Abstract
Background: The aim of the study was to evaluate clinical manifestations, T-SPOT, endoscopy and CT enterography to differentiate Crohn’s disease (CD) from intestinal tuberculosis (ITB). Methods: 128 in patients with suspected CD and ITB were prospectively enrolled in the study. Demographic, clinical, laboratory, endoscopic and CT enterographic data were collected. After treatment for 6 months, when a definite diagnosis was reached, the differential diagnostic value of each parameter was analyzed. Multivariable logistic regression was used to analyze further, parameters of statistical significance to establish a mathematical regression equation. Receiver operating characteristic curves were plotted. Results: Clinical parameters helpful in differentiating CD from ITB included diarrhea, night sweat and perianal disease. Endoscopic parameters were useful in differentiating CD from ITB including transverse ulcers, longitudinal ulcers, rodent-like ulcers and patulous ileocecal valve. CT enterographic parameters aided the identification of the two conditions. The sensitivity, specificity, accuracy, positive predictive value and negative predictive value of a mathematical regression model established for 6 parameters of clinical endoscopy and CT enterography were 97.8%, 96.8%, 97.6%, 98.9% and 93.7% respectively, whereas those for T-SPOT were 96.8%, 91.3%, 92.7%, 78.9% and 98.8% respectively. Conclusions: T-SPOT is useful to exclude a diagnosis of ITB. Differentiating CD from ITB is a difficult clinical problem that requires a consideration of clinical, T-SPOT, endoscopic and CT enterographic parameters for accurate diagnosis.
Keywords: Intestinal tuberculosis, Crohn’s disease, differential diagnosis
Introduction
Crohn’s disease (CD) and intestinal tuberculosis (ITB) are both ulcerative diseases that can occur in any segment of the gastrointestinal tract, especially the distal ileum, ileocecal region or the ascending colon. The past two decades have seen a great increase in the incidence of CD, with an estimated 3-fold increase in China. These changes may be due to an increased contact with the West, westernization of diet, increased use of antibiotics, improved hygiene, vaccinations or changes in the gut microbiota [1,2]. Also, as a developing country, China still suffers a high TB prevalence compared to western countries, with ITB remaining as common as extra-pulmonary tuberculosis [3].
Distinguishing CD from ITB is always a tough problem for clinicians due to their overlapping manifestations in clinical, laboratory, endoscopy and radiology tests. In recent few years, numerous studies have focused on the differential diagnosis of CD and ITB [4-6], with a number of differentiating parameters having been described. However, in clinical practice, most of these parameters lack specificity and only exist during specific stages of the diseases. Furthermore, none of the studies has weighed all of the differentiating parameters. In most cases, physicians make a diagnosis according to their own experience, which leads to bias. Steroid and other immunosuppressants that are effective in the treatment of CD patients may be disastrous in ITB patients. It is an inconvenient truth that the rate of administration of anti-TB chemotherapy remains high, which may delay effective medical treatment, increase drug resistance, add unnecessary medical costs and furthermore, make patients suffer from adverse drug side effects [7].
The past decade has seen T-cell based interferon-gamma release assays (IGRAs) evolve to be an effective diagnostic tool in detecting both active and latent TB [8,9]. T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) is one of the two commercially available methods for IGRAs that has been introduced to our hospital. In recent years, numerous studies have been carried out focusing on T-SPOT alone as a diagnostic tool in differentiating CD from ITB [10,11]. Ng et al. carried out a meta-analysis that proved T-SPOT had high sensitivity for the diagnosis of ITB. Furthermore, negative findings with T-SPOT may be very helpful in excluding a diagnosis of ITB [12].
Since it was introduced clinically, endoscopy has played a crucial role in the detection of gastrointestinal luminal lesions. Although CD and ITB have overlapping features, some parameters revealed by endoscopy are still helpful in distinguishing one condition from the other [5,13].
CT enterography is a new imaging tool that can produce better visualization of the bowel wall and extraluminal lesions, and several studies have proved its important role in the differential diagnosis of CD versus ITB [14,15].
In recent years, our department has carried out much practical research into the differential diagnosis of lower GI tract ulcers, particularly those associated with CD and ITB. In this study, we prospectively enrolled 128 patients with suspected CD or ITB, and evaluated the value of clinical, laboratory, endoscopic and CT enterographic parameters to make an accurate diagnosis.
Methods
Patients enrolled
The institutional review board approved our study and informed written consent was obtained from all patients. We enrolled 128 inpatients suspected for CD or ITB based on clinical, laboratorial, endoscopic and radiologic findings at the Department of Gastroenterology, Shanghai Ruijin Hospital from March 2013 to December 2014. Exclusion criteria included patients who didn’t give consent, those with human immunodeficiency virus (HIV) infection, inherited or acquired immunodeficiency, those who already had a definite diagnosis. Furthermore, patients who received anti-TB chemotherapy or immunosuppressive medication within the previous 3 months were excluded from the study.
Clinical evaluation
All enrolled patients suspected of having CD or ITB were prospectively evaluated. Baseline information that included demographic, clinical, laboratory, endoscopic and CT enterographic data were collected by two researchers, and the data entered into a Microsoft Excel spreadsheet.
Demographic features included age and gender. The clinical parameters were duration of symptoms, abdominal pain, anorexia, diarrhea, constipation, hematochezia, fatigue, fever, night sweat, weight loss, perianal disease, appendectomy history, history of bowel obstruction or pulmonary TB, extraintestinal symptoms and ascites. Hemoglobin, hematocrit, serum albumin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and T-SPOT were documented as laboratory parameters. T-SPOT regarded positive for either the following two criteria was met. (1) If the value of blank control (BC) is 0-5, either ESAT-6 or CFP-10 minus BC is greater than or equal to 6. (2) If the value of BC is 6-10, either ESAT-6 or CFP-10 is greater than or equal to twice the value of BC.
Colonoscopic findings mainly included transverse ulcers, longitudinal ulcers, rodent-like ulcers, aphthoid ulcers, patulous ileocecal valve, cobblestone appearance, stricture of the bowel and anorectal lesions.
All of our patients had undergone CT enterography at least once. Features included bowel wall thickness, skip lesions, asymmetric pattern of involvement, contracture of ileocecal valve, fixed patulous ileocecal valve, intraluminal pseudopolyp formation, lymph node with central necrosis, lymph node with calcification, ascites, comb sign, phlegmon, abscess, fistulas and bowel obstruction.
Diagnostic criteria and follow-up
A primary diagnosis of CD was based on morphological (radiological, endoscopic or surgical findings) and pathological criteria suggesting focal, asymmetrical, transmural or granulomatous features [16]: (1) morphological: (a) discontinuous/segmental and asymmetrical mucosal involvement, (b) deep mucosal longitudinal fissures/ulcers, (c) transmural inflammation, (d) rigid and strictured intestinal wall, (e) presence of entero-cutaneous/entero-enteric fistula and/or chronic perianal disease and/or other extraintestinal complications; (2) pathological: (a) normal mucus content in the goblet cells of the inflamed region, (b) lymphocyte aggregation in the mucosa and submucosa, (c) non-caseating granuloma, (d) longitudinal ulcers/fissures, (e) transmural inflammation or inflammation beyond the mucosa. For a primary diagnosis of CD, the following criteria were used: presence of at least 3 different criteria or presence of non-caseating granuloma on histology with at least 1 other criterion, exclusion of TB (by the criteria listed below). After a diagnosis of CD, we evaluated the classification, grading, high risk factors for the patient, and then gave the patient 5-aminosalicylic acid, steroids, immunosuppressants or biological agents as therapy.
Primary diagnosis of ITB was established when any of the following criteria were met [17]: (1) presence of caseous necrosis granuloma on histology of biopsy tissue; (2) demonstration of acid-fast bacilli (AFB) on smear or histological sections; (3) positive culture for M. tuberculosis; (4) histologically or microbiologically confirmed TB at the extra-intestinal site; (5) a positive result of T-SPOT; (6) rodent-like or transverse ulcers observed during endoscopy; (7) contracture of ileocecal valve, fixed patulous ileocecal valve or lymph nodes with central necrosis under CT enterography. For the primary diagnosis of ITB, at least one of the first three criteria or two of the latter four criteria had to be fulfilled. After a diagnosis of ITB, we gave the patient diagnostic anti-TB chemotherapy (HREZ2/HR8-10).
For those patients when it was difficult to establish the primary diagnosis, diagnostic anti-TB chemotherapy (HREZ2/HR8-10) was given. After treatment for 6 months, definite diagnoses of all patients were reached according to the resolution of symptoms and morphological (endoscopic and radiologic) features.
Statistical analysis
SPSS 19.0 was used for data analysis and to screen for potential valuable parameters for the differential diagnosis of CD and ITB. Continuous variables were expressed as mean ± SD and a comparison was performed using Student’s t-test depending on a normal data distribution. Binary categorical variables were expressed as a frequency and a percentage, while comparisons were made using a chi-square test or Fisher’s exact test. A probability (P) value of less than 0.05 was considered to be statistically significant. Valuable parameters, which reached statistical significance, were further analyzed by multivariate logistic regression to establish a differentiating mathematical model. Confidence intervals and the odds ratios of these parameters were evaluated and receiver operating characteristic (ROC) curves plotted and predictive diagnosis points obtained. Sensitivity, specificity, accuracy, PPV and NPV were calculated to evaluate the diagnostic efficacy of this model.
Results
Demographic, clinical and laboratory features of patients with CD and ITB
After a median of 6 months’ follow-up, 123 out of a total of 128, where there was difficulty in making a differential diagnosis between CD and ITB, finally received a definite diagnosis, with 92 cases of CD and 31 cases of ITB. The other 5 cases were diagnosed as Bechet disease (1/5), lymphoma (1/5), non-specific colitis (2/5) and an unconfirmed diagnosis (1/5).
Demographic and clinical features of CD and ITB are listed in Table 1. No significant difference between the 2 groups was found with respect to the patients’ gender, age of onset of the disease and the duration of symptoms. For other parameters, the occurrence of diarrhea and perianal disease in CD was significantly higher than in ITB (P < 0.05). In contrast, the occurrence of night sweat was significantly higher in ITB patients than in CD patients (P < 0.05).
Table 1.
Demographic, clinical and laboratory parameters of CD and ITB patients
| Parameters | CD (n = 92) | ITB (n = 31) | P | Code |
|---|---|---|---|---|
| Demographic and clinical parameters | ||||
| Gender (male/female) | 47/45 | 17/14 | 0.718 | X1 |
| Age | 36.54 ± 15.98 | 32.80 ± 16.14 | 0.328 | X2 |
| Duration of symptoms (months) | 22.77 ± 26.32 | 14.42 ± 15.21 | 0.067 | X3 |
| Abdominal pain | 75 (81.5) | 24 (77.4) | 0.618 | X4 |
| Anorexia | 23 (25.0) | 13 (41.9) | 0.073 | X5 |
| Diarrhea | 73 (79.3) | 13 (41.9) | < 0.001 | X6 |
| Constipation | 5 (5.4) | 2 (6.5) | 0.835 | X7 |
| Hematochezia | 22 (23.9) | 6 (19.4) | 0.601 | X8 |
| Fatigue | 25 (27.1) | 14 (45.2) | 0.063 | X9 |
| Fever | 21 (22.8) | 12 (38.7) | 0.084 | X10 |
| Night sweat | 3 (3.3) | 8 (25.8) | 0.001 | X11 |
| Weight loss | 58 (63.0) | 22 (71.0) | 0.424 | X12 |
| Perianal disease | 33 (35.9) | 3 (9.7) | 0.006 | X13 |
| Appendectomy | 10 (10.9) | 2 (6.5) | 0.455 | X14 |
| Bowel obstruction | 14 (15.2) | 3 (9.7) | 0.424 | X15 |
| Pulmonary TB | 6 (6.5) | 5 (16.1) | 0.108 | X16 |
| Extraintestinal symptoms | 9 (9.8) | 1 (3.2) | 0.207 | X17 |
| Ascites | 7 (7.6) | 6 (19.4) | 0.066 | X18 |
| Laboratory parameters | ||||
| Hemoglobin (g/L) | 116.41 ± 15.86 | 119.04 ±16.58 | 0.477 | X19 |
| Hematocrit | 33.30 ± 5.43 | 35.64 ± 4.77 | 0.060 | X20 |
| Albumin (g/L) | 34.77 ± 5.83 | 35.15 ± 6.91 | 0.102 | X21 |
| Erythrocyte sedimentation rate | 24.53 ± 22.47 | 26.47 ± 25.11 | 0.722 | X22 |
| C-reactive protein | 22.13 ± 27.17 | 19.1 ± 30.73 | 0.648 | X23 |
| T-SPOT.TB | 8 (8.7) | 30 (96.8) | < 0.001 | X24 |
The routine laboratory tests carried out are listed in Table 1. CD and ITB patients exhibited no significant difference in level of serum hemoglobin, hematocrit, albumin, ESR and CRP. T-SPOT was found to be positive in 8.7% (8/92) of CD patients and much higher 96.8% (30/31) in ITB patients (P < 0.05).
Endoscopic and CT enterographic features of patients with CD and ITB
Endoscopic and CT enterographic parameters of CD and ITB are summarized in Table 2. The morphology of ulcers under endoscopy was quite different in CD and ITB patients. Transverse and rodent-like ulcers (Figures 1D, 3A, 3B) were more apparent in patients with ITB (P < 0.05), whereas longitudinal ulcers (Figures 2B, 4B, 4D) were more common in patients with CD (P < 0.05). We also found that patulous ileocecal valve (Figures 1D, 3A) was more frequently observed in ITB patients through endoscopy (P < 0.05).
Table 2.
Endoscopic and CT enterographic parameters of CD and ITB patients
| Parameters | CD (n = 92) | ITB (n = 31) | P | Code |
|---|---|---|---|---|
| Endoscopic parameters | ||||
| Transverse ulcers | 17 (18.5) | 20 (64.5) | < 0.001 | X25 |
| Longitudinal ulcers | 74 (80.4) | 18 (58.1) | 0.013 | X26 |
| Rodent-like ulcers | 4 (4.3) | 10 (32.3) | < 0.001 | X27 |
| Aphthoid ulcers | 41 (44.6) | 9 (29.0) | 0.128 | X28 |
| Patulous ileocecal valve | 7 (7.6) | 12 (38.7) | < 0.001 | X29 |
| Cobblestone appearance | 31 (33.7) | 10 (32.3) | 0.883 | X30 |
| Stricture of bowel | 17 (18.5) | 4 (12.9) | 0.465 | X31 |
| Anorectal lesions | 16 (17.4) | 2 (6.5) | 0.109 | X32 |
| CT enterograhic parameters | ||||
| Bowel wall thickness | 89 (96.7) | 29 (93.5) | 0.458 | X33 |
| Skip lesions | 75 (81.5) | 6 (19.4) | < 0.001 | X34 |
| Asymmetric pattern of involvement | 37 (40.2) | 0 (0.0) | < 0.001 | X35 |
| Contracture of ileocecal valve | 7 (7.6) | 8 (25.8) | 0.007 | X36 |
| Fixed patulous ileocecal valve | 2 (2.2) | 13 (41.9) | < 0.001 | X37 |
| Intraluminal pseudopolyp formation | 56 (60.9) | 13 (41.9) | 0.066 | X38 |
| Lymph node with central necrosis | 0 (0.0) | 11 (35.5) | < 0.001 | X39 |
| Lymph node with calcification | 0 (0.0) | 2 (6.5) | 0.018 | X40 |
| Ascites | 7 (7.6) | 9 (29.0) | 0.002 | X41 |
| Comb sign | 84 (91.3) | 10 (32.3) | < 0.001 | X42 |
| Phlegmon | 13 (14.1) | 0 (0.0) | 0.018 | X43 |
| Abscess | 11 (12.0) | 2 (3.2) | 0.313 | X44 |
| Fistula | 36 (39.1) | 3 (9.7) | 0.001 | X45 |
| Bowel obstruction | 18 (19.6) | 2 (6.5) | 0.070 | X46 |
Figure 1.
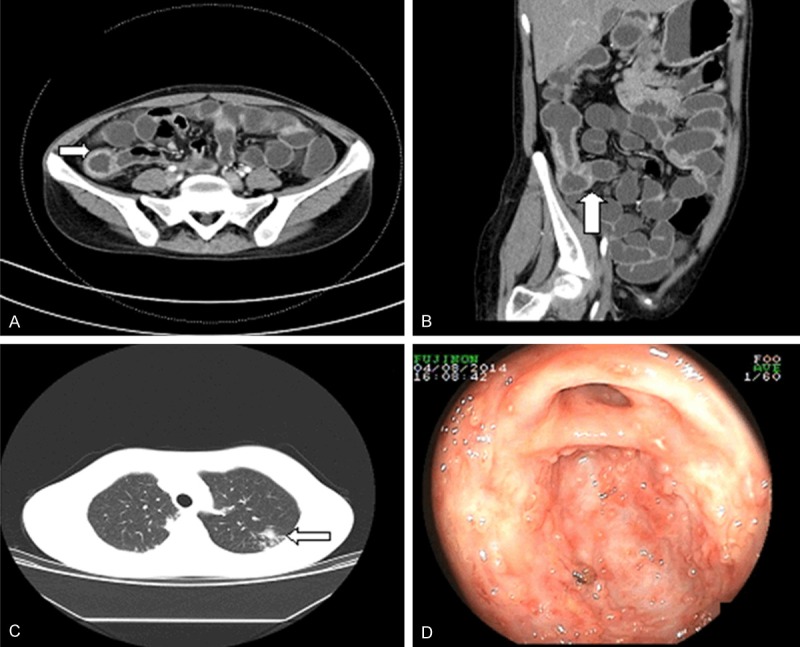
ITB and pulmonary TB in a 26-year-old woman who was referred to our department with a complaint of weight loss and abdominal pain. A. CT enterography indicated bowel wall thickening with a symmetric involvement and mucosal enhancement in the ileocecal region. B. Coronary reconstructed mode reflected a fixed patulous ileocecal valve (arrow). C. Pulmonary CT revealed nodules in the left upper lobe with exudation and proliferation (arrow). D. Colonoscopy showed transverse ulcers and a patulous ileocecal valve.
Figure 3.
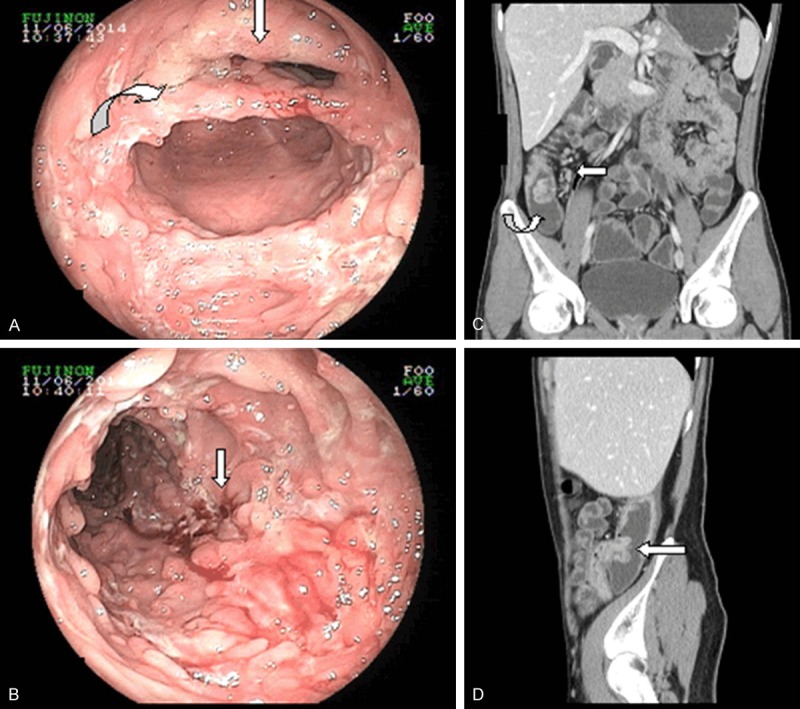
ITB in a 23-year-old woman, who was referred to our department with a complaint of night sweat and anorexia. A. Colonoscopy revealed a patulous ileocecal valve (arrow) and transverse ulcers (curve). B. Ulcers in ITB patients appeared to be rodent-like, with an unclear base and bleeding as a result of TB infection (arrow). C. Coronary reconstructed mode of a CT enterography reflected bowel wall thickness with enhancement and a fixed patulous ileocecal valve (curve). Enlarged lymph nodes are distributed along the right colic artery (arrow). D. Sagittal reconstructed mode showing a patulous ileocecal valve with a fish-mouth-shape.
Figure 2.
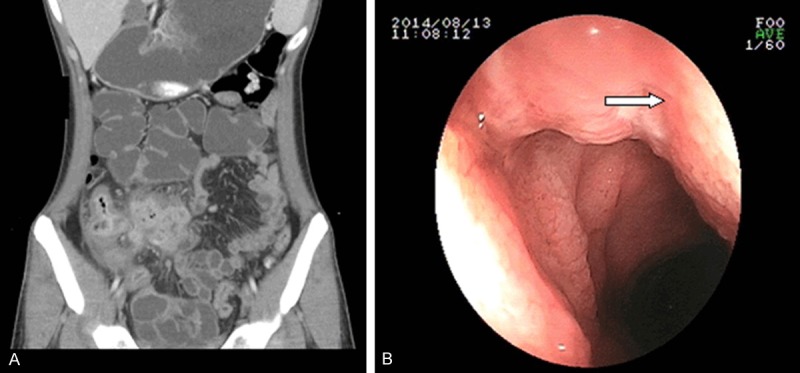
CD of a 30-year-old man who complained of intermittent fever for 2 months. A. Coronary reconstructed CT enterography revealed phlegmon (arrow). B. Endoscopy revealed a longitudinal ulcer that stretched across several intestinal folds (arrow).
Figure 4.
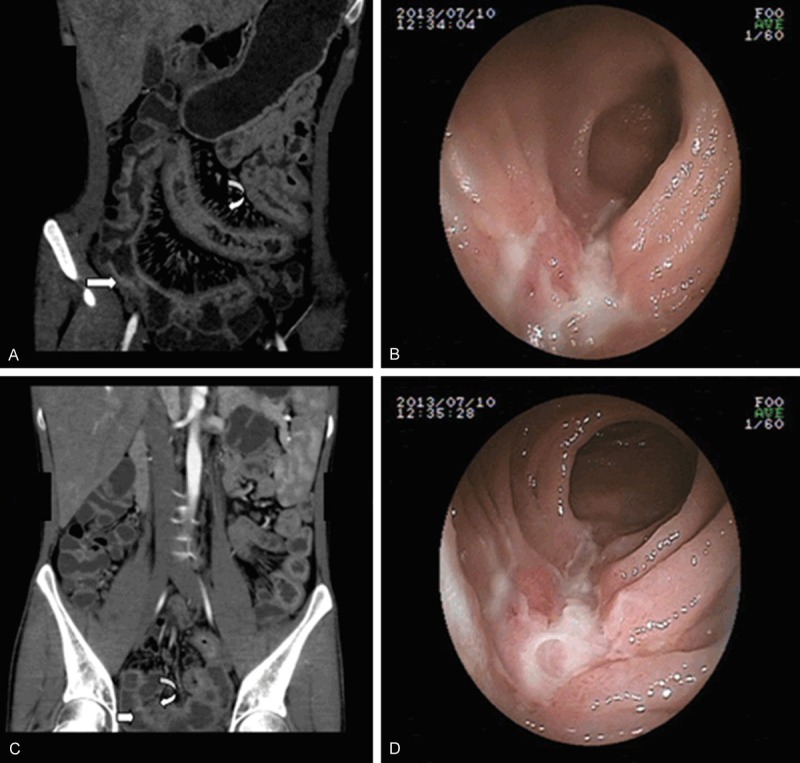
CD of a 25-year-old woman who complained of perianal disease for 5 years. A, C. Coronary reconstructed CT enterography showing asymmetric bowel wall thickening (arrow) and hypervascularity, with vascular dilatation and wide spacing of the vasa recta, the so-called comb sign (curve). B, D. Endoscopy images showing longitudinal ulcers that stretched across several intestinal folds (arrow).
For CT enterographic parameters, we found that the occurrences of skip lesions, and asymmetric pattern of involvement (Figure 4A, 4C) in patients with CD, were significantly higher than those in patients with ITB (P < 0.05). In ITB, malformation of the ileocecal valve was detected more often compared with CD, such as contracture of the ileocecal valve and a fixed patulous ileocecal valve (Figures 1B, 3C, 3D) (P < 0.05). In terms of extraluminal manifestations, characteristic lesions of lymph nodes such as calcification and central necrosis indicated a diagnosis of ITB (P < 0.05). ITB patients also had a predilection of developing ascites compared with CD patients (P < 0.05). In patients with CD, parenteral complications such as comb sign (Figure 4A), phlegmon (Figure 2A) and fistula were more frequently observed than in patients with ITB (P < 0.05), suggesting that these parameters were indicative of a CD diagnosis.
Multivariate analysis to differentiate CD from ITB
All of the parameters that had significance in differentiating CD from ITB were enrolled to further perform multivariate binary logistic regression. This analysis demonstrated that perianal disease, transverse ulcer, rodent-like ulcer, skip lesions, fixed patulous ileocecal valve and comb sign were valuable in differentiating CD from ITB (P < 0.05) (Table 3). Perianal disease, skip lesions and comb sign were indicative of CD diagnosis, whereas transverse ulcer, rodent-like ulcer and fixed ileocecal valve were indicative of ITB diagnosis in this multivariate logistic regression. T-SPOT, as it yielded high sensitivity and NPV, could not be included in the multivariate binary logistic regression.
Table 3.
Multivariate logistic regression analysis of different parameters in CD and ITB patients
| Indexes (Variable code) | B | BE | Wald X2 | P | OR (95% CI) |
|---|---|---|---|---|---|
| Perianal disease (X13) | 4.814 | 2.218 | 4.709 | 0.030 | 123.226 (1.594-9526.372) |
| Transverse ulcer (X25) | -5.151 | 1.862 | 7.653 | 0.006 | 0.006 (0.000-0.223) |
| Rodent-like ulcer (X27) | -3.622 | 1.617 | 5.020 | 0.025 | 0.027 (0.001-0.635) |
| Skip lesion (X34) | 5.399 | 1.834 | 8.668 | 0.003 | 221.240 (6.079-8051.221) |
| Fixed patulous ileocecal valve (X37) | -3.897 | 1.865 | 4.365 | 0.037 | 0.020 (0.001-0.786) |
| Comb sign (X42) | 4.477 | 1.369 | 10.697 | 0.001 | 87.946 (6.014-1286.154) |
| Constant | -1.279 | 1.284 | 4.991 | 0.026 | 0.278 |
Predictive multivariate model to differentiate CD from ITB
Based on statistically significant parameters identified by chi-square tests (x2 = 116.080, P < 0.001), a predictive multivariate equation was developed (P, predictive value; e, natural logarithm) to help predict the differential diagnosis between CD and ITB, with a high sensitivity (97.8%), specificity (96.8%), accuracy (97.6%), positive predictive value (98.9%), and a negative predictive value (93.7%) (Table 4). The diagnostic point of 0.508 was obtained from ROC curve (P > 0.508, predictable diagnosis of CD; P < 0.508, diagnosis of ITB) and the area under the ROC curve was 0.994 (Figure 5). P = 1/[1+e-(-1.279+4.814*X13-5.151*X25-3.622*X27+5.399*X34-3.897*X37+4.477*X42)].
Table 4.
Diagnostic efficacy of the multivariate equation and T-SPOT (%)
| Models | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|
| Multivariate equation | 97.8 | 96.8 | 97.6 | 98.9 | 93.7 |
| T-SPOT | 96.8 | 91.3 | 92.7 | 78.9 | 98.8 |
Figure 5.
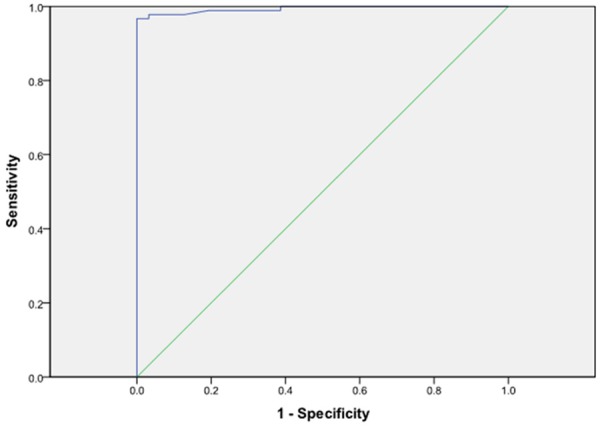
ROC curve of multivariate logistic regression model (area under the ROC curve = 0.994).
Further analysis in the patients that T-SPOT did not produce the correct diagnosis
We further check T-SPOT alone as a diagnostic tool to differentiate CD from ITB, and evaluate the sensitivity (96.8%), specificity (91.3%), accuracy (92.7%), PPV (78.9%) and NPV (98.8%) (Table 4). For cases of T-SPOT that were not correctly diagnosed (8 CD patients and 1 ITB patient), we used predictive multivariate equation for further analysis. Of the 8 patients with CD, the predictive value lay in [0.6208, 0.9999] (P > 0.508), whereas the patient with ITB was 0.1658 (P < 0.508). Through the equation established by multivariate logistic regression, the 9 patients finally received the correct diagnosis.
Discussion
As an opportunistic infection, tuberculosis still remains a major health concern in the world, especially in developing countries like China. It is of great importance to make a correct differential diagnosis between these two diseases as misdiagnosis may produce disastrous effects for the patient [7]. In our study, we collated numerous parameters including demographic, clinical, radiological and endoscopic data, and analyzed their value in differentiating CD from ITB. We developed an equation based on several certain parameters and explored a protocol of differential diagnosis to make diagnosis more objective and easier for inexperienced clinicians.
For various demographic and clinical factors, our study found that only three of them, including diarrhea, night sweats and perianal disease, are helpful in the differential diagnosis of CD and ITB. Among them, diarrhea and perianal disease favored a diagnosis of CD, whereas night sweat favored a diagnosis of ITB. These results further confirm that CD and ITB exhibit overlapping manifestations in symptom [4,18]. Compared with laboratory, endoscopic and radiologic parameters, demographic and clinical features were not objective enough. There may be bias in data-collecting process, which may have lead to minor inconsistencies in our study and previously published research [19].
Our study showed that CD and ITB patients displayed no significant difference in routine laboratory tests, including serum hemoglobin, hematocrit, albumin, ESR, CRP. T-SPOT is a relatively new tool for detecting TB infection [8]. Both active and latent TB can be detecting using T-SPOT, which is of great significance in tuberculosis pandemic countries like China [9,10]. Moreover, T-SPOT will not be affected by BCG vaccination, which is superior to the tuberculin skin test (TST). In our study, only 1 of 31 patients with ITB was T-SPOT negative. These findings have further proven that T-SPOT has high sensitivity and NPV in detecting TB, which was in accordance with other published studies [11,12]. Compared with endoscopy, CT enterography and many other evaluating methods, T-SPOT only requires a blood sample from a patient and thus highlighting its convenience, relative non-invasiveness, and high value in excluding a diagnosis of TB. Thus, in the process of differentiating CD from ITB, T-SPOT should be a fundamental test carried out early on in the clinical investigation.
Endoscopy is the first choice for clinical practitioners to detect bowel lesions and to evaluate the therapeutic response. Our team has much experience in the differential diagnosis of GI disease using endoscopy. The morphology of bowel ulcers was different in CD and ITB patients. Ulcers of CD patients were longitudinal and may stretch across several intestinal folds. In contrast, morphology of ITB ulcers is characterized by transverse ulcers and the base of ulcers are irregular in shape; so-called rodent-like ulcers. The data provided by our study has further proven that longitudinal ulcers are more likely to be found in CD patients with transverse and rodent-like ulcers mainly found in ITB patients. We also found that patulous ileocecal valve favored a diagnosis of ITB. Our findings were quite similar to those reported by Lee et al. [13].
CT enterography is an emerging technology for the diagnosis and evaluation of small bowel lesions [20]. It offers an unparalleled tool to detect bowel wall lesions as well as extra-enteric complications, which is a necessary addition to other examination methods [21]. Furthermore, many of the parameters under CT enterography correlate closely with disease activity and the response to therapy [22,23]. Inside the bowel cavity, our study illustrated that skip lesions and asymmetric patterns of involvement indicated a probable diagnosis of CD, whereas contracture of the ileocecal valve and fixed patulous ileocecal valve suggested a diagnosis of ITB. Regarding extra-enteric manifestations, our study has shown that comb sign, phlegmon and fistula were more likely to be found in CD patients, while lymph nodes with central necrosis, lymph nodes with central calcification and ascites in ITB patients. Our findings are in good agreement with Zhao et al. and Park et al. [14,15]. These findings provide us a new prospective that extraluminal manifestations should not be neglected in differentiating CD from ITB.
Although several valuable diagnostic parameters have been identified, the sensitivity and specificity for each parameter was not very high. For those parameters that had not been weighed, there were some intricate cases that we could not easily make a differential diagnosis. Thus, it was urgent for us to develop an equation, which included multi-variables and was easy for ordinary gastrointestinal clinicians to understand and facilitate an accurate diagnosis. Hence, we performed a multivariable logistic regression to further analyze the parameters that had univariate significance. In total 6 parameters, including clinical, endoscopic and CT enterographic, were screened out, namely perianal disease, transverse ulcers, rodent-like ulcers, skip lesions, fixed patulous ileocecal valve and comb sign. Then, a mathematical equation was developed. The sensitivity, specificity, accuracy, PPV and NPV of our model were 97.8%, 96.8%, 97.6%, 98.9% and 93.7%, respectively. Our mathematical equation produced a high diagnostic efficacy, with the area under the ROC curve being 0.994.
T-SPOT, with its high sensitivity and NPV, correlated closely with the diagnosis of ITB. Thus, T-SPOT could not be included in the multivariate logistic regression model for this mathematical reason. However, we should never overlook the importance of T-SPOT in our clinical practice, for its convenience, non-invasiveness and high value of diagnostic efficacy. We further reviewed 9 cases in which T-SPOT had given an incorrect diagnosis but our mathematical model a correct diagnosis. For 3 cases that the mathematical model did not provide the correct diagnosis, T-SPOT results were invaluable. In conclusion, both T-SPOT and the results of the mathematical model should be taken into consideration when making a differential diagnosis between CD and ITB.
In our study, pathological features were only some of the criteria used to make a differential diagnosis. One concern was that the context of small-sized endoscopic mucosal biopsies and their superficial nature further complicated differential diagnosis [7]. Various studies have focused on pathology to differentiate CD from ITB. On review of these studies, we found that the diagnostic accuracy was low and largely depended on the experience of the endoscopist in acquiring biopsy specimens, and the experience of the pathologist reviewing the specimens [24-26]. As we only had a limited number of patients that may lead to unintentional bias, pathological findings were not included in our analysis.
There have been other few studies that tried to explore a multivariate model to differentiate CD from ITB [5,15,19]. Compared to them, our multivariate model includes clinical, endoscopic and CT enterographic parameters, and is superior in diagnostic efficacy. Furthermore, all this studies did not include T-SPOT into analysis. In our study, we found that a negative T-SPOT result could almost exclude a diagnosis of ITB. In our hospital, T-SPOT, CT enterography and endoscopy would all be routinely performed when there was difficulty in differentiating CD from ITB. Through the analysis of this article, we recommend other clinical practitioners to carry out T-SPOT, CT enterography and endoscopy in differentiating these two diseases. Final diagnosis could be formed using our multivariate equation with a consideration of T-SPOT.
There are a number of limitations in our study. First, we found it difficult to establish grading criteria, mainly due to insufficient patient numbers, especially ITB patients. In future, a multi-center collaboration should be carried out to enroll a larger cohort of patients and to establish grading criteria that incorporate as many parameters as possible. Second, although CT enterography can help us detect many radiological signs, which cannot be detected by endoscopy, this technique exposes patients to radiation. This will prevent CT enterography being used frequently, especially since IBD patients are already at an increased risk of malignancies [27]. Clinicians should be aware of the increased risk of cumulative doses of radiation during CT enterography to maximize the benefit to each patient. For those with identifiable risk factors of malignancy, MR enterography may be an alternative evaluation tool [28].
Conclusions
CD and ITB have overlapping clinical manifestations, which continuously perplex us. As it is convenient, non-invasive and provides high-value diagnostic efficacy, T-SPOT should be used to differentiate CD from ITB, especially to exclude a diagnosis of TB infection in a TB-endemic region like China. For some intricate cases, clinical, T-SPOT, endoscopic and CT enterographic parameters will all need to be taken into consideration.
Disclosure of conflict of interest
None.
Abbreviations
- CD
Crohn’s disease
- ITB
intestinal tuberculosis
- TB
tuberculosis
- IBD
inflammatory bowel disease
- CT
computed tomography
- MR
magnetic resonance
- IGRAs
interferon-gamma release assays
- ROC
receiver operating characteristic
- PPV
positive predictive value
- NPV
negative predictive value
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- HREZ
isoniazid rifampicin ethambutol pyrazinamide
- HR
isoniazid rifampicin
- P
probability
- TST
tuberculin skin test
References
- 1.Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: A systematic review. J Gastroenterol Hepatol. 2012;27:1266–1280. doi: 10.1111/j.1440-1746.2012.07150.x. [DOI] [PubMed] [Google Scholar]
- 2.Zheng JJ, Zhu XS, Huangfu Z, Gao ZX, Guo ZR, Wang Z. Crohn’s disease in mainland China: a systemic analysis of 50 years of research. Chin J Dig Dis. 2005;6:175–181. doi: 10.1111/j.1443-9573.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Yang Z, Fu Y, Zhang G, Wang X, Zhang Y, Wang X. Insight to the epidemiology and risk factors of extrapulmonary tuberculosis in Tianjin, China during 2006-2011. PLoS One. 2014;12:e112213. doi: 10.1371/journal.pone.0112213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson G, Shenoy T, Ramasubramanian R, Balakumaran LK, Småstuen MC, Bjune GA, Moum BA. Rountine diagnosis of intestinal tuberculosis and Crohn’s disease in Southern India. World J Gastroenterol. 2014;17:5017–5024. doi: 10.3748/wjg.v20.i17.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Liu X, Zou Y, Ouyang C, Wu X, Zhou M, Chen L, Ye L, Lu F. Predictors of clinical and endoscopic findings in differentiating Crohn’s disease from intestinal tuberculosis. Dig Dis Sci. 2011;56:188–196. doi: 10.1007/s10620-010-1231-4. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Chen M, Cao Z, Liu S, Ding B. Differential diagnosis of intestinal tuberculosis from Crohn’s disease and primary intestinal lymphoma in China. Saudi J Gastroenterol. 2014;20:241–247. doi: 10.4103/1319-3767.136979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein D, Watermeyer G, Kirsch R. Review article: the diagnosis and management of Crohn’s disease in populations with high-risk rates for tuberculosis. Aliment Pharmacol Ther. 2007;25:1373–1388. doi: 10.1111/j.1365-2036.2007.03332.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazurek GH, Jereb J, LoBue P, Lademarco MF, Metchock B, Vernon A Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC) Guidelines for using QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54:49–56. [PubMed] [Google Scholar]
- 9.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Zhang LF, Qian JM, Liu XQ, Wang L, Wang X, Wang J. The role of in vitro interferon γ-release assay in differentiating intestinal tuberculosis from Crohn’s disease in China. J Crohn’s Colitis. 2012;6:317–323. doi: 10.1016/j.crohns.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Lei Y, Yi FM, Zhao J, Yang GF, Xia B. Utility of in vitro interferon-γ release assay in differential diagnosis between intestinal tuberculosis and Crohn’s disease. J Dig Dis. 2013;14:68–75. doi: 10.1111/1751-2980.12017. [DOI] [PubMed] [Google Scholar]
- 12.Ng SC, Hirai HW, Tsoi KK, Wong SH, Chan FK, Sung JJ, Wu JC. Systematic review with meta-analysis: Accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J Gastroenterol Hepatol. 2014;29:1664–1670. doi: 10.1111/jgh.12645. [DOI] [PubMed] [Google Scholar]
- 13.Lee YJ, Yang SK, Byeon JS, Myung SJ, Chang HS, Hong SS, Kim KJ, Lee GH, Jung HY, Hong WS, Kim JH, Min YI, Chang SJ, Yu CS. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn’s disease. Endoscopy. 2006;38:592–597. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 14.Park YH, Chung WS, Hong SP, Lim JS, Park SJ, Cheon JH, Kim TI, Kim WH. Diagnostic role of computed tomographic enterography differentiating Crohn disease from intestinal tuberculosis. J Comput Assist Tomogr. 2013;37:834–839. doi: 10.1097/RCT.0b013e31829e0292. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XS, Wang ZT, Miao F, Zhong J, Wu ZY, Yin QH, Yan FH. Differentiation of Crohn’s disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014;20:916–925. doi: 10.1097/MIB.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaus S, Schreiber S. Diagnostics of inflammatory bowel disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, Gupte P. Gastrointestinal luminal tuberculosis: establishing the diagnosis. J Gastroenterol Hepatol. 2004;19:1240–1246. doi: 10.1111/j.1440-1746.2004.03485.x. [DOI] [PubMed] [Google Scholar]
- 18.Amarapurkar Deepak N, Patel Nikhi D, Rane Priyamvada S. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14:741–746. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makharia GK, Srrivastava S, Das P, Goswami P, Singh U, Tripathi M, Deo V, Aggarwal A, Tiwari RP, Sreenivas V, Gupta SD. Clinical, endoscopic and histopathological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642–651. doi: 10.1038/ajg.2009.585. [DOI] [PubMed] [Google Scholar]
- 20.Engin G. Computed tomography enteroclysis in the diagnosis of intestinal diseases. J Comput Assist Tomogr. 2008;32:9–16. doi: 10.1097/rct.0b013e318059bed7. [DOI] [PubMed] [Google Scholar]
- 21.Park MJ, Lim JS. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin Endosc. 2013;46:327–366. doi: 10.5946/ce.2013.46.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YW, Tang YH, Hao NX, Tang CY, Miao F. Crohn’s disease: CT enterography manifestations before and after treatment. Eur J Radiol. 2012;81:52–59. doi: 10.1016/j.ejrad.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Lo Re G, Cappello M, Tudisca C, Galia M, Randazzo C, Craxì A, Cammà C, Giovagnoni A, Midiri M. CT enterography as a powerful tool for evaluation of inflammatory activity in Crohn’s disease: relationship of CT findings with CDAI and acute phase reactants. Radiol Med. 2014;119:658–666. doi: 10.1007/s11547-013-0377-5. [DOI] [PubMed] [Google Scholar]
- 24.Pulimood AB, Peter S, Ramakrishna B, Chacko A, Jeyamani R, Jeyaseelan L, Kurian G. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn’s disease. J Gastroenterol Hepatol. 2005;20:688–696. doi: 10.1111/j.1440-1746.2005.03814.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn’s disease and intestinal tuberculosis. J Clin Pathol. 2006;59:840–844. doi: 10.1136/jcp.2005.032383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin XJ, Kim JM, Kim HK, Kim L, Choi SJ, Park IS, Han JY, Chu YC, Song JY, Kwon KS, Kim EJ. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn’s disease. World J Gastroenterol. 2010;20:2496–2503. doi: 10.3748/wjg.v16.i20.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–1529. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 28.Swanson G, Behara R, Braun R, Keshavarzian A. Diagnostic medical radiation in inflammatory bowel disease: how to limit risk and maximize benefit. Inflamm Bowel Dis. 2013;19:2501–2508. doi: 10.1097/MIB.0b013e31828dc6b6. [DOI] [PubMed] [Google Scholar]


