Abstract
The mechanisms underlying the growth inhibitory effect of Benzyl isothiocyanate (BITC) against breast cancer are still not fully understood. Therefore, we further investigated the mechanism in BITC triggering breast cancer. In the present study, we found that the overexpression of FOXH1 in breast cancers tissues and cells, and FOXH1 significantly promoted cell proliferation, invasion and tumorigenesis in vitro. FOXH1 significantly increased the expression levels of β-catenin, cyclinD1, and c-myc proteins in breast cancer cells. Furthermore, siβ-catenin reduced FOXH1 promotion of cell proliferation and invasion in breast cancer cells. Taken together, these results suggest that FOXH1 promoted breast cancer cell growth and invasion by potentiating the Wnt/β-catenin pathway, suggesting that FOXH1 may be a potential molecular target for breast cancer prevention and therapy. Furthermore, BITC treatment has remarkable effect on the expression level of FOXH1 and β-catenin mRNA and protein in MCF-7 cells, MDA-MB-231 cells and SUM 159 cells. BITC treatment has an obvious significance on transcriptional activity of FOXH1. Cell growth and invasion inhibition resulting from BITC exposure were significantly augmented by FoxH1 knockdown. In conclusion, the present study provides novel insights into the molecular circuitry of BITC-induced cell death involving FoxH1-mediated tumorigenesis. Thus, the present study provides a novel insight into the underlying mechanism of tumorigenesis in BITC triggering breast cancer, indicating the therapeutic potential of FOXH1 in the treatment of breast cancer.
Keywords: Benzyl isothiocyanate, FoxH1, tumorigenesis, Wnt, β-catenin
Introduction
Breast cancer is a significant prevalent cause of cancer-related death in women worldwide, which usually results from morbidity and mortality [1]. Estrogen play an major role in promoting breast cancer development and progression Brown, agents that block the ER, such as tamoxifen and aromatase inhibitors, could markedly increase ER positive patient survival [2,3]. But for estrogen receptor-negative breast cancer, these approaches tend to be ineffective, so chemoprevention agents of breast cancer such as Benzyl isothiocyanate (BITC) are clinically attractive [4]. BITC is a naturally occurring constituent of edible cruciferous vegetables with in vivo breast cancer preventive efficacy [5,6], BITC is a not only a potent inhibitor of EMT [7], but also has a suppression function in various oncogenic pathways such as nuclear factor-jB [8], leptin-induced Stat3 phosphorylation and cyclin D1 transactivation [9], and Akt [10].
FoxH1 (also named FAST-1), is a member of the Forkhead-box (FOX) family of transcription factors, more and more evidence implies the critical role for FOX protein involved in the carcinogenesis [11,12], such as FOXA1, FOXQ1 [13,14], but the function of FOXH1 implicated in cancer progression was still poorly understood [15]. And whether the involvement of FOXH1 was in the development of breast cancer remains unclear [16].
Wnt/β-catenin signaling transduction pathway, which functions by regulating cell proliferation, polarity, and cell fate not only in the embryonic stem cells development but also the tissue homeostasis [17,18]. Aberrant activation of the Wnt/β-catenin pathway by amounts of transcription factors (TFs) is linked to various human cancers [19-21]. We are interested in the whether there are more TFs included in the activation β-catenin and its downstream target gene, such as c-Myc, cyclinD1 in breast cancer cells, thus promote cell proliferation and invasion.
In the present study, the biological function and associated molecular mechanisms of FOXH1 in breast cancer were analyzed, and the role of FOXH1 involved in Wnt/β-catenin signaling to BITC anticancer responses was investigated.
Materials and methods
Patients and ethics statement
All breast cancer tissues and their adjacent normal tissues examined from breast cancer patients who underwent surgical resection at the Department of Breast Surgery, West China Second University Hospital, Sichuan University (Chengdu, China) from 2007 to 2012. The patients included 35 females, aged 33-62 years old, the median age was 53 years. Informed consent letter for research was obtained from all the samples. The tumors were classified according to the criteria standard of the WHO. Samples were collected and maintained in ice-cold buffer [10 mM Tris (pH 7.2) and 0.9% NaCl] after surgical resection for future analysis.
Cell lines
Human breast adenocarcinoma cell lines (MCF-7 cells, MDA- MB-231 cells and SUM159 cells) and a normal human mammary epithelial cell line (MCF-10A) were purchased from the American Type Culture Collection (Manassas, VA, USA), and were cultured in DMEM or 1640 medium supplemented with 10% fetal bovine serum added with antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin; Sigma-Aldrich, USA).
Reagents
Antibodies against Forkhead Box H1 transcription factor (FOXH1), β-catenin, cyclinD1 and GAPDH were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondly antibody was purchased from Abgent (San Diego, CA, USA).
RNA isolation and reverse transcription-PCR
Total RNA was isolated using the RNeasy kit (Invitrogen Life Technologies), according to the manufacture’s introduction. First-strand cDNA was transcribed using reverse transcriptase (Invitrogen Life Technologies) with oligo (deoxythymidine) 20 primer.
Real-time PCR
Quantitative real-time PCR was done using 2× SYBR Green master mix (Applied Biosystems Life Technologies) on the condition as 95°C (30 s) and 60°C (60 s) for 40 cycles. Relative gene expression was calculated using the method described earlier and normalized against to the House-keeping gene GAPDH. Amplification conditions for FOXH1 were 95°C for 5 min, 30 cycles 95°C for 30 s, 58°C for 30 s, 72°C for 1 min and 72°C for 10 min.
RNA interference
The FOXH1 stable transfected cells were transfected with a control nonspecific siRNA or β-catenin targeted siRNA (100 nM), using RNAimax (Invitrogen Life technology). Forty-eight hours after transfection, subsequently, the cells were collected and used for western blotting, cell proliferation or cell migration assays.
Stable FOXH1 over expression cell lines
MCF-7 or MDA-MB-231 cells were transfected with pcDNA4/TO/myc-his-FOXH1 (ABGENT, CA, USA) to create tet-inducible FOXH1 over expression cell lines, respectively. Cell selection was achieved using zeocin (Invitrogen Life Technologies) for stable over expression cells. After the selection period, stability of the cell lines was confirmed by immunoblotting analysis. The expression of FOXH1 was maintained in the presence of doxycycline constitutively.
Western blot analysis
Total protein was extracted from relative breast cancer cells using radioimmunoprecipitation lysis buffer (TransGen, Beijing, China) according to the manufacturer’s instruction. 30 μg lysate was resolved on 10% SDS denaturing gels (Sigma-Aldrich). After SDS gel electrophoresis, the proteins were transferred to NC membranes (Millipore, Boston, MA, USA), 5% skim milk was used to block the NC membranes, and immunoblotted with primary antibodies, mouse anti-FOXH1 (1:1000) or mouse anti-β-catenin (1:1500), or rabbit anti-cyclinD1 (1:1000), or mouse anti-β-actin (1:2000) overnight at 4°C. After washing with PBS with Tween-20 buffer, the membranes were incubated with Goat anti-Rabbit secondary antibodies (1:3000) or Goat anti-Mouse secondary antibodies (1:3000) for 1 hour, chemiluminescence reagent was used and the fluorescence was scanned to visualize the protein bands using a Typhoon scanner (9400, GE Healthcare Life Sciences, USA).
Luciferase reporter assays
Luciferase reporter assay was performed to determine the effect of BITC treatment on FOXH1 transcription. A total of 1×105 cells were seeded in 12-well plates and incubated at 37°C overnight. For β-catenin luciferase assay, cells were co-transfected with 6 μg of pGL-3 Basic-β-catenin-Luc plasmid (Addgene, Cambridge, USA) and 0.6 μg of pGL-3 basic plasmid using Fugene6 (Roche Applied Science, Indianapolis, IN). 24 hours after transfection, cells were treated with DMSO or BITC for the same specified time periods. Luciferase activity was determined and normalized to protein concentration and expressed as a ratio of renilla luciferase units.
Cell proliferation assay
To determine the relative transfected cell growth, 2000 cells of each group were plated in triplicate in 96-well plates and assessed by MTT assay. At 24, 48, 72 or 96 h, MTT reagent [diluted from a 4-mg/ml solution with PBS to a final concentration of 0.8 mg/ml] (Sigma-Aldrich)was added to the wells, the treated cells were further incubated for 4 h at 37°C 200 μL/well dimethyl sulfoxide (Sigma-Aldrich) was used to terminate the reaction. The absorbance was read at 570 nm on an ELISA plate reader (Nanjing Perlove Medical Equipment Company, China).
Migration and invasion assays
To detect the role of FOXH1 and BITC treatment on migration and invasion of breast cancer cellsin vitro, Transwell Boyden chamber (Corning, NY, USA) with a pore size of 8 μm polycarbonate filter was used. For invasion assay, the chamber was covered with 30 μL of Matrigel (BD Biosciences). Briefly, a total of 1×105 cells in 0.5 ml of serum-free media were mixed and added in the upper compartment of the chamber, and complete medium were added to the lower compartment of the chambers.
Following incubation at 37°C for 24 h, non-motile cells in the upper membrane were removed gently by a cotton swab, the motile cells on the bottom face were stained with hematoxylin and eosin, then counted under a microscope. Triplicate measurements in each experiment were carried out.
Statistical analyses
All statistical tests were confirmed by at least three independent experiments and performed using SPSS 17.0 software. Statistical significance differences expression of FOXH1 between control and treated groups was detected using paired t-test by comparing mean values (± SD) or one-way ANOVA comparison tests. P<0.05 were considered as significantly differences.
Results
Theexpression of FOXH1 is upregulated in breast cancer cell lines and tissues
In order to investigate the role of FOXH1 in breast cancer, RT-qPCR analysis (Figure 1A) and Western blot analysis (Figure 1B) were firstly performed to detect the FOXH1 expression in MCF-7 cells, MDA-MB-231 cells, SUM159 cells and MCF-10A cells. As compared with the normal breast cell line MCF-10A, the FOXH1 expression was shown to be significantly upregulated in MCF-7 cells, MDA-MB-231 cells and SUM159 cells. Further, using the patients samples collected, FOXH1 expression was then examined, we found in primary breast cancer tissues and in their corresponding adjacent healthy tissues, FOXH1 expression was increased in breast cancer tissues (Figure 1C and 1D). These results indicated that the overexpression of FOXH1 in breast cancer.
Figure 1.
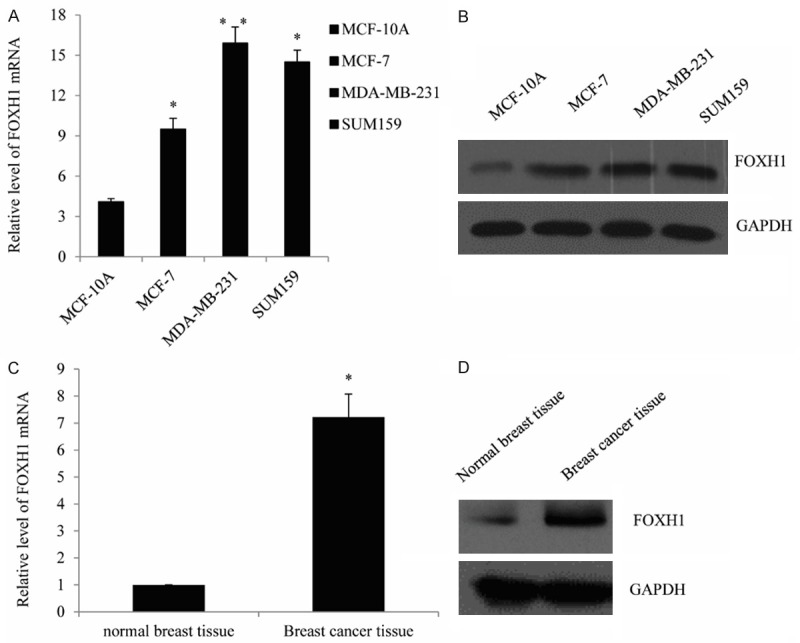
The expression of FOXH1 is upregulated in breast cancer cell lines and tissues. Representative mRNA expression of FOXH1 in the breast cancer cell lines, compared with the normal cell MCF-10A, determined by RT-qPCR. Data were represented as mean ± standard of three independent experiments. *P<0.05. A. Representative protein expression of FOXH1 in the breast cancer cell lines, measured by Western blot. B. Representative results of FOXH1 mRNA expression in breast cancer tissues, compared with the corresponding normal breast tissues as detected by RT-qPCR. C. Representative of FOXH1 protein expression in breast cancer tissues.
FOXH1 promotes breast cancer cell proliferation and invasion
To further understand the impact of FOXH1 in breast cancer progression, we firstly established an FOXH1 expression construct and transfected it in the breast cancer cell lines MCF-7 cells and MDA-MB-231 cells and detect whether FOXH1 could participate in cell proliferation. The transfection efficiency was measured by RT-qPCR and western blot analysis. As shown, FOXH1 mRNA expression was significantly increased compared with those transfected with the vector in MCF-7 cells and MDA-MB-231 cells (P<0.05) (Figure 2A). Consistent with the RT-qPCR, protein expression of FOXH1 was markedly increased in the breast cancer cells (Figure 2B). Furthermore, cell proliferation assay indicated that ectopic expression of FOXH1 significantly promotes the MCF-7 cells (Figure 2C left panel) and MDA-MB-231 cells proliferation (Figure 2C right panel), when compared with the vector groups. The function of FOXH1 on cell invasion was also investigated. As shown in Figure 2D, enforced expression of FOXH1 resulted in a obviously increase effect in the number of MCF7 cells or MDA-MB-231 cells migrating across the filter. These results indicated that FOXH1 promotes breast cancer cell proliferation and invasion. Knockdown β-catenin reduced the promotion of FOXH1 in cell proliferation and invasion Since the aberrant activation of different signaling pathway was significantly included in the breast cancer pathogenesis, such as Wnt/β-catenin, HIF1a. To investigate the effects of FOXH1 on the potential signaling pathway, the expression of the relative signaling molecules was examined by RT-qPCR and western blot analysis. As measured, the mRNA and protein levels of β-catenin and cyclinD1 were significantly increased in FOXH1 transfected MCF-7 cells (Figure 3A) or MDA-MB-231 cells (Figure 3B), compared with the vector group (P<0.05). To further investigate the role of β-catenin in FOXH1 triggering breast cancer, upon treatment with siRNA targeting β-catenin in the FOXH1-transfected MCF-7 cells, the promotion effect of FOXH1 on breast cancer cell proliferation and invasion was identified. The data indicated the knockdown of β-catenin could significantly reduced the effect of FOXH1 in MCF-7 cells (Figure 3C). We created a MDA-MB-231 cell line with stable expression of FOXH1 to further test its role, the similar tendency was also observed in MDA-MB-231 cells (Figure 3D) (P<0.05). These results revealed that FOXH1 promoted breast cancer cell proliferation and invasion through the activation of the Wnt/β-catenin pathway.
Figure 2.
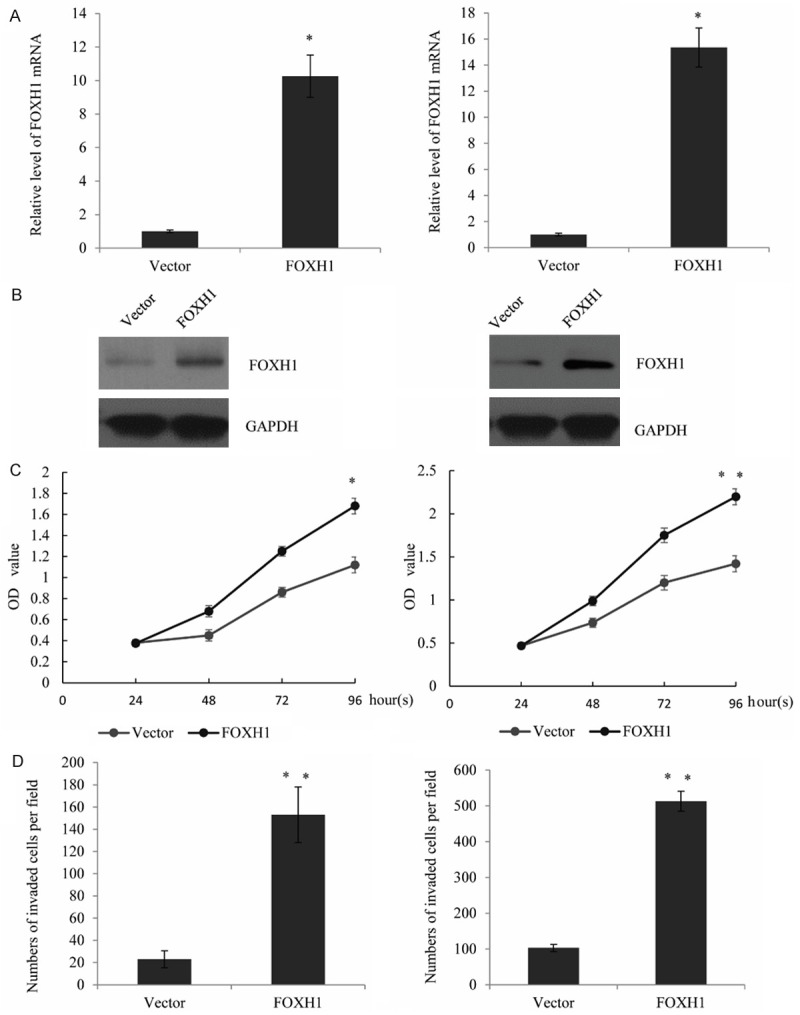
FOXH1 promotes breast cancer cell proliferation and invasion. A. Expression of FOXH1 mRNA in transfected breast cancer cell lines MCF-7 and MDA-MB-231, when compared with the vector group. analyzed using RT-qPCR. Data were represented as mean ± standard of three independent experiments. *P<0.05. B. Western blotting analysis was used to measure the FOXH1 protein expression in the above cell lines. C. FOXH1 markedly promoted the proliferation of MCF-7 cells and MDA-MB-231 cells, using cell proliferation assay, when compared with the relative vector group (vector). D. FOXH1 promotes cell invasion. Cell invasion was detected using transwell assay. The number of invaded cells in FOXH1-transfected MCF-7 and MDA-MB-231 cells was counted and analyzed. Representative photos were shown. Data were presented as mean ± standard, repeated three times. *P<0.05.
Figure 3.
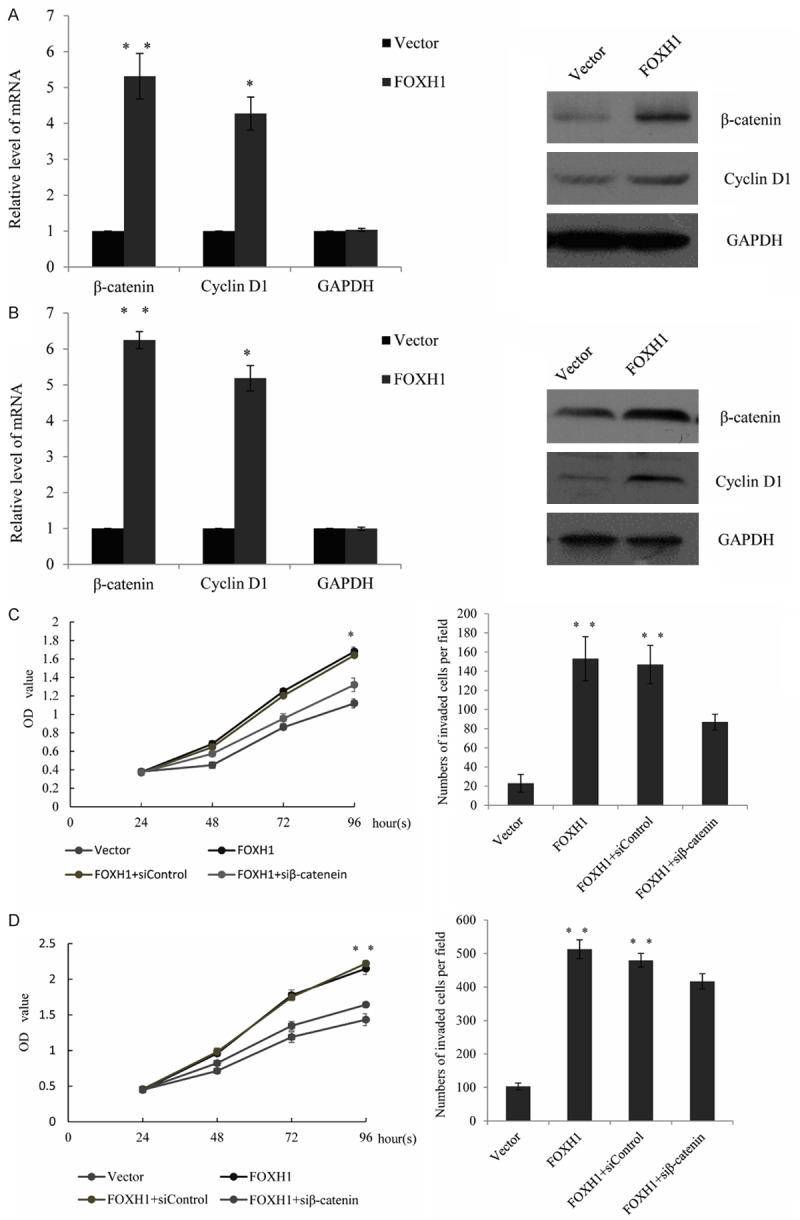
Knockdown β-catenin reduced the promotion of FOXH1 in cell proliferation and invasion. A. RT-qRCR for β-catenin and cyclinD1 mRNA and Immunoblotting for the relative protein using lysate from MCF-7 cells stably transfected with a vector or FOXH1-transfected cells. B. The above experiments were performed in MDA-MB-231 cells stably transfected with a vector or FOXH1-transfected cells. C. Cell proliferation assay and transwell assay were performed in FOXH1 stable transfected MCF-7 cells, follow by transiently transfected with a control siRNA or a β-catenin targeted siRNA and treated for 24 hours. D. The similar experiments were performed in FOXH1 stable transfected MDA-MB-231 cells, follow by transiently transfected with a control siRNA or a β-catenin targeted siRNA and treated for 24 hours.
BITC treatment has remarkable effect on transcriptional activity of FOXH1
As mentioned early, BITC treatment are shown to inhibit breast cancer cell growth and EMT, and the suppression of FOXQ1 was an important mechanism by which BITC triggering metastasis. So we focus our interest on whether BITC has an effect on the FOXH1. First, we measure BITC treatment on the expression level of FOXH1 and β-catenin in MCF-7 cells, levels of relative mRNA and protein were decreased markedly upon 12 and 24 h treatment with BITC in MCF-7 cells (estrogen-responsive with wild-type p53) (Figure 4A). The BITC-mediated suppression of FOXH1 and β-catenin was also observed in the MDA-MB-231 cell line (estrogen-independent cell line with mutant p53), and the MDA-MB-231 was relatively more sensitive compared with MCF-7 (Figure 4B). The BITC treatment also leading to a remarkable change on levels of FOXH1 and β-catenin in SUM159 (Figure 4C), which is a triple-negative human breast cancer cell line. Further, the transcriptional activity of FOXH1 was also detected in MCF-7 cells, MDA-MB-231 cells, and SUM159 cells. We declare the overexpression of FOXH1 could remarkably increase the β-catenin luciferase reporter activity. However, after exposure to BITC for 6 h-24 h, the BITC treatment resulted in a significant decrease in β-catenin luciferase reporter activity especially upon 24 hours treatment at the 5 μM concentration in each cell line (Figure 4D).
Figure 4.
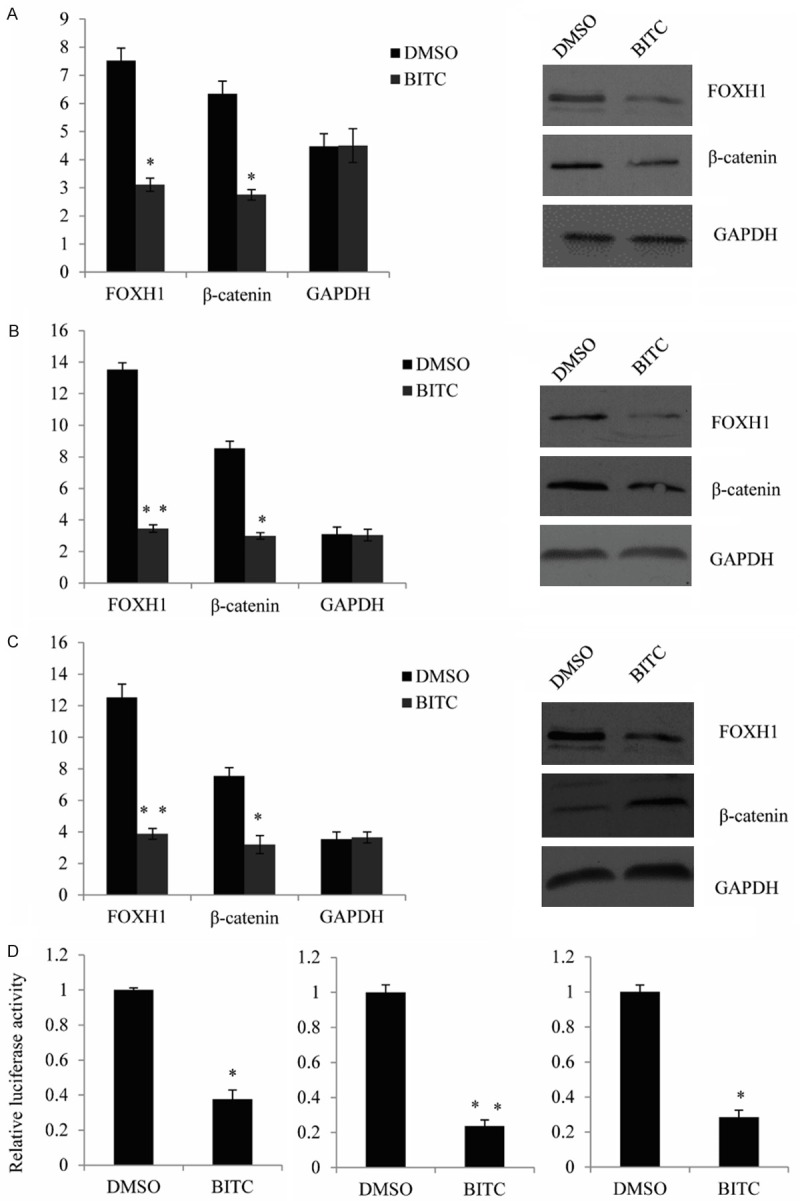
BITC treatment has remarkable effect on transcriptional activity of FOXH1. A. qRT-PCR and western blot analysis for FOXH1, β-catenin and GAPDH mRNA levels and protein levels in MCF-7 cells treated with DMSO (control) or the indicated concentrations of BITC for 16-24 h. Results shown are mean ± SD (n = 3). *Significantly different (P<0.05). B. qRT-PCR and western blot analysis for FOXH1, β-catenin and GAPDH mRNA levels in MDA-MB-231 cells. C. qRT-PCR and western blot analysis for FOXH1, β-catenin and GAPDH mRNA levels and protein levels in SUM159 cells. D. β-catenin luciferase reporter activity (as measurement of FOXH1 transcriptional activity) in MCF-7, MDA-MB-231, and SUM159 cells after treated with DMSO or the indicated concentrations of BITC for 24 h Each experiment was repeated at least three times. Results were shown as mean ± SD. *Significantly different (P<0.05).
Knockdown of FOXH1 augments BITC-mediated inhibition of cell proliferation and invasion
mRNA and protein levels of FOXH1 were decreased by nearly 90% in the MDA-MB-231 cells and SUM159 cells transfected with the FOXH1-targeted siRNA compared with the control siRNA transfection cells (Figure 5A). Similar to siRNA transfection experiments, inhibition of cell proliferation resulting from BITC exposure was affected by FOXH1 knockdown in either MDA-MB-231 cells or SUM159 cells, as detected by the MTT assay (Figure 5B). In other word, knockdown of FOXH1 had obviously meaningful impact on BITC’s ability to inhibit cell proliferation. Furthermore, we also detect the BITC-mediated inhibition of MDA-MB-231 cell and SUM159 cell migration by knockdown of FOXH1, upon treatment with BITC, the invasion was markedly augmented by RNA interference of FOXH1, compared with the control siRNA-transfected cells, as detected by transwell assay (Figure 5C). Collectively, these results indicated that FOXH1 repression by BITC impeded its inhibitory effect on cell proliferation and invasion.
Figure 5.
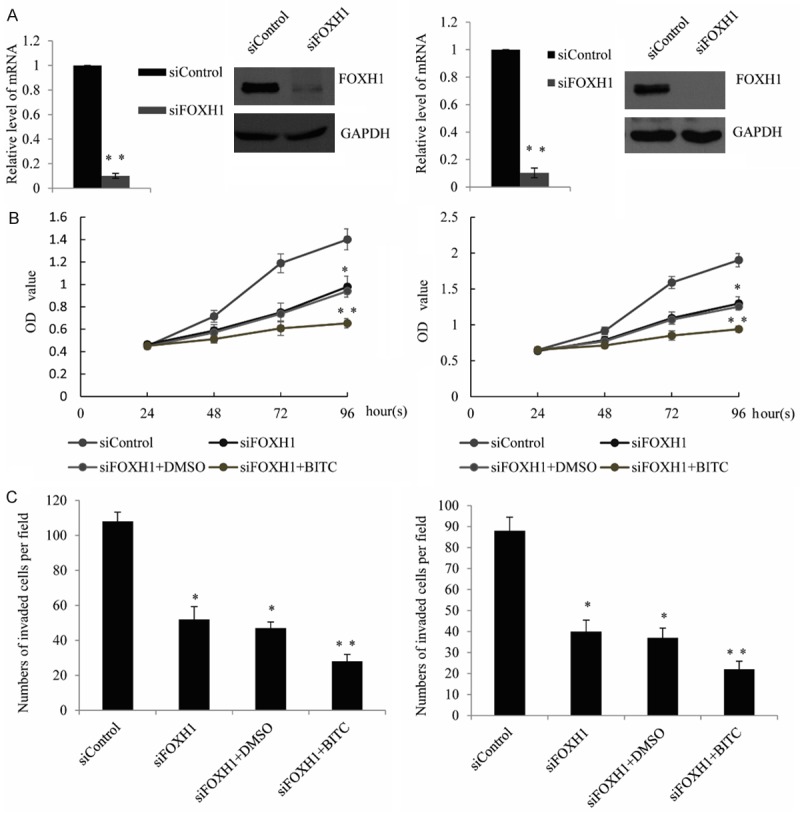
Knockdown of FOXH1 augments BITC-mediated inhibition of cell proliferation and invasion. A. qRT-PCR and Western blot analysis was performed inMDA-MB-231 cells or SUM159 cells transfected with FOXH1-specific siRNA for 48 hours. FOXH1 expression was observed to be downregulated both at the protein and mRNA levels. qRT-PCR results are presented as the mean ± S.D. of three independent experiments in triplicates. B. MTT assay was performed in the siFOXH1-transfected MDA-MB-231 cells or SUM159 cells, followed by treat with BITCor DMSO (control) for 24 hours. C. Transwell assay was performed in the siFOXH1-transfected MDA-MB-231 cells or SUM159 cells, followed by treat with BITC or DMSO (control) for 24 hours.
Discussion
Recently, more and more evidence indicated that epigenetic activation and genetic amplification of potential oncogenes contribute to the development of the breast cancer progression. In the present study, we observed the expression of FOXH1 was upregulated in breast cancer cell lines and tissues, and the ectopic expression of FOXH1 appeared to promote cancer cell proliferation and invasion.
Aberrant activation of Wnt signaling pathway has been implicated by a large body of data in many cancer types, such as breast ,colorectal, and prostate cancer, Wnt pathway was usually considered as key regulator in the cancer cell growth and differentiation [22]. Via translocate to the nucleus, β-catenin was stabilized and modulated the target genes expression, such as c-Myc and cyclin D1 [23], the role of which is promoting tumor cell proliferation and migration [24].
We found increased FOXH1 expression level induced the β-catenin expression, and siβ-catenin significantly reduced the FOXH1-induced promotion of breast cancer cells proliferation and invasion. These results demonstrated that FOXH1 in triggering breast cancer cell proliferation and invasion via activation of the Wnt/β-catenin signaling pathway.
Furthermore, we declared BITC treatment has remarkable effect on the FOXH1 and β-catenin expression as well as on the transcriptional activity of FOXH1, since FOXH1 could activate the β-catenin luciferase reporter activity, while the BITC treatment in the FOXH1 transfected MCF-7 cells, MDA-MB-231 cells, and SUM159 cells, resulted in a significant decrease in β-catenin luciferase reporter activity. Knockdown of FOXH1 augments BITC-mediated inhibition of cell proliferation and invasion in MDA-MB-231 cells and SUM159 further support our argument.
The functions of FOXH1 may not be limited to the regulation of â-catenin transcriptional activity, in the further, additional signaling pathways required for FOXH1 tumor transformed function need further investigations. In summary, our findings are of importance in understanding the breast cancer aetiology, with potential implications for breast cancer diagnosis and therapy.
Disclosure of conflict of interest
None.
References
- 1.Howlader N, Chen VW, Ries LA, Loch MM, Lee R, DeSantis C, Lin CC, Ruhl J, Cronin KA. Overview of breast cancer collaborative stage data items--their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010. Cancer. 2014;120(Suppl 23):3771–80. doi: 10.1002/cncr.29059. [DOI] [PubMed] [Google Scholar]
- 2.Breast International Group (BIG) 1-98 Collaborative Group. Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 3.Dahhan T, Balkenende E, van Wely M, Linn S, Goddijn M. Tamoxifen or letrozole versus standard methods for women with estrogen-receptor positive breast cancer undergoing oocyte or embryo cryopreservation in assisted reproduction. Cochrane Database Syst Rev. 2013;11:CD010240. doi: 10.1002/14651858.CD010240.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011;32:359–367. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warin R, Xiao D, Arlotti JA, Bommareddy A, Singh SV. Inhibition of Human Breast Cancer Xenograft Growth by Cruciferous Vegetable Constituent Benzyl Isothiocyanate. Mol Carcinog. 2010;49:500–507. doi: 10.1002/mc.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warin R, Chambers WH, Potter DM, Singh SV. Prevention of Mammary Carcinogenesis in MMTV-neu Mice by Cruciferous Vegetable Constituent Benzyl Isothiocyanate. Cancer Res. 2009;69:9473–9480. doi: 10.1158/0008-5472.CAN-09-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res (Phila) 2011;4:1107–1117. doi: 10.1158/1940-6207.CAPR-10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011;32:359–367. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic Tumor Suppression by Benzyl Isothiocyanate Is Associated with Inhibition of PI3K/AKT/FOXO Pathway. Clin Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright TM, Wardell SE, Jasper JS, Stice JP, Safi R, Nelson ER, McDonnell DP. Delineation of a FOXA1/ER alpha/AGR2 Regulatory Loop That Is Dysregulated in Endocrine Therapy-Resistant Breast Cancer. Mol Cancer Res. 2014;12:1829–1839. doi: 10.1158/1541-7786.MCR-14-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabowska MM, Elliott AD, DeGraff DJ, Anumanthan G, Yamashita H, Sun Q, Friedman DB, Hachey DL, Yu X, Sheehan JH, Ahn JM, Raj GV, Piston DW, Gronostajski RM, Matusik RJ. NFI Transcription Factors Interact with FOXA1 to Regulate Prostate-Specific Gene Expression. Mol Endocrinol. 2014;28:949–964. doi: 10.1210/me.2013-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y, Tindall DJ, Huang HJ. Modulation of Androgen Receptor by FOXA1 and FOXO1 Factors in Prostate Cancer. Int J Biol Sci. 2014;10:614–619. doi: 10.7150/ijbs.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng X, Luo Z, Kang Q, Deng D, Wang Q, Peng H, Wang S, Wei Z. FOXQ1 mediates the crosstalk between TGF- and Wnt signaling pathways in the progression of colorectal cancer. Cancer Biol Ther. 2015;16:1099–1109. doi: 10.1080/15384047.2015.1047568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Q, Lim SK, Zhao B, Hoffmann FM. Selective inhibition of TGF-beta responsive genes by Smad-interacting peptide aptamers from FoxH1, Lef1 and CBP. Oncogene. 2005;24:3864–3874. doi: 10.1038/sj.onc.1208556. [DOI] [PubMed] [Google Scholar]
- 16.Yum J, Jeong HM, Kim S, Seo JW, Han Y, Lee KY, Yeo CY. PKA-mediated stabilization of FoxH1 negatively regulates ERalpha activity. Mol Cells. 2009;28:67–71. doi: 10.1007/s10059-009-0099-7. [DOI] [PubMed] [Google Scholar]
- 17.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhl SJ, Kuhl M. On the role of Wnt/beta-catenin signaling in stem cells. Biochim Biophys Acta. 2013;1830:2297–2306. doi: 10.1016/j.bbagen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Zhou D, Bai F, Zhang X, Hu M, Zhao G, Zhao Z, Liu R. SOX10 is a novel oncogene in hepatocellular carcinoma through Wnt/beta-catenin/TCF4 cascade. Tumour Biol. 2014;35:9935–9940. doi: 10.1007/s13277-014-1893-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X, Yuan J, Li M. DEPDC1B enhances migration and invasion of non-small cell lung cancer cells via activating Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2014;450:899–905. doi: 10.1016/j.bbrc.2014.06.076. [DOI] [PubMed] [Google Scholar]
- 21.Sadras T, Perugini M, Kok CH, Iarossi DG, Heatley SL, Brumatti G, Samuel MS, To LB, Lewis ID, Lopez AF, Ekert PG, Ramshaw HS, D’Andrea RJ. Interleukin-3-mediated regulation of beta-catenin in myeloid transformation and acute myeloid leukemia. J Leukoc Biol. 2014;96:83–91. doi: 10.1189/jlb.2AB1013-559R. [DOI] [PubMed] [Google Scholar]
- 22.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury MK, Montgomery MK, Morris MJ, Cognard E, Shepherd PR, Smith GC. Glucagon phosphorylates serine 552 of beta-catenin leading to increased expression of cyclin D1 and c-Myc in the isolated rat liver. Arch Physiol Biochem. 2015;121:88–96. doi: 10.3109/13813455.2015.1048693. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


