Abstract
Objectives: This study is to investigate the role and mechanism of microRNA-202 (miR-202) in endometriosis. Methods: Forty-five cases of ectopic endometrial tissues, 25 cases of eutopic endometrial tissues and 26 cases of normal endometrial tissues were collected. MiR-202 expression was detected by quantitative RT-PCR. The protein expressions of SOX6 (sex determining region Y-box 6) and its downstream proteins (p21, cyclin D1 and pRb (retinoblastoma protein)) were detected by immunochemistry and western blot. MTT and transwell assays were used to examine cell proliferation and cell migration. The dual luciferase assay was applied to validate whether miR-202 can directly target SOX6 gene. Results: MiR-202 was highly expressed in eutopic and ectopic endometrial tissues than normal endometrial tissues (P < 0.05), and the expression was higher in tissues with III/IV stages than I/II stages (P < 0.05). The expression of SOX6 protein was lower in ectopic endometrial tissues than in normal endometrial tissues. In ectopic endometrial tissues, the expression of p21 was decreased while cyclin D1 and pRb was up-regulated than in normal endometrial tissues (P < 0.05). In cultured endometrial cells, miR-202 down-regulation induced up-regulation of SOX6 and p21 whereas down-regulation of cyclin D1 and pRb. MiR-202 promoted the proliferation and metastasis of endometrial cells. And, miR-202 could complementary bind to SOX6 3’UTR to regulate the expression of SOX6. Conclusion: MiR-202 was up-regulated in the endometriosis. Through targeting SOX6 and its downstream proteins (p21, cyclin D1 and pRb), miR-202 can promote the progression of endometriosis.
Keywords: EMs, miR-202, SOX6
Introduction
Endometriosis (EMs) is an inflammation related and hormone dependent disease, which means functional endometrial tissues appear outside the uterine mucous membrane (not including the myometrium) [1]. The ovarian endometriosis is the main manifestations, and the dysmenorrhea and infertility are the main clinical symptoms [2,3]. EMs is usually regarded as benign in pathology, while ectopic EMs has malignant characters like infiltration, proliferation and metastasis [4,5]. In recent years, EMs incidence increased gradually, which threatens the life quality and health of women [6].
The etiology of EMs is complex, which is highly related with the inflammatory factors and hormone levels [7-9]. Recent studies indicate that microRNA (miRNA), as a new post-transcriptional regulator, may play important roles in the progression of Ems [10-13]. Braza-Boïls et al showed that the expression of many miRNAs was dysregulated in EMs tissues, among which miRNA-202 (miR-202) expression significantly decreased compared with normal control [14]. On the contrary, Hawkins et al found that miR-202 increased significantly in Ems [15]. Bioinformatics prediction indicated that miR-202 could target SOX6 (sex determining region Y-box 6) whose expression was associated with the cell proliferation, invasion and metastasis [16].
In this study, quantitative real-time PCR (qRT-PCR) was performed to detect the expression level of miR-202 in ectopic tissues. The protein changes of SOX6, p21, cyclin D1 and p-Rb were identified by Western blot and immunohistochemistry. We further explored the biological functions and molecular mechanisms of miR-202 on the primary cultured endometrial cells. The results may present novel diagnosis and treatment biomarkers for EMs.
Materials and methods
Sample collection and cell culture
This study included 45 patients with ectopic EMs, 25 patients with eutopic EMs, and 26 patients with abnormal uterine Obstetrics and Gynecology of the Huaihe Hospital from Jan, 2013 to Apr, 2014. All the patients did not receive hormone treatment and did not have serious complication. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Henan University. Of the 45 ectopic patients (21-49 years old), the average age was 35.7 and the median age was 31. According to the criteria of American Fertility Association [17], samples of EMs were classified into 16 cases of I/II stage, and 29 cases of III/IV stage. Endometrial cells (ESC) were cultured according to methods published by Li MQ [18]. 293T cells were cultured in 10% FBS-1640 medium.
Immunohistochemistry (IHC)
The tissues were quickly frozen with liquid nitrogen after surgery resection, then preserved in -80°C. Tissues were fixed in 10% formalin and embedded in paraffin. Then, tissues were cut into 4 m sections. The sections were placed in xylene with serial dilutions and 100% ethanol for dewaxing and dehydration. Freshly prepared 3% hydrogen peroxide was added and incubated at room temperature for 10 min to inactivate endogenous peroxidase. After washing with PBS and antigen retrieval, primary antibodies of SOX6 (Bioworld, Minnesota, USA), p21 (Bioworld, Minnesota, USA), cyclin D1 (Bioworld, Minnesota, USA), and p-Rb (Bioworld, Minnesota, USA) (rabbit anti-human) were added respectively. After extensive washing, the slides were incubated with biotinylated secondary antibody at 37°C for about 30 min. Then sections were developed with DAB chromogenic reagent. Finally, sections were counterstained with haematoxylin. After hydrochloric acid differentiation and xylene transparency, sections were mounted with neutral gum.
Scoring of IHC
Under high-power field, the positive cells were selected if the brown or brown granules appeared in the cytoplasm or membrane. In each section, 5 fields of tumor cells were counted randomly, and the scores were determined by combining the proportion of positively stained cells and the intensity of staining. The proportion of positively stained tumor cells was graded from 0 to 3 (0, < 5% positive cells; 1, 5-25%; 2, 26-50%; 3, > 50%). The intensity of staining was recorded on a scale of 0 (no staining), 1 (weak staining, light blue or yellow), 2 (moderate staining, blue or yellow), and 3 (strong staining, dark blue or yellow). The staining index (SI) was calculated as follows: staining index = proportion of positively stained cells × staining intensity. The expression of target proteins was evaluated by the SI and scored as 0, 1, 2, 3, 4, 6, or 9. A score in 0-1 was regarded as negative, in 2-3 was weakly positive, in 4-6 was middle positive, and more than 6 was strong positive.
RNA extraction and qRT-PCR
Total RNA was isolated from tissues using 1 ml Trizol reagent (Invitrogen, California, USA) per 100 mg, according to the manufacture’s protocol. The RNA integrity was checked by gel electrophoresis, and the purity of RNA was detected by Spectrophotometer 260/280. cDNA was synthesized from total RNA by the reverse transcription with PrimeScript RT Regent Kit (TAKARA, Dalian, China) and stored in -20°C. The qRT-PCR was performed by the protocol of SYBR PrimeScript RT-PCR Kit (TAKARA, Dalian, China). The reaction system included 10 μl qRT-PCR-Mix, 0.5 μl forward primer and 0.5 μl reverse primer, 2 μl cDNA and 7 μl ddH2O. The cycle conditions were the following: 95°C for 10 min, and followed by 40 cycles at 95°C for 1 min and 60°C for 30 s.
Western blot analysis
For protein isolation, each 100 mg tissue was ground into powder, and lysed with RIPA (containing 1% PMSF) lysis buffer. After centrifugation at 4°C for 10 min, the supernatant was reserved. The isolated protein was loaded into SDS-PAGE and then transferred to PVDF membrane. The primary antibody was rabbit anti-human SOX6 (1:800) and mouse anti-human GAPDH antibody (1:3000). The second antibody was HRP-conjugated goat anti-mouse and goat anti-rabbit IgG (1:3000). All the antibodies were purchased from the Bioworld Company (Minnesota, USA). Finally, the membrane was developed by enhanced chemiluminescence plus reagent.
Transfection of ESC
Logarithmic growth ESC cells were seeded in 24-well plates (3 × 105) in medium without antibiotic. The antagomiR-202 were transfected using LipofectamineTM 2000 reagent (Invitrogen, California, USA) according to the manufacturer’s instructions. After 48 h, the transfected cells were collected to detect the protein change of SOX6 and associated downstream proteins of p21, cyclin D1 and p-Rb. Cells without transfection were used as negative control (NC).
MTT assay to detect the proliferation
Three groups of cells were seeded in 96-well plates (2 × 103) respectively, and each group had 3 replicates. The MTT solution was added to each well at 0 h, 24 h, 48 h, and 72 h respectively. In the last day, 150 ul DMSO was added to dissolve purple crystals. After incubation at 37°C for 4 h, the absorbance of each well at 490 nm wavelength was measured to generate cell growth curves.
Transwell migration and invasion assay
The migration and invasion assays were performed using the Transwell chamber (Corning, Michigan, USA). The transfected cells were seeded into the upper chambers (1 × 105 cell/well) that were incubated by Matrigel in 200 µl of serum free RPMI1640 medium, while the bottom of the chamber was incubated with 500 µl of medium containing 10% fetal bovine serum. After culture for 48 h, cells were fixed by formaldehyde, washed by PBS, and stained by GIMSA (Beyotime Biotechnology Company, Beijing, China). Finally, pictures of the cells were taken under a microscope with 5 random views. The number of invaded cells was counted. All experimental operations were incubated on ice.
Dual luciferase assay
The wild-type 3’UTR and the mutant 3’UTR of SOX6 were synthesized in vitro and were cloned into the downstream of pMIR-REPORT luciferase vector by Spe-I and HindIII enzyme. 293 T cells were co-transfected with miR-202 mimic and wild-type SOX6 3’UTR or the mutant 3’UTR. After transfection for 24 h, cells were lysed and luciferase intensity was measured by GloMax 20/20 luminometer (Promega, Wisconsin, USA). The intensity of Renilla was used as control, and all step followed by the protocol of the luciferase kit (Sigma, Saint Louis, USA). Cells without transfection were used as negative control (NC).
Statistical analysis
All the data were shown as the mean ± SD, and difference were determined by two-tailed Student’s t-test of SPSS. P < 0.05 was considered as statistically significant.
Results
MiR-202 is up-regulated in eutopic and ectopic endometrium tissues
To examine the roles of miR-202 in endometriosis progression, the expression level of miR-202 was detected by qRT-PCR. Compared with normal control, the expression of miR-202 was both increased significantly (P < 0.05), as shown in Figure 1A. And the expression of miR-202 was significantly lower in tissues of I/II stages than III/IV stages (Figure 1B). The results indicate that miR-202 expression is increased ineutopic and ectopic endometrium tissues.
Figure 1.
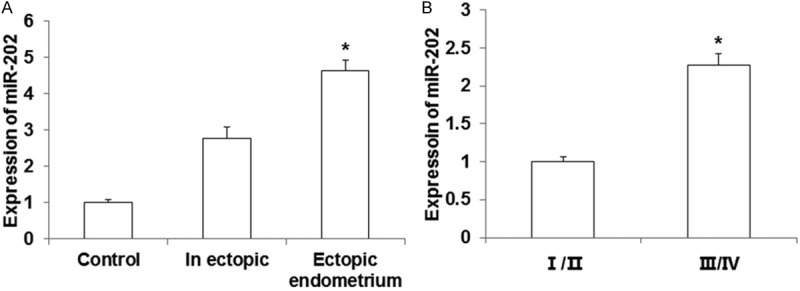
Expression of miR-202 in endometrial tissues. The expression of miR-202 was detected using qRT-PCR. A. miR-202 expression in eutopic, ectopic and normal endometrial tissues. Compared with endometrial tissues, *P < 0.05. B. miR-202 increased in eutopic endometrial tissues of III tissues of etri/II stages. Compared with I/II stages, *P < 0.05.
The expressions of SOX6, p21, cyclin D1 and p-Rb by IHC
To detect the expression of SOX6 and its associated down-stream proteins, IHC method was applied. As shown in Figure 2A, the positive rate of SOX6 decreased in ectopic and eutopic tissues compared with normal tissues, and the expression of SOX6 was mainly weak positive in ectopic and eutopic tissues. The positive rate of p21 protein in ectopic and eutopic tissues was lower than normal tissues (Figure 2B). The expressions of cyclin D1 (Figure 2C) and p-Rb (Figure 2D) were strong positive in ectopic and eutopic tissues although no significant different was found between the two groups.
Figure 2.
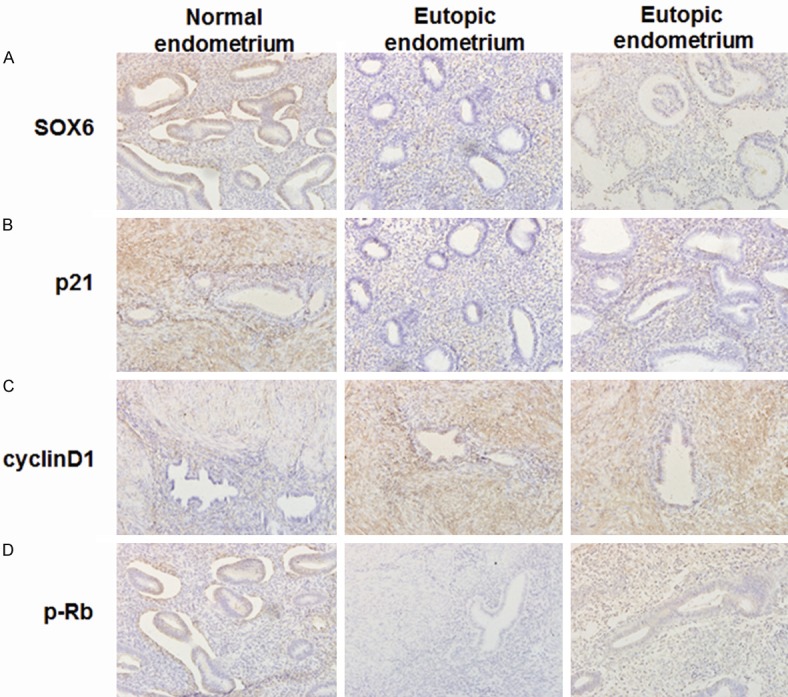
Immunohistochemistry to detect the expression of SOX6 and its regulated proteins (200 ×). A. The expression of SOX6 protein; B. The expression of p21 protein; C. The expression of cyclin D1 protein; D. The expression of p-Rb protein.
The expressions of SOX6, p21, cyclin D1 and p-Rb by Western-blot
To further validate the expression of SOX6 and associated downstream proteins, Western blot was applied in different endometrial tissues. The expression of SOX6 and p21 was obviously lower in ectopic and eutopic tissues than normal endometrium. On the contrary, the expression of cyclin D1 and p-Rb was increased significantly (Figure 3A). Similarly, SOX6 and p21 were decreased in ectopic tissues of III/IV stages than I/II stages, while cyclin D1 and p-Rb increased significantly in III/IV stages than I/II stages (Figure 3B). Combined with the IHC results, we can speculate that SOX6 and associated down-stream signals (p21, cyclin D1 and p-Rb) may participate in the progression of endometriosis.
Figure 3.
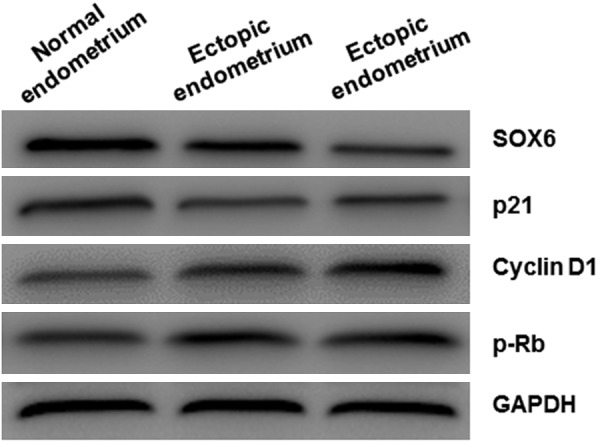
The expression of SOX6 and its regulated proteins in endometrial tissues. Western Blot analysis was performed to detect SOX6 and its regulated proteins expression.
MiR-202 promotes the proliferation and invasion of ESC
To investigate whether miR-202 is related to tumor proliferation and metastasis, we detected the effect of miR-202 on cell proliferation, migration and invasion. After transfection with antagomiR-202, the proliferation of ESC cells was inhibited significantly, as detected by MTT assay (P < 0.05) (Figure 4A), which indicates that highly expressed miR-202 can promote the proliferation of ESC cells. From Transwell migration and invasion analysis, we found that antagomiR-202 transfection significantly reduced the migration and invasion of ESC cells (P < 0.05) (Figure 4B and 4C). Overall, these results suggest that miR-202 may promote the proliferation and invasion of ESC cells.
Figure 4.
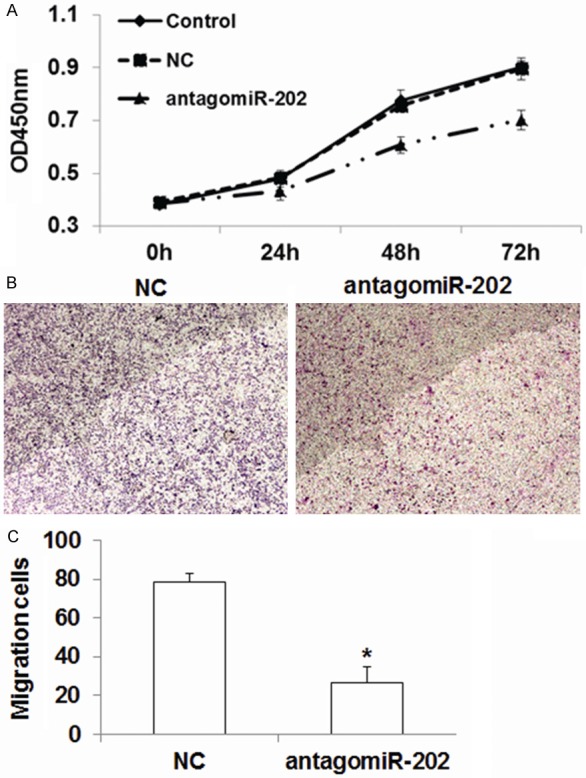
MiR-202 promotes the proliferation and invasive behaviors of ESC cells. A. The proliferation of ESC cells was detected with MTT assay; Compared with NC, *P < 0.05. B. Microscope to detect the effect of antagomiR-202 on the invasion of ESC cells. C. Statistical analysis of the effect of antagomiR-202 on the invasion of ESC cells. Compared with NC, *P < 0.05.
SOX6 is a direct target gene of miR-202
To identify the target genes of miR-202, we predicted candidate targets of miR-202 by using bioinformatics method. After bioinformatic prediction, we selected SOX6 as our candidate target for further study. We detected the GFP intensity by dual luciferase assay to validate whether miR-202 can directly target SOX6. As shown in Figure 5A, the wild type and mutant binding sites of miR-202 on SOX6 3’UTR were cloned. ESC cells were co-transfected with miR-202 mimic and pMIR-REPORT vectors with wild-type or mutant SOX6 3’UTR, respectively. We found that miR-202 reduced the GFP intensity of wild type SOX6 3’UTR (Figure 5B). The results indicated that SOX6 can be directly regulated by miR-202.
Figure 5.
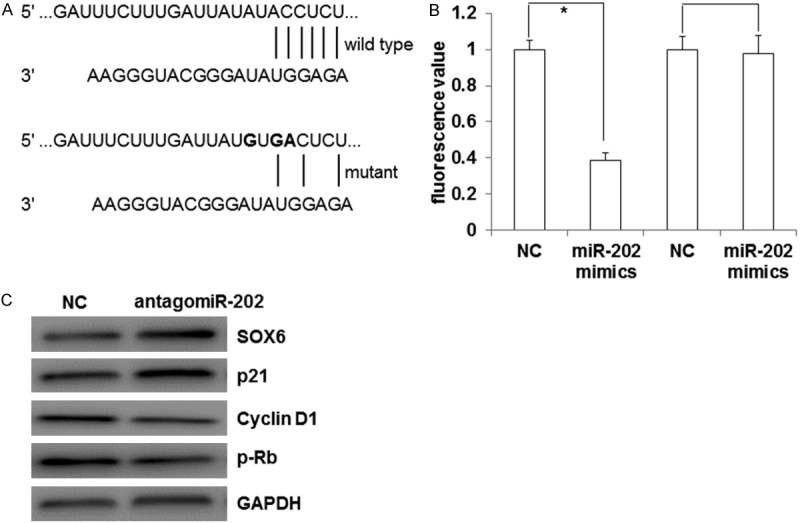
SOX6 is a direct target gene of miR-202. A. The wild type and mutant binding sites of miR-202 on SOX6 3’UTR. B. Dual luciferase assay was used to detect that whether SOX6 is regulated by miR-202. Compared with NC, *P < 0.05. C. Western Blot analysis was performed to detect SOX6 and its regulated proteins’ expression after transfection of antagomiR-202.
In order to further explore whether miR-202 participates in the proliferation and invasion of ESC through targeting SOX6 and its regulated down-stream proteins, we detected the expression of SOX6, p21, cyclin D1 and p-Rb after transfection of antagomiR-202 in ESC cells. As shown in Figure 5C, the ESC cells with transfected antagomiR-202 increased expression of SOX6 and p21, while decreased the expression of cyclin D1 and p-Rb. The results indicated that miR-202 may participate in the proliferation and cell cycle of ESC cells through regulating SOX6 and its regulated down-stream proteins.
Discussion
Current studies reported that miRNA can play important roles in almost all pathophysiological processes, such as tumor cell proliferation, invasion and metastasis, hypertension, diabetes, atherosclerosis [19-22]. Although the functions of miRNA was understood deeply, the roles of miRNA in endometriosis is still unclear. Several miRNAs such as miR-451, miR-29c, miR-210, miR-143/145 and miR-20a were reported to be associated with the endometriosis [23-26]. Many miRNAs were found to promote tumor invasion and metastasis. Actually, the characters of invasion and metastasis also appear in the processes of endometriosis [27]. It is valuable to explore new endometriosis-related miRNA in clinical practice. Some research demonstrates that there is abnormal expression of miR-202 in endometriosis [15]. But the mechanisms of how miR-202 participated in the processes are unclear.
In this study, we found that the expression of miR-202 was higher in endometriosis tissues than in normal control. The expression of miR-202 was also higher in ectopic tissues than in eutopic tissues, and higher in III/IV stages than in I/II stages. Bioinformatics prediction indicated that SOX6 was a candidate target gene of miR-202. It is reported that the expression of SOX6 is highly related to the expression of p21, cyclin D1 and p-Rb proteins [16,28]. So we applied IHC and western blot to detect the expression of SOX6 and its down-stream proteins in endometrial tissues. The results demonstrated that the expression of SOX6 and p21 was lower in endometriosis tissues than in normal whereas expression of cyclin D1 and p-Rb was higher in endometriosis tissues. We hypothesize that miR-202 can impact the cell proliferation and other biological functions through targeting SOX6, p21, cyclin D1, and p-Rb. For validating this hypothesis, we transfected antagomiR-202 into cultured ESC cells. Through MTT and transwell assay, we explored the proliferation and invasion of ESC cells. The results showed that miR-202 promoted the proliferation and invasion in ESC cells. The expression of SOX6 and p21 was up-regulated and that of cyclin D1 and p-Rb was down-regulated after the transfection of antagomiR-202 in ESC cells. Dual luciferase assay showed that miR-202 could directly target SOX6 gene.
In summary, we showed that miR-202 played important roles in the progression of endometriosis. The high expression of miR-202 in endometriosis tissues promoted the cell proliferation and cell invasion through targeting a transcription factor, SOX6. The change of SOX6 stimulated downstream signaling proteins, such as p21, cyclin D1 and pRb. Our findings may have clinical value to identify miR-202 as the potential biomarker for the diagnosis and treatment of endometriosis.
Acknowledgements
We would like to thank Professor AipingBian’s great help. From the topic selection, experimental design, result integration, and manuscript preparation, Prof. Bian contributed great efforts.
Disclosure of conflict of interest
None.
References
- 1.Verit FF, Yucel O. Endometriosis, leiomyoma and adenomyosis: the risk of gynecologic malignancy. Asian Pac J Cancer Prev. 2013;14:5589–5597. doi: 10.7314/apjcp.2013.14.10.5589. [DOI] [PubMed] [Google Scholar]
- 2.Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L, Missmer SA. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21:500–516. doi: 10.1093/humupd/dmv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somigliana E, Benaglia L, Paffoni A, Busnelli A, Vigano P, Vercellini P. Risks of conservative management in women with ovarian endometriomas undergoing IVF. Hum Reprod Update. 2015;21:486–499. doi: 10.1093/humupd/dmv012. [DOI] [PubMed] [Google Scholar]
- 4.Kodaman PH. Current strategies for endometriosis management. Obstet Gynecol Clin North Am. 2015;42:87–101. doi: 10.1016/j.ogc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Grimstad FW, Carey E. Periclitoral endometriosis: the dilemma of a chronic disease invading a rare location. J Minim Invasive Gynecol. 2015;22:684–686. doi: 10.1016/j.jmig.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Tandrasasmita OM, Sutanto AM, Arifin PF, Tjandrawinata RR. Anti-inflammatory, antiangiogenic, and apoptosis-inducing activity of DLBS1442, a bioactive fraction of Phaleria macrocarpa, in a RL95-2 cell line as a molecular model of endometriosis. Int J Womens Health. 2015;7:161–169. doi: 10.2147/IJWH.S74552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H. Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation (Review) Mol Med Rep. 2014;9:9–15. doi: 10.3892/mmr.2013.1755. [DOI] [PubMed] [Google Scholar]
- 8.Abu Hashim H. Potential role of aromatase inhibitors in the treatment of endometriosis. Int J Womens Health. 2014;6:671–680. doi: 10.2147/IJWH.S34684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu L, Ma YY. Expression of focal adhesion kinase in endometrial stromal cells of women with endometriosis was adjusted by ovarian steroid hormones. Int J Clin Exp Pathol. 2015;8:1810–1815. [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Y, Qian WP, Zhang CH, Zhou L, Hou ZH. Study on microRNA expression in endometrium of luteal phase and its relationship with infertility of endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2013;48:907–910. [PubMed] [Google Scholar]
- 11.Dong M, Yang P, Hua F. MiR-191 modulates malignant transformation of endometriosis through regulating TIMP3. Med Sci Monit. 2015;21:915–920. doi: 10.12659/MSM.893872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastingschafer CS, Schafer SD, Kiesel L, Gotte M. miR-142-3p is a novel regulator of cell viability and proinflammatory signalling in endometrial stroma cells. Reprod Biomed Online. 2015;30:553–556. doi: 10.1016/j.rbmo.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Mari-Alexandre J, Garcia-Oms J, Barcelo-Molina M, Gilabert-Aguilar J, Estelles A, Braza-Boils A, Gilabert-Estelles J. MicroRNAs and angiogenesis in endometriosis. Thromb Res. 2015;135(Suppl 1):S38–40. doi: 10.1016/S0049-3848(15)50439-8. [DOI] [PubMed] [Google Scholar]
- 14.Braza-Boils A, Mari-Alexandre J, Gilabert J, Sanchez-Izquierdo D, Espana F, Estelles A, Gilabert-Estelles J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum Reprod. 2014;29:978–988. doi: 10.1093/humrep/deu019. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25:821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Zheng D, Zhang B, Liu L, Ou J, Chen W, Xiong S, Gu Y, Yang J. Mir-208 promotes cell proliferation by repressing SOX6 expression in human esophageal squamous cell carcinoma. J Transl Med. 2014;12:196. doi: 10.1186/1479-5876-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamson GD. Endometriosis Fertility Index: is it better than the present staging systems? Curr Opin Obstet Gynecol. 2013;25:186–192. doi: 10.1097/GCO.0b013e32836091da. [DOI] [PubMed] [Google Scholar]
- 18.Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30:1677–1689. doi: 10.1093/humrep/dev100. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Zhou J, Chen X, Yang B, Wang D, Yang P, He X, Li H. miRNA-449a is downregulated in osteosarcoma and promotes cell apoptosis by targeting BCL2. Tumour Biol. 2015;36:8221–9. doi: 10.1007/s13277-015-3568-y. [DOI] [PubMed] [Google Scholar]
- 20.Fang F, Chang RM, Yu L, Lei X, Xiao S, Yang H, Yang LY. MicroRNA-188-5p Suppresses Tumor Cell Proliferation and Metastasis by Directly Targeting FGF5 in Hepatocellular Carcinoma. J Hepatol. 2015;63:874–85. doi: 10.1016/j.jhep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Junker F, Chabloz A, Koch U, Radtke F. Dicer1 imparts essential survival cues in Notch driven T-ALL via miR-21 mediated tumor suppressor Pdcd4 repression. Blood. 2015;126:993–1004. doi: 10.1182/blood-2014-12-618892. [DOI] [PubMed] [Google Scholar]
- 22.Di Francesco A, Falconi A, Di Germanio C, Micioni Di Bonaventura MV, Costa A, Caramuta S, Del Carlo M, Compagnone D, Dainese E, Cifani C, Maccarrone M, D’Addario C. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J Nutr Biochem. 2015;26:250–258. doi: 10.1016/j.jnutbio.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Graham A, Falcone T, Nothnick WB. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum Reprod. 2015;30:642–652. doi: 10.1093/humrep/dev005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long M, Wan X, La X, Gong X, Cai X. miR-29c is downregulated in the ectopic endometrium and exerts its effects on endometrial cell proliferation, apoptosis and invasion by targeting c-Jun. Int J Mol Med. 2015;35:1119–1125. doi: 10.3892/ijmm.2015.2082. [DOI] [PubMed] [Google Scholar]
- 25.Zheng B, Xue X, Zhao Y, Chen J, Xu CY, Duan P. The differential expression of microRNA-143,145 in endometriosis. Iran J Reprod Med. 2014;12:555–560. [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto M, Nasu K, Abe W, Aoyagi Y, Kawano Y, Kai K, Moriyama M, Narahara H. Enhanced miR-210 expression promotes the pathogenesis of endometriosis through activation of signal transducer and activator of transcription 3. Hum Reprod. 2015;30:632–641. doi: 10.1093/humrep/deu332. [DOI] [PubMed] [Google Scholar]
- 27.Fettback PB, Pereira RM, Domingues TS, Zacharias KG, Chamie LP, Serafini PC. Uterine rupture before the onset of labor following extensive resection of deeply infiltrating endometriosis with myometrial invasion. Int J Gynaecol Obstet. 2015;129:268–270. doi: 10.1016/j.ijgo.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Qin YR, Tang H, Xie F, Liu H, Zhu Y, Ai J, Chen L, Li Y, Kwong DL, Fu L, Guan XY. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma. Clin Cancer Res. 2011;17:46–55. doi: 10.1158/1078-0432.CCR-10-1155. [DOI] [PubMed] [Google Scholar]


