Abstract
The NOD-like receptor family-pyrin domain containing 3 (NLRP3) inflammasome is an essential factor in enhancing inflammation and autoimmunity. Anesthetic isoflurane (ISO) exerts a novel pharmacological action in anti-inflammation. However, whether ISO hinders the pathogenesis of lupus nephritis (LN) by inhibiting NLRP3 inflammasome activation remains unclear. In this study, 12-week-old MRL/lpr and C57BL/6 mice were treated with and without 1.4% ISO for eight weeks. ISO administration significantly reduced mortality, serum anti-dsDNA level, renal immune complex deposition, and the ratio of Th17 to Treg cells in MRL/lpr mice. ISO treatment remarkably reduced the levels of blood urea nitrogen, proteinuria, interleukin (IL)-17, IL-1β, and tumor necrosis factor-α, as well as the infiltration of macrophages. ISO also abrogated renal NLRP3 inflammasome formation and activation. These results suggest that ISO may be a promising therapeutic agent for LN partly because it restricts NLRP3 inflammasome activation.
Keywords: Lupus nephritis, isoflurane, NLRP3 inflammasome
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease involving multiple systems; however, the kidney is the most commonly affected organ [1]. Lupus nephritis (LN) is one of the most common and severe complications of SLE and results in approximately 50% incidence of morbidity and mortality in lupus patients [2]. Despite considerable advances in therapeutic strategy, a large number of LN patients eventually progress to end-stage renal disease [3]. Thus, identifying the underlying mechanisms of LN and establishing a novel therapy approach are urgently needed.
Inflammation is involved in the pathogenesis of LN with macrophage infiltration [4]. The inflammasome is known to switch on the inflammatory responses to various stress signals [5,6]. The NOD-like receptor family-pyrin domain containing 3 (NLRP3) inflammasome is one of the best-characterized members implicated in various mammalian cells. NLRP3 inflammasome is composed of the regulatory subunit NLRP3, an adaptor apoptosis-associated speck-like protein (ASC), and effector caspase-1. Caspase-1 is triggered and activated to promote the cleavage and secretion of pro-inflammatory cytokine interleukin (IL)-1β [7]. Recent studies have reported on the enhancement of NLRP3 inflammasome activation in the pathogenesis of SLE, including LN [7-10]. Thus, reducing NLRP3 inflammasome activation may be an effective therapeutic strategy for LN.
Isoflurane (ISO) induces a novel pharmacological action in anti-inflammation [11]. Previous studies have confirmed that ISO treatment significantly attenuates experimental lung and liver injury by inhibiting inflammatory responses [12-14]. However, whether the protective effect of ISO on LN depends on its anti-inflammatory activity remains unknown.
In this study, the inhibitory effect of ISO on LN in MRL/lpr mice and the underlying mechanisms were investigated. We found that ISO significantly increased the survival rate and attenuated the renal damage of the mice. ISO treatment abrogated renal NLRP3 inflammasome formation and activation to reduce inflammatory responses. These results suggest that ISO may be a promising therapeutic agent for hindering LN progression partly by the inactivation of NLRP3 inflammasome.
Materials and methods
Reagents
Rabbit anti-mouse NLRP3, ASC, caspase-1 p20, and β-actin monoclonal antibodies (mAbs) were purchased from Abcam (Cambridge, UK). ISO was provided by Baxter Healthcare Corporation (Deerfield, IL, USA). Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG (Santa Cruz, Dallas, TX, USA) and FITC-conjugated goat IgG fraction to mouse complement C3 (Cedarlane Labs, Canada) were commercially obtained. Calf thymus dsDNA (Sigma-Aldrich, St. Louis, MO, USA), rabbit anti-mouse dsDNA mAb (Chemicon International, USA), and goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technology Inc., Danvers, MA, USA) were commercial products. Other reagents were purchased from Sigma-Aldrich unless otherwise noted.
Mice
Twelve-week-old female MRL/lpr mice were purchased from Shanghai SLAC Laboratory Animal Company (Shanghai, China) and maintained under specific pathogen-free conditions in the Laboratory Animal Center of the First Affiliated Hospital of Zhengzhou University. Sex- and age-matched C57BL/6 mice were obtained from the Laboratory Animal Center of Henan Province as the normal control. All experiments were conducted in accordance with the National Institute of Health Guide for Care and Use of Animals and adhered to the rules of the ethical committee of the First Affiliated Hospital of Zhengzhou University. Euthanasia by sodium pentobarbital was performed following the American Veterinary Medical Association Guidelines on Euthanasia (June 2007).
Treatment protocol
MRL/lpr mice were randomly divided into two groups (n = 8), namely, room air (RA)-treated group (MRL + RA) and ISO-treated group (MRL + ISO). The mice in the MRL + ISO group received 1.4% ISO inhalation (1 h per time, thrice a week) for eight weeks. The mice in the MRL + RA group were exposed to RA instead of ISO treatment in the same manner. The sex- and age-matched C57BL/6 mice as the control (Ctrl) were also randomly categorized into two groups (n = 8), namely, Ctrl + RA and Ctrl + ISO. The animals were treated in the same manner as the MRL/lpr mice. Briefly, the mice were placed in a sealed Plexiglas chamber with inflow and outflow outlets and allowed to inhale ISO for the indicated times, as previously described [12-14]. The gas concentration in the outflow hose of the chamber was continuously monitored with a gas analyzer (Brüel & Kjae, Naerum, Denmark). ISO concentration was maintained at 1.4%, and oxygen concentration was maintained at 21% in the chamber during ISO treatment. Carbon dioxide was removed from the chamber gases with baralyme (Allied Healthcare Products, Inc., St. Louis, MO, USA). The animals not subjected to ISO treatment were exposed to RA in the chamber in the same manner. The room and chamber temperatures were maintained between 22°C and 24°C. All animals were sacrificed after various treatments for eight weeks. Kidney tissues were harvested after perfusion and removal of residual blood. Some of the samples were fixed in 10% neutral-buffered formalin and embedded in paraffin, and the others were snap-frozen in liquid nitrogen and stored at -80°C.
Measurement of survival rate
To measure the survival rate, the 12-week-old MRL/lpr mice and sex- and age-matched C57BL/6 mice were treated with and without 1.4% ISO for eight weeks; the survival state was examined for 18 weeks, from 12 weeks to 30 weeks of age (n = 12).
Biochemistry assays
Blood samples were collected upon completion of the experiment, and the levels of blood urea nitrogen (BUN) were determined with a commercial autoanalyzer (Beckman Coulter, Inc., USA). To assess urinary protein excretion, the mice were placed in metabolic cages, and 24 h urine was collected biweekly from 12 weeks to 20 weeks of age. Urinary protein excretion was tested with Multistix 10SG reagent strips (Bayer Health Care) and graded on a scale of 0-4, where 0 = none, 1 = 30-100 mg/dl, 2 = 100-300 mg/dl, 3 = 300-2,000 mg/dl, and 4 > 2,000 mg/dl, as described previously [15].
Assessment of histopathology and immune complex deposition
The paraffin kidney sections were stained with hematoxylin and eosin (HE) reagents. According to the histopathology findings, the severities of the glomerular and renal vascular lesions were graded blindly on a scale of 0 to 3 as reported previously [16].
To detect immune complex deposition, the frozen sections were blocked with 10% fetal bovine serum and then stained with FITC-conjugated rabbit anti-mouse IgG (Santa Cruz) or FITC-conjugated goat IgG fraction to mouse complement C3 (Cedarlane). Fluorescence intensity was calculated and scored from 0 to 3 as described previously [17].
Immunolohistochemistry (IHC)
The paraffin kidney sections were deparaffinized, rehydrated, quenched with 0.3% H2O2, and incubated in 10% normal goat serum (Gibco). Primary anti-CD68 polyclonal antibody (Abcam) was added to the sections and incubated overnight, followed by a biotinylated secondary antibody. The reaction products were visualized with a diaminobenzidine (DAB) substrate (Maixin Biotech., Fuzhou, China). The slides were counterstained with commercial hematoxylin and observed under a light microscope (Leica, Wetzlar, Germany). All sections were blindly evaluated for DAB-positive staining by two experienced pathologists.
Enzyme-linked immunosorbent assay (ELISA)
Serum anti-double-stranded DNA (anti-dsDNA) level was determined by ELISA as described previously [18]. Briefly, 96-well plates were pre-coated with 5 μg/ml calf thymus dsDNA. Serum was then added into the 96-well plates, and absorbance was measured at 450 nm. A mouse anti-dsDNA mAb (Chemicon International, USA) was utilized to prepare a reference standard curve. The anti-dsDNA concentrations were quantified according to the standard curve. Normal mouse IgG was employed as negative control.
Serum and kidney homogenates were collected to measure IL-1β, IL-17, and tumor necrosis factor-α (TNF-α) by using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Optical density was measured with an ELISA plate scanner (CA94089, Molecular Devices, Sunnyvale, Canada).
Western blotting
Total proteins were extracted from fresh kidney tissues using tissue lysis buffer (Cell Signaling Technology). Equal amounts of protein (20 μg) were separated through SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Millipore, USA). Subsequently, the membranes were incubated with rabbit anti-mouse mAbs targeting NLRP3 (1:3,000), ASC (1:2,000), caspase-1 p20 (1:2,000), and β-actin (1:4,000) at 4°C overnight. Blots were then incubated with goat anti-rabbit secondary antibody (1:2,000) conjugated to horse radish peroxidase for 2 h at room temperature. Peroxidase reaction was visualized with an enhanced chemiluminescence kit (Santa Cruz). Protein band density was quantified with Quantity One software (Bio-Rad, Berkeley, CA, USA).
Isolation of mouse splenocytes
Mice were euthanatized with sodium pentobarbital, and the fresh spleens were removed and dissociated by using the plunger end of the syringe. After the cell suspension was passed through a 70 μm cell strainer, erythrocytes were lysed with red blood cell lysis buffer at room temperature for 5 min. Other cells were collected by centrifugation at 300 × g for 5 min and washed thrice for flow cytometry analysis.
Flow cytometry
To evaluate the T cell subsets, single-cell suspensions dissociated from splenocytes were incubated with FITC-conjugated anti-mouse CD4, phycoerythrin (PE)-conjugated anti-mouse CD25, PE-Cy5-conjugated anti-mouse Foxp3 (FJK-16 s), PE-conjugated anti-mouse IL-17, or their respective isotype controls. The CD4+, CD25+, and Foxp3+ cells were considered Treg cells. To detect the amount of Thl7 cells, splenocytes (1 × 106 cells/well) were incubated with 50 ng/ml phorbol myristate acetate and 1 μg/ml ionomycin (MultiSciences) supplemented with brefeldin A/monensin mixture (MultiSciences) for 5 h before intracellular staining. The CD4+ and IL-17+ cells were considered Th17 cells. Cell groups were analyzed with FACS Aria II (BD Biosciences, San Jose, CA, USA). Analyses were performed with CellQuest 3.0 software (BD).
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed with student’s t test or one-way analysis of variance. Kaplan-Meier method and log-rank analysis were employed to compare the survival rates. All data were analyzed with SPSS 16.0 software. P < 0.05 was considered statistically significant.
Results
ISO reduced the mortality of MRL/lpr mice
The effect of ISO on the mortality of MRL/lpr mice was investigated. As expected, the mice in the control groups survived. A significant survival benefit was observed in ISO-treated MRL/lpr mice as compared with the mice in the MRL + RA group (Figure 1). These results indicate that ISO significantly increased the survival rate of MRL/lpr mice.
Figure 1.
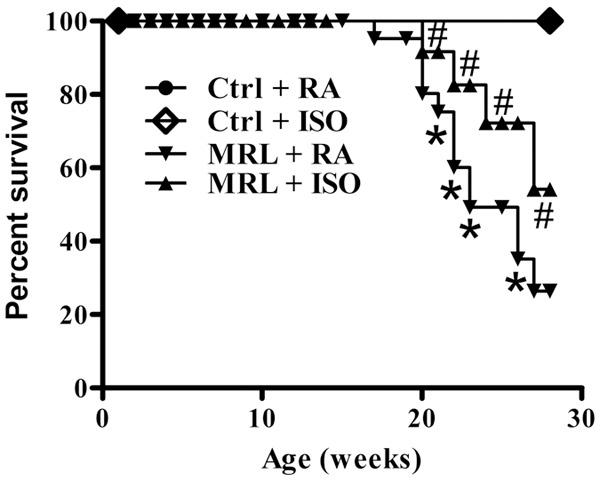
ISO treatment increased the survival rate of MRL/lpr mice. Treatment began at 12 weeks of age, and the mice were observed for 18 weeks. Each experiment was performed in triplicate. Values are presented as mean ± SD. *P < 0.05 versus (vs.) Ctrl groups; #P < 0.05 vs. MRL + RA group. Ctrl: control; RA: room air; ISO: isoflurane.
ISO ameliorated renal dysfunction and injury in MRL/lpr mice
The effect of ISO on renal function in MRL/lpr mice was also investigated. The BUN level was significantly higher in RA-treated MRL/lpr mice than that in the control groups. However, a notable reduction by ISO treatment was observed in MRL/lpr mice (P < 0.05; Figure 2A). A similar effect on proteinuria development by ISO treatment was observed (Figure 2B). The HE staining results showed that RA-treated MRL/lpr mice exhibited severe kidney damage, which was attenuated by ISO administration (P < 0.05; Figure 2C). All the data demonstrate that ISO improved renal function and reduced renal injury in MRL/lpr mice.
Figure 2.
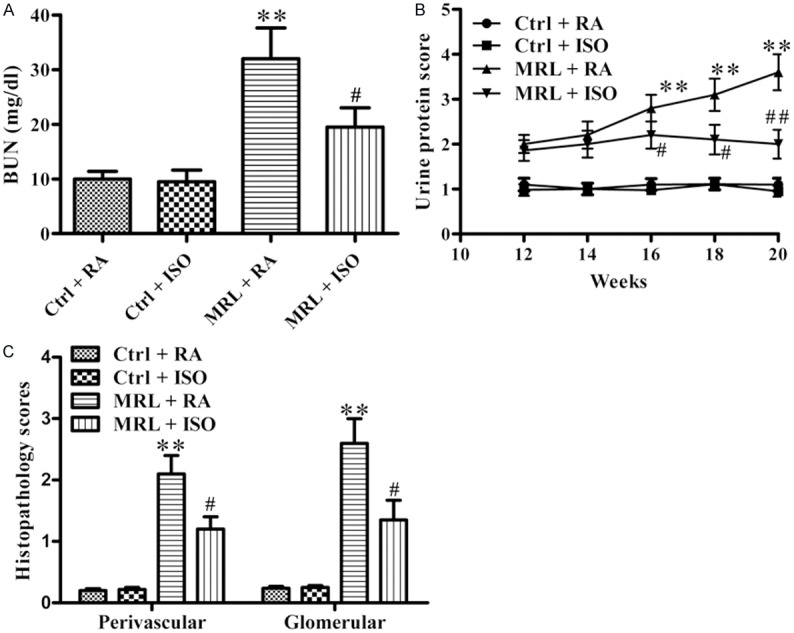
ISO ameliorated renal dysfunction and injury in MRL/lpr mice. A. BUN levels of the different mice groups. B. Urine protein excretion was measured every two weeks. C. HE staining of kidney sections was histopathologically assessed using a scoring system based on the damage index of kidneys. Each experiment was performed in triplicate. Values are presented as mean ± SD. **P < 0.01 vs. Ctrl groups; #P < 0.05, ##P < 0.01 vs. MRL + RA group. Ctrl: control; RA: room air; ISO: isoflurane.
ISO reduced autoantibody production and renal immune complex deposition in MRL/lpr mice
Circulating anti-dsDNA antibody level is a key marker for LN [18]. The level of anti-dsDNA antibody was much higher in the RA-treated MRL/lpr mice than that in the control groups, whereas ISO treatment significantly inhibited anti-dsDNA antibody production (P < 0.05; Figure 3A). Pronounced deposition of IgG and C3 was observed in the kidneys of MRL/lpr mice as compared with the control mice, which was remarkably reduced by ISO inhalation (P < 0.05; Figure 3B). These results indicate that ISO inhibited anti-dsDNA antibody production and kidney immune complex deposition in MRL/lpr mice.
Figure 3.
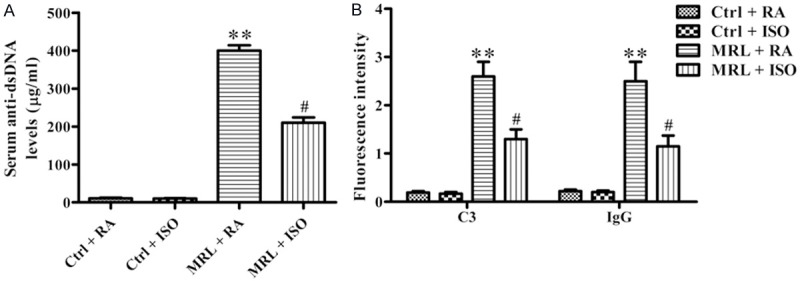
ISO reduced serum anti-dsDNA antibody production and renal immune complex deposition in MRL/lpr mice. A. Serum anti-dsDNA antibody was assessed by ELISA. B. Deposition of immune complex in the kidney was detected and scored based on fluorescence intensity using FITC-conjugated anti-C3 and anti-IgG antibodies. Each experiment was performed in triplicate. Values are presented as mean ± SD. **P < 0.01 vs. Ctrl groups; #P < 0.05 vs. MRL + RA group. Ctrl: control; RA: room air; ISO: isoflurane.
ISO treatment reduced Thl7/Treg cell ratio, macrophage accumulation, and pro-inflammatory cytokine production
The Thl7/Treg cell ratio in the spleens was determined by flow cytometry analysis. A conspicuous reduction in the Thl7/Treg cell ratio was observed in MRL/lpr mice with ISO treatment, suggesting an increase in Treg cells and a diminution in Thl7 cells compared with the RA-treated group (P < 0.05; Figure 4A). ISO treatment also resulted in a conspicuous decrease in serum IL-17 levels in MRL/lpr mice (P < 0.05; Figure 4B). In addition, macrophage accumulation in the kidney was sharply decreased in MRL/lpr mice with ISO treatment as compared with the RA-treated group (P < 0.05; Figure 4C), as evidenced by CD68-positive cell numbers. The ELISA results showed a significant reduction in TNF-α in renal and serum of ISO-treated MRL/lpr mice (P < 0.05; Figure 4D and 4E). These results suggest that ISO administration reduced Thl7/Treg cell ratio and inflammatory response in MRL/lpr mice.
Figure 4.
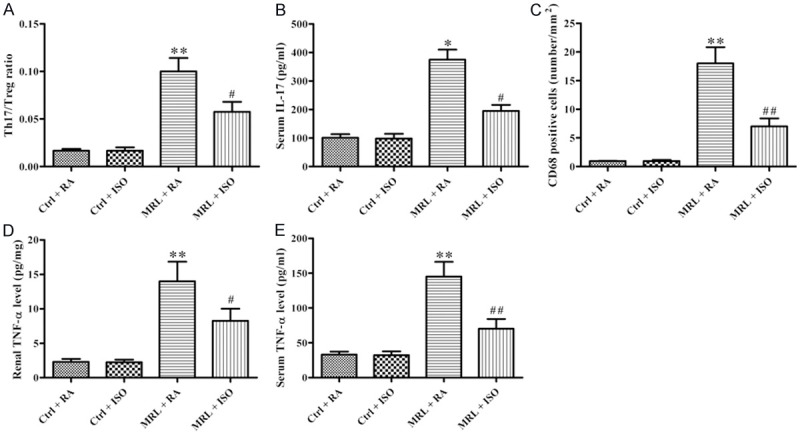
ISO treatment resulted in the reduction of Thl7/Treg cell ratio, macrophage infiltration, and IL-17 and TNF-α production in MRL/lpr mice. A. Thl7/Treg cell ratio was determined by flow cytometry analysis. B. ELISA analysis of serum IL-17 level. C. Macrophage accumulation was assessed based on the number of CD68-positive cells. D and E. Renal and serum TNF-α levels were measured by ELISA. Each experiment was performed in triplicate. Values are presented as mean ± SD. **P < 0.01 vs. Ctrl groups; #P < 0.05, ##P < 0.01 vs. MRL + RA group. Ctrl: control; RA: room air; ISO: isoflurane.
ISO treatment inhibited NLRP3 inflammasome activation in the kidneys of MRL/lpr mice
The effects of ISO on NLRP3 inflammasome activation in MRL/lpr mice were examined to decipher the underlying mechanisms by which ISO attenuates murine LN. The upregulation of NLRP3, ASC, and caspase-1 p20 was significantly reduced by ISO treatment in the kidneys of MRL/lpr mice (Figure 5A and 5B). The renal and serum levels of IL-1β were also reduced after ISO treatment in MRL/lpr mice (P < 0.05; Figure 5C and 5D). These results demonstrate that ISO treatment inhibited NLRP3 inflammasome activation in MRL/lpr mice.
Figure 5.
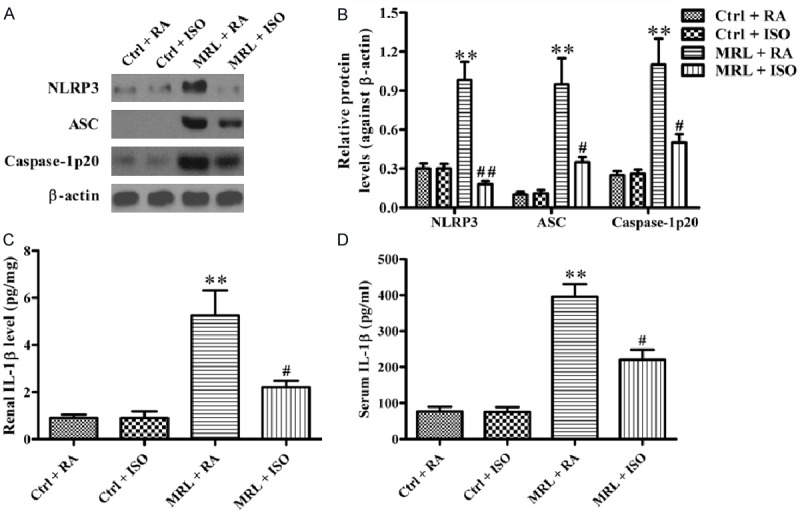
ISO suppressed NLRP3 inflammasome activation in the kidney of MRL/lpr mice. A. Western blot was performed to analyze the expression of NLRP3, ASC, and caspase-1 p20. b-actin was used as the endogenous control. B. Quantitative expression of NLRP3, ASC, and caspase-1 p20 was normalized against b-actin. C and D. Renal and serum IL-1β levels were measured by ELISA. Each experiment was performed in triplicate. Values are presented as mean ± SD. **P < 0.01 vs. Ctrl groups; #P < 0.05, ##P < 0.01 vs. MRL + RA group. Ctrl: control; RA: room air; ISO: isoflurane.
Discussion
Despite significant improvements in LN therapy, the pathogenesis of LN remains unclear, and its prognosis is still dismal [19]. Thus, clarifying the underlying mechanisms and discovering new means of LN treatment are necessary. In this study, we found that ISO significantly attenuated LN in lupus-prone MRL/lpr mice. The key findings were as follows: First, ISO markedly reduced the mortality of MRL/lpr mice and improved kidney dysfunction and injury. Second, ISO significantly reduced serum anti-dsDNA levels and renal immune complex deposition. Third, the Thl7/Treg cell ratio, serum IL-17, macrophage accumulation in the kidney, and levels of TNF-α and IL-1β in renal and serum were significantly reduced by ISO treatment. Lastly, ISO administration inhibited NLRP3 inflammasome activation.
BUN and proteinuria are two indices of kidney inflammation and dysfunction [20]. Antibodies for dsDNA/nucleosomes are most closely linked with nephritis, and the presence of autoantibody is a requirement for LN development [21,22]. Immune complex deposition in the kidney can trigger a series of events that result in kidney inflammation and injury [23]. This study showed that ISO significantly reduced the mortality of lupus-prone MRL/lpr mice that benefited from decreased BUN level, proteinuria, serum anti-dsDNA production, and renal immune complex deposition.
The imbalance in Thl7/Treg cells is closely associated with the pathogenesis of LN. Previous studies have shown that decreased Thl7 cell numbers is positively associated with LN attenuation [24], and expansion of Treg cells protects against chronic graft-versus-host disease-induced LN in mice [25]. Increased IL-17 production contributes critically to the development of lethal pathologic features [26]. In addition, IL-17 has strong pro-inflammatory effects by inducing the production of other cytokines (e.g., TNF-α), promoting inflammatory cell recruitment, and facilitating T-cell infiltration [27-29]. Macrophage infiltration is a prominent feature in both humans and mice LN [30]. In this study, ISO administration reduced the Thl7/Treg cell ratio probably by inhibiting IL-17 generation, macrophage infiltration, and renal and serum TNF-α production.
Inflammation plays an indispensable role in LN progression [4]. The NLRP3 inflammasome/IL-1β signaling pathway is reportedly implicated in a mouse model of LN [31]. The NLRP3 inflammasome is activated in the kidney of lupus-prone NZBWF1 mice [32]. In addition, NLRP3 expression is highly elevated in LN patients [33]. ASC is significantly upregulated in peripheral lymphocytes and renal biopsy tissues of LN patients [34,35]. ASC acts as an adaptor by linking NLRP3 and procaspase-1, thereby activating caspase-1 to promote IL-1β production [36]. Moreover, self dsDNA can induce IL-1β production from human monocytes by activating the NLRP3 inflammasome in the presence of anti-dsDNA antibodies [37]. In the present study, ISO administration significantly inhibited NLRP3 inflammasome activation, as evidenced by the downregulation of NLRP3, ASC, and caspase-1 p20 expression, as well as IL-1β production. These results suggest that the protective effects of ISO on LN are partly attributed to the inhibition of the NLRP3 inflammasome/IL-1β signaling pathway.
In conclusion, ISO treatment attenuated LN by inhibiting NLRP3 inflammasome formation and activation and subsequently reducing inflammatory responses in MRL/lpr mice. Thus, ISO may be a promising therapeutic strategy to prevent LN progression by inhibiting the NLRP3 inflammasome/IL-1β signaling pathway.
Disclosure of conflict of interest
None.
References
- 1.Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis Res Ther. 2011;13:250. doi: 10.1186/ar3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18:468–475. doi: 10.1097/01.bor.0000240356.45550.13. [DOI] [PubMed] [Google Scholar]
- 3.Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010;62:873–880. doi: 10.1002/acr.20116. [DOI] [PubMed] [Google Scholar]
- 4.Grande JP. Mechanisms of progression of renal damage in lupus nephritis: pathogenesis of renal scarring. Lupus. 1998;7:604–610. doi: 10.1191/096120398678920721. [DOI] [PubMed] [Google Scholar]
- 5.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloidbeta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2014;52:286–293. doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 12.Mu J, Xie K, Hou L, Peng D, Shang L, Ji G, Li J, Lu Y, Xiong L. Subanesthetic dose of isoflurane protects against zymosan-induced generalized inflammation and its associated acute lung injury in mice. Shock. 2010;34:183–189. doi: 10.1097/SHK.0b013e3181cffc3f. [DOI] [PubMed] [Google Scholar]
- 13.Li JT, Wang H, Li W, Wang LF, Hou LC, Mu JL, Liu X, Chen HJ, Xie KL, Li NL, Gao CF. Anesthetic isoflurane posttreatment attenuates experimental lung injury by inhibiting inflammation and apoptosis. Mediators Inflamm. 2013;2013:108928. doi: 10.1155/2013/108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Fan J, Li NL, Li JT, Yuan SF, Yi J, Wang L, Chen JH, Lv YG, Yao Q, Wang T, Wang YC, Ling R. A subanesthetic dose of isoflurane during postconditioning ameliorates zymosan-induced neutrophil inflammation lung injury and mortality in mice. Mediators Inflamm. 2013;2013:479628. doi: 10.1155/2013/479628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muraoka M, Hasegawa H, Kohno M, Inoue A, Miyazaki T, Terada M, Nose M, Yasukawa M. IK cytokine ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2006;54:3591–3600. doi: 10.1002/art.22172. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Yang N, Wang S, Huang B, Li F, Tan H, Liang Y, Chen M, Li Y, Yu X. Adenosine 2A receptor is protective against renal injury in MRL/lpr mice. Lupus. 2011;20:667–677. doi: 10.1177/0961203310393262. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz N, Goilav B, Putterman C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol. 2014;26:502–509. doi: 10.1097/BOR.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaelson JS, Wisniacki N, Burkly LC, Putterman C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J Autoimmun. 2012;39:130–142. doi: 10.1016/j.jaut.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Zhang H, Huang Y, Wang H, Wang S, Zhao C, Liang Y, Yang N. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kB activation. Int Immunopharmacol. 2013;17:116–122. doi: 10.1016/j.intimp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Arbuckle MR, McClain MT, Rubertone MV, Scofi eld RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 22.Fenton KA, Rekvig OP. A central role of nucleosomes in lupus nephritis. Ann N Y Acad Sci. 2007;1108:104–113. doi: 10.1196/annals.1422.012. [DOI] [PubMed] [Google Scholar]
- 23.Rovin BH, Parikh SV. Lupus nephritis: the evolving role of novel therapeutics. Am J Kidney Dis. 2014;63:677–690. doi: 10.1053/j.ajkd.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, Ninichuk V, Segerer S, Vielhauer V. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas) lpr mice. J Am Soc Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JL, Sun DJ, Hou CM, Wei YL, Li XY, Yu ZY, Feng JN, Shen BF, Li Y, Xiao H. CD3 mAb treatment ameliorated the severity of the cGVHD-induced lupus nephritis in mice by up-regulation of Foxp3+ regulatory T cells in the target tissue: kidney. Transpl Immunol. 2010;24:17–25. doi: 10.1016/j.trim.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, Mayadas TN, Illei GG, Siebenlist U. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–1115. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4509. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolidis SA, Crispin JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120–124. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 30.Hu Q, Yang C, Wang Q, Zeng H, Qin W. Demethylzeylasteral (T-96) Treatment Ameliorates Mice Lupus Nephritis Accompanied by Inhibiting Activation of NF-κB Pathway. PLoS One. 2015;10:e0133724. doi: 10.1371/journal.pone.0133724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, Wang S, Gaskin F, Yang N, Fu SM. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, Hua KF, Chang WL, Huang JJ, Yang SS, Chen A. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187:6143–6156. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eggleton P, Harries LW, Alberigo G, Wordsworth P, Viner N, Haigh R, Donnelly S, Jones HW, Chikanza IC. Changes in apoptotic gene expression in lymphocytes from rheumatoid arthritis and systemic lupus erythematosus patients compared with healthy lymphocytes. J Clin Immunol. 2010;30:649–658. doi: 10.1007/s10875-010-9429-y. [DOI] [PubMed] [Google Scholar]
- 36.Saleh M. The machinery of Nod-like receptors: refining the paths to immunity and cell death. Immunol Rev. 2011;243:235–246. doi: 10.1111/j.1600-065X.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA 3rd. Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]


