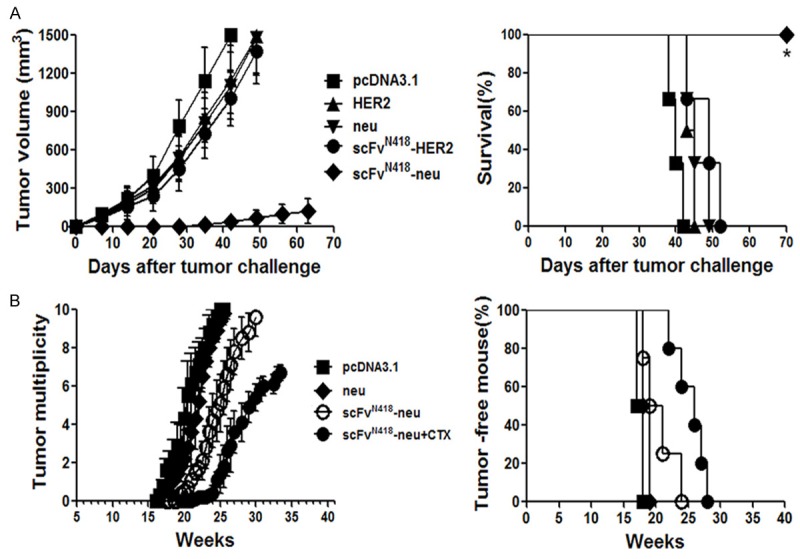
Protective effects of scFvN418-neu in transgenic BALB-neuT mice. A. Female BALB-neuT mice (10 mice per group) were vaccinated with neu, HER2, scFvN418-HER2 or scFvN418-neu in left hind limb on days -21 and -7. Control animals received pcDNA3.1. On day 0, mice were inoculated s.c. with neu-expressing TUBO cells in opposite flank. Left panel, kinetics of tumor growth; Right panel, survival curve. *P < 0.01, scFvN418-neu compared with other groups. B. Animals were immunized neu or scFvN418-neu twice at weeks 8 and 10. One group mice with scFvN418-neu vaccination also received CTX injection 4 days before the first vaccination. Control animals received pcDNA3.1. Development of mammary tumors was monitored by manual examination of the mammary glands once every week. Measurable masses of > 2 mm diameter were regarded as tumors. Points, mean number of tumors in each group (tumor multiplicity; left panel) and percentage of tumor-free mice (right panel); bars, SE. All results were representative of two to three independent experiments. *P < 0.01, scFvN418-neu /CTX compared with other groups.
The effect of scFvN418-neu vaccination in the prevention of spontaneous mammary tumors that naturally arise in BALB-neuT mice was also evaluated. The scFvN418-neu was given to the mice at week 8 from birth when diffuse atypical hyperplasia is already evident in the mammary glands but before in situ carcinoma is evident and repeated at week 10. Mice in every group also received CTX injection 4 days before the first vaccination. As shown in Figure 5B, scFvN418-neu/CTX vaccination resulted in a significant prolongation of tumor-free survival. This corresponded with a marked delay (~3 weeks) in the appearance of macroscopically detectable tumors in the mammary glands of these mice. By week 35, all of the mice that were vaccinated with scFvN418-neu/CTX remained alive. In contrast, by week 26, all of the mice in the control groups had large tumors and required euthanasia.
Discussion
In the present study, we investigated protective and therapeutic effects of DNA vaccines that consist of scFvN418 fused to the extracellular domain of the HER2/neu antigen. The results showed that scFvN418-antigen DNA vaccine induced potent antigen-specific T-cell and antibody responses and protected mice from subsequent challenge with antigen-positive tumor cells. Furthermore, scFvN418-HER2 immunization combination with Treg depletion by low-dose CTX eradicated established HER2-expressing tumors in a therapeutic setting. More importantly, scFvN418-neu vaccination significantly protected against a subsequent challenge with neu-expressing tumor cells and combination with low-dose CTX markedly delayed the onset of spontaneous mammary carcinomas in immunotolerant BALB-neuT mice.
Dendritic cells are known to express several relative receptors with the potential to boost antigen uptake [25,26]. In our research CD11c was selected as target receptor, because the rationale originated in prior research showing that its involvement in endocytosis, and it could greatly increase antigen presentation and immune responses, and specific monoclonal antibodies were available against these receptors that could be used for scFv design. To date, indicating antigens delivered by CD11c enter into both MHC classes I and II processing and presentation pathways.
DNA vaccines are usually injected into muscle or skin. Transfected muscle cells at sites of DNA injection clearly express antigen and act as a target for immune effector cells. The secretion of scFvN418 vaccines expressed in myocytes or keratinocytes after the vaccination. Encoded antigen then would be transferred to DCs targeted by the anti-CD11c. This indirect process of transfer of antigenic material is termed cross presentation because muscle cells synthesize the vaccine antigen, which then crosses into DCs for processing and presentation to T cells [27]. A small proportion of DNA is also taken up directly by DCs and the encoded antigen can then be processed and presented endogenously. Cross presentation performs as a capital mechanism for the induction of T cell responses following DNA vaccination. Moreover, scFvN418 targeting DNA vaccines can induce immunity with no additional external adjuvant, presumably because the DNA itself provides some signals for DC maturation [28,29].
As we know that T regulatory cells (Tregs) might induce tolerance status through modulation of dendritic cell number and/or activity, and render dendritic cells inefficient as antigen-presenting cells and this effect was accompanied with increased TGF-β and IL-10 secretion and reduced expression of costimulatory molecules on dendritic cells [30]. From previous study, a low dose of cyclophosphamide significantly decreases the number while inhibits the immunosuppression activity of the residual Tregs [31]. Hence, we decided to investigate the antitumor activity of the DC targeted vaccines association with cyclophosphamide, as a effective therapeutic combination for mammary cancer. As what we expected, our experiment interpret that a low dose of cyclophosphamide and optimal doses of scFvN418-HER2 eradicate the established tumors induced by breast carcinoma. This immunomodulatory method seems to be a feasible strategy to break the tolerance induced by breast carcinoma. Moreover, a single injection of low-dose cyclophosphamide not only deplets of CD4+ CD25+ Foxp3+ Tregs but also can switch towards Th1 response, resulted to tip the balance towards the generation of antitumor immunity [32].
In conclusion, this study shows that targeting vaccine antigens to DCs association with CTX in vivo may offer us with a effective way to eliminate pre-established tumors and to treat diseases. The CD11c molecule is expressed by human DCs, so coupling of tumor antigens to it, in combination with cyclophosphamide treatment, has important implications for tumor gene therapy, especially as a useful target for developing vaccination strategies in clinical trials.
Acknowledgements
We thank Yao Xu and Jing Zhang in Shanghai Shidong Hospital for their excellent technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Mwangi W, Brown WC, Lewin HA, Howard CJ, Hope JC, Baszler TV, Caplazi P, Abbott J, Palmer GH. DNA-encoded fetal liver tyrosine kinase 3 ligand and granulocyte macrophage-colony-stimulating factor increase dendritic cell recruitment to the inoculation site and enhance antigen-specific CD4+ T cell responses induced by DNA vaccination of outbred animals. J Immunol. 2002;169:3837–3846. doi: 10.4049/jimmunol.169.7.3837. [DOI] [PubMed] [Google Scholar]
- 2.Pokorna D, Cerovska N, Smahel M, Moravec T, Ludvikova V, Mackova J, Synkova H, Duskova M, Hozak P, Veleminsky J. DNA vaccines based on chimeric potyvirus-like particles carrying HPV16 E7 peptide (aa 44-60) Oncol Rep. 2005;14:1045–1053. [PubMed] [Google Scholar]
- 3.Qin H, Zhou C, Wang D, Ma W, Liang X, Lin C, Zhang Y, Zhang S. Enhancement of antitumour immunity by a novel chemotactic antigen DNA vaccine encoding chemokines and multiepitopes of prostate-tumour-associated antigens. Immunology. 2006;117:419–430. doi: 10.1111/j.1365-2567.2006.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zong J, Peng Q, Wang Q, Zhang T, Fan D, Xu X. Human HSP70 and modified HPV16 E7 fusion DNA vaccine induces enhanced specific CD8+ T cell responses and anti-tumor effects. Oncol Rep. 2009;22:953–961. doi: 10.3892/or_00000522. [DOI] [PubMed] [Google Scholar]
- 5.Mahnke K, Qian Y, Fondel S, Brueck J, Becker C, Enk AH. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005;65:7007–7012. doi: 10.1158/0008-5472.CAN-05-0938. [DOI] [PubMed] [Google Scholar]
- 6.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 7.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, Vremec D, Pietersz S, Lahoud MH, Schofield L, Hansen DS, O’Keeffe M, Smyth MJ, Bedoui S, Davey GM, Villadangos JA, Heath WR, Shortman K. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, Brockstedt D, Dubensky TW, Stins MF, Lanier LL, Pardoll DM, Housseau F. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 10.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 11.Beyer M, Wang H, Peters N, Doths S, Koerner-Rettberg C, Openshaw PJ, Schwarze J. The beta2 integrin CD11c distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Respir Res. 2005;6:70. doi: 10.1186/1465-9921-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro FV, Tutt AL, White AL, Teeling JL, James S, French RR, Glennie MJ. CD11c provides an effective immunotarget for the generation of both CD4 and CD8 T cell responses. Eur J Immunol. 2008;38:2263–2273. doi: 10.1002/eji.200838302. [DOI] [PubMed] [Google Scholar]
- 13.Kurts C. CD11c: not merely a murine DC marker, but also a useful vaccination target. Eur J Immunol. 2008;38:2072–2075. doi: 10.1002/eji.200838645. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 15.Bei R, Masuelli L, Moriconi E, Visco V, Moretti A, Kraus MH, Muraro R. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene. 1999;18:1267–1275. doi: 10.1038/sj.onc.1202442. [DOI] [PubMed] [Google Scholar]
- 16.Klapper LN, Kirschbaum MH, Sela M, Yarden Y. Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv Cancer Res. 2000;77:25–79. [PubMed] [Google Scholar]
- 17.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 18.Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the immunotherapy and prevention of cancer. J Clin Invest. 2004;113:1515–1525. doi: 10.1172/JCI21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, Wang H, Lu B, Li B, Hou S, Qian W, Fan K, Dai J, Zhao J, Guo Y. Cancer immunotherapy using in vitro genetically modified targeted dendritic cells. Cancer Res. 2008;68:3854–3862. doi: 10.1158/0008-5472.CAN-07-6051. [DOI] [PubMed] [Google Scholar]
- 21.Demangel C, Zhou J, Choo AB, Shoebridge G, Halliday GM, Britton WJ. Single chain antibody fragments for the selective targeting of antigens to dendritic cells. Mol Immunol. 2005;42:979–985. doi: 10.1016/j.molimm.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–7740. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 23.Rolla S, Nicolo C, Malinarich S, Orsini M, Forni G, Cavallo F, Ria F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol. 2006;177:7626–7633. doi: 10.4049/jimmunol.177.11.7626. [DOI] [PubMed] [Google Scholar]
- 24.Nagata Y, Furugen R, Hiasa A, Ikeda H, Ohta N, Furukawa K, Nakamura H, Furukawa K, Kanematsu T, Shiku H. Peptides derived from a wild-type murine proto-oncogene c-erbB-2/HER2/neu can induce CTL and tumor suppression in syngeneic hosts. J Immunol. 1997;159:1336–1343. [PubMed] [Google Scholar]
- 25.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 26.You Z, Huang X, Hester J, Toh HC, Chen SY. Targeting dendritic cells to enhance DNA vaccine potency. Cancer Res. 2001;61:3704–3711. [PubMed] [Google Scholar]
- 27.Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 28.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, Bouchard P, Wolf S, Modesti A, Musiani P, Lollini PL, Colombo MP, Forni G. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589–596. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC, Berzofsky JA. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol. 2005;174:4228–4236. doi: 10.4049/jimmunol.174.7.4228. [DOI] [PubMed] [Google Scholar]
- 30.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 31.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom I, Lieber A. Effect of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Liu J, Weng D, Chen Y, Song L, He Q, Chen J. CD4+CD25+Foxp3+ regulatory T cells depletion may attenuate the development of silica-induced lung fibrosis in mice. PLoS One. 2010;5:e15404. doi: 10.1371/journal.pone.0015404. [DOI] [PMC free article] [PubMed] [Google Scholar]


