Abstract
Objective: We observed the effects of Masson pine pollen aqueous extracts (MPPAE) on CCl4-induced oxidative damage of the human hepatic cell line L-02. Methods: We created an in vitro model of oxidative liver damage by treating L-02 human hepatic cells with 40 mmol/L CCl4. Effects of different concentrations of MPPAE on cell proliferation, morphology, and change of functional indexes were observed after addition of CCl4. Results: CCl4 was toxic to proliferation, cell morphology, and functionality of hepatic cells. It decreased proliferation by 29.3-38.4% and increased AST and ALT activities by 22.3% and 99.2%, respectively. The oxidative stress also disrupted hepatic cell growth and induced pyknosis. Although MPPAE did not prevent decreased proliferation of L-02 cells, the treatment alleviated some CCl4-induced cell morphology changes and inhibited the abnormal rise of ALT (39.8%-70.1%) and AST (14.75-27.25%) activities in a dose dependent manner. A high dose of MPPAE (400 mg/L) ameliorated nucleus deformation to an almost normal appearance. Conclusions: According to our in vitro model, MPPAE specifically prevented the changes in cell morphology and functional injury caused by CCL4 treatment; however, it offered limited protection against damage-induced reduction of proliferation.
Keywords: Aqueous extract, masson pine pollen, hepatic cells, oxidative damage, CCl4
Introduction
Liver disease is a major risk to human life and health. In addition to cancer and cirrhosis, chronic liver diseases are extremely harmful. If left untreated, these chronic diseases can develop into liver fibrosis, cirrhosis, and potentially cancer. In fact, liver cirrhosis and the corresponding complications have become main contributors to global mortality. Prevention of chronic liver diseases is a hot topic of current research [1].
Masson pine pollen that is derived from plants is rich in nutrients. It provides health benefits such as bolstering immunity, being anti-aging as well as reducing fatigue, regulating metabolism, lowering blood pressure and blood glucose levels and protecting the liver. The pollen is a highly efficient and versatile nutritional product, and it enjoys the reputation of providing “complete nutrition and as a natural health food” [2]. Recent studies have elucidated the protective effects of Masson pine pollen on the liver [3,4]. Pine pollen demonstrated a protective effect on acute alcoholic liver injury by accelerating liver function recovery, and inducing ascites absorption [4]. Experiments indicate that 70% of Masson pine pollen is composed of lignin, which is not absorbed by the human body. Purification improves the functionality of Masson pine pollen, and extracts can be used during and after the treatment of liver diseases.
Three approaches are commonly used to treat liver fibrosis: (1) inhibiting or reducing the risk factors causing liver cell injury; (2) inhibiting immune cells or stellate cells by regulating the expression of cytokines and associated signaling proteins and (3) suppressing or enhancing degradation of collagen [5,6].
Based on these three treatments we first selected normal human liver cell line L-02 for the study. We observed MPPAE’s impact on oxidative damage of human liver cells using morphological and immunohistochemical methods.
Materials and methods
Freeze-dried Masson pine pollen aqueous extract powder (MPPAE)
Natural Masson pine pollen was collected and broken up using low-temperature, low-speed jet milling for 20 min. Then, it was soaked in water at room temperature and normal pressure for 30 min. The mixture was ultrasonically extracted for 30 min, cold centrifuged at 10000-16000 rpm for 15 min, and the supernatant was collected. The water-soluble matter with a molecular weight less than 3 kD was separated by molecular sieve ultrafiltration at 1-5°C. The material was freeze-dried to give a lyophilized powder and stored at -20°C. For experiments, 0.1 g lyophilized powder was diluted with purified water to 100 ml (1.0 mg/mL).
Normal human liver cell line L-02
Purchased from Shanghai-Cheung Biological Technology Co., Ltd.
Equipment
Hitachi 7600 automatic biochemical analyzer (Hitachi, Japan), Nikon Ti-Si inverted microscope (Nikon, Japan), Sn-69513 Immune Counter (Shanghai Nuclear Institute Rihuan Photoelectric Instrument Co. Ltd.), 5810R/5415D centrifuge (Eppendorf, Germany), Sartorius electronic balance (Goettingen Sartorius Ltd., Germany), LabSystems Clean Bench (Thermo Fisher Scientific, USA), LDZ5-2 automatically balanced centrifuge (Beijing Medical Centrifuge Factory), Ultra-low temperature freezer (SANYO, Japan) and Muse cytometer (Merck, USA).
Reagents and material
AR carbon tetrachloride (CCl4) (Beijing Chemical Reagent Company), DMEM high glucose medium (Gibco, USA), 0.25% trypsin (Gibco, USA), PBS buffer, Hematoxylin and eosin dye, Dimethyl sulfoxide (DMSO) and WST-1 cell proliferation and cytotoxicity assay kit (Beyotime Biotechnology Co. Ltd.). Penicillin-streptomycin solution (Gibco, USA), Immunohistochemical goat anti-rabbit monoclonal antibodies (Biotechnology Co., Ltd. and Tianjin Sungene Biotech Co., Ltd.), Superoxide dismutase (SOD) activity detection kit (Nanjing Jiancheng Bioengineering Institute), Malondialdehyde (MDA) content detection kit (Nanjing Jiancheng Bioengineering Institute), 20% CCl4- ethanol solution-prepared as follows: Ethanol is required as a solvent to dissolve CCl4 in water. 20 ml of AR CCl4 was dissolved in 80 ml anhydrous ethanol and filtered using a 0.22 μm sterile filter (4 mol/L).
Preparation of CCl4 intervention solution
Under sterile conditions, 2 ml of 20% CCl4 ethanol solution was added to 48 ml serum-free DMEM high glucose culture medium. The final concentration was 80 mmol/L CCl4, and it was stored at 4°C.
Preparation of MPPAE application solution
MPPAE lyophilized powder (100 mg) was added to 10 ml PBS (final concentration: 10 mg/ml or 10,000 mg/L). It was filtered under sterile conditions and stored at 4°C. For experiments, it was diluted with serum-free DMEM to the desired concentration.
Experimental methods
WST-1 cell proliferation assay
L-02 cells were maintained in high glucose DMEM medium containing 10% fetal bovine serum and 2% antibiotic solution and incubated at 37°C with 5% CO2 using a LabSystems series thermostat incubator (Thermo Fisher Scientific, USA). When cells reached the logarithmic growth phase, they were detached from the culture vessel with 0.25% trypsin. Upon neutralizing the trypsin, the supernatant was centrifuged and the cells were diluted and counted. Cells were seeded into 96-well plates as follows. The outermost wells were kept as moisturizing wells and contained only PBS. 100 μL of a 2-5 × 104 cells/mL suspension was added to each of the remaining 60 wells (approximately 2-5 × 103 cells/well), and the cells were incubated under for 12 hours. To create working stocks of CCL4 intervention solution at various concentrations, different volumes of CCL4 intervention solution were mixed with serum-free DMEM so that the final volume of each was the same. Each concentration of CCL4 intervention solution + DMEM mixture was added to 6 wells and serum-free DMEM alone was used as a negative control. In experiments with MPPAE, solutions of DMEM with varying concentrations of MPPAE were added at the same time as CCL4 intervention solution (final concentration of CCL4 was 40 mmol/L). Cell proliferation was measured at 24 and 48 h after addition of CCL4 or CCL4 plus MPPAE using the WST-1 kit. 10 μL of prepared WST-1 solution was added to each well, and then the plate was incubated in the dark at 37°C for 3 h. Micro-oscillator vibration was applied for 10 s, and the absorbance was measured with a Multiskan MK3 microplate reader (Thermo Fisher Scientific, USA) at 450 nm.
Cell function and antioxidant activity assays
L-02 cells were seeded into a 24-well plate and incubated for 12 h. There were 4 replicate wells for each condition tested. In control wells, the solution was changed to 1 ml serum-free DMEM. In the test groups, 0.5 ml serum-free DMEM medium containing 80 mmol/L CCl4 (CCl4 final concentration 40 mmol/L) was first added to each well, then 0.5 ml of 0, 100, 200, 400 or 800 mg/L MPPAE in serum-free DMEM medium was added (MPPAE final concentration of 0, 50, 100, 200 and 400 mg/L). The solutions were mixed by pipetting and incubated at 37°C for 24 or 48 hours. Cell supernatants were collected, and the alanine aminotransferase (ALT) and aspartic aminotransferase (AST) activities were measured. Superoxide dismutase (SOD) activity was measured using xanthine oxidase, and malondialdehyde (MDA) content was determined by thiobarbituric acid (TBA) colorimetric assay.
Morphology assay
L-02 cells were seeded in an 8-well plate and incubated for 12 h. They were divided into four groups with each containing two duplicate wells. Control wells contained 2 ml serum-free DMEM. In the test groups, 1 ml serum-free DMEM medium containing 80 mmol/L CCl4 (CCl4 final concentration 40 mmol/L) and 1 ml of 0, 200, or 800 mg/L MPPAE in serum-free DMEM medium were added to each well (MPPAE final concentration of 0, 100 or 400 mg/L). After mixing and incubation at 37°C for 24 h, the supernatant was discarded. The remaining residue was washed with PBS and fixed with cold 95% ethanol for 30 min. After one more wash, hematoxylin-eosin staining was performed and morphological changes were observed under a microscope.
Statistical methods
Experimental data were expressed as mean ± standard deviation (m ± SD). SPSS 11.0 software was used for statistical analysis. We first tested the data for normality and homogeneity of variance. Normally distributed data with homogenous variance were analyzed by multi-factor analysis of variance (ANOVA), while the non-normal data were analyzed using Kruskal-Wallis rank-sum test with P<0.05 considered statistically significant.
Results and discussion
Establishing a model of oxidative injury in L-02 cells
Lower concentrations of CCl4 had a mild stimulatory effect on the proliferation of L-02 liver cells, but CCL4 became toxic at concentrations greater than 20 mmol/L (Table 1; Figure 1). Cytotoxicity peaked at 70-80 mmol/L of CCL4. This trend was consistent between cells treated for 24 or 48 hours. The larger absorbance for the 48-hour dataset was due to the overall presence of more number of cells. The effective concentration range of CCl4 to inhibit proliferation of cells without becoming too toxic was between 40-60 mmol/L. To prevent irreversible cytotoxic damage, we decided to use 40 mmol/L of CCl4 for 24 hours as the condition for further experiments.
Table 1.
The impact of different concentrations of CCl4 on L-02 cell proliferation
| Ethanol-CCl4 final concentration (mmol/L) | n | 24 h OD value | 48 h OD value |
|---|---|---|---|
| 0 | 6 | 0.571±0.080 | 0.770±0.168 |
| 10 | 6 | 0.658±0.052 | 0.838±0.064 |
| 20 | 6 | 0.611±0.056 | 0.846±0.039 |
| 30 | 6 | 0.563±0.040 | 0.787±0.066 |
| 40 | 6 | 0.446±0.058 | 0.681±0.087 |
| 50 | 6 | 0.394±0.064 | 0.546±0.100 |
| 60 | 6 | 0.316±0.086 | 0.354±0.124 |
| 70 | 6 | 0.255±0.006 | 0.268±0.018 |
| 80 | 6 | 0.233±0.012 | 0.268±0.019 |
| 100 | 6 | 0.227±0.020 | 0.271±0.024 |
Figure 1.
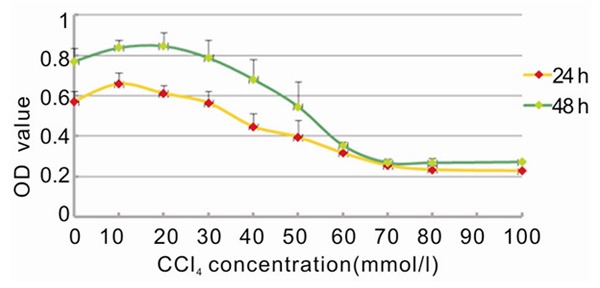
Graph showing the effect of CCl4 concentration on L-02 proliferation at different action period.
MPPAE imparts modest protection against CCL4-induced proliferation reduction of L-02 cells
Low concentrations of MPPAE (20-60 mg/L) restored proliferation of CCL4-damaged L-02 cells (Table 2; Figure 2). Interestingly, higher concentrations of MPPAE did not rescue proliferation. If damaged cells were incubated with low concentrations of MPPAE for a longer period of time (48 hours), the protective effect was enhanced (Table 2; Figure 2). Therefore, the protective effect on liver cell proliferation was not significant.
Table 2.
The impact of different concentrations of MPPAE on CCl4 damaged L-02 cell proliferation
| CCl4 final concentration (mmol/L) | MPPAE final concentration (mg/L) | 24 h OD value | 48 h OD value |
|---|---|---|---|
| 0 | 0 | 0.470±0.038 | 0.78±0.14 |
| 40 | 0 | 0.332±0.008 | 0.48±0.04 |
| 40 | 20 | 0.442±0.076 | 0.66±0.06 |
| 40 | 40 | 0.419±0.025 | 0.64±0.14 |
| 40 | 60 | 0.387±0.023 | 0.62±0.07 |
| 40 | 80 | 0.343±0.016 | 0.48±0.04 |
| 40 | 100 | 0.348±0.016 | 0.49±0.05 |
| 40 | 120 | 0.338±0.024 | 0.50±0.08 |
| 40 | 140 | 0.359±0.013 | 0.53±0.10 |
| 40 | 160 | 0.352±0.025 | 0.57±0.08 |
Figure 2.
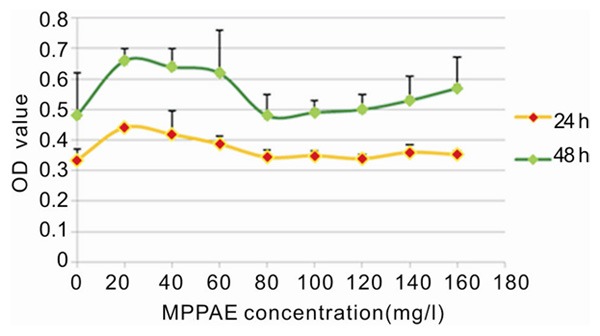
Graph demonstrating the effect of MPPAE on CCl4 damaged L-02 cell proliferation over time.
Influence of MPPAE on damaged liver cell function
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are common enzymes found in liver cells. When the cell membrane is damaged or cells are dying, these enzymes are released into the supernatant. The detection of ALT and AST activity in the supernatant is a sensitive measure of the degree of liver cell damage. ALT levels are very sensitive to a variety of acute viral hepatitis, drug or alcohol-induced acute liver cell damage and is the main sign of liver cell damage. The level of change often correlates with the severity of disease. In patients with chronic hepatitis and cirrhosis, AST levels increase more than ALT, and, as a result, AST is the primary indicator of the degree of liver damage [7].
After CCl4 damage to liver cells, ALT and AST levels both increased in L-02 cells (Table 3 and Figure 3, compare control to model). MPPAE intervention significantly inhibited elevation of both the transaminase enzymes (Table 3 and Figure 3, compared to model group). The rate of inhibition increased modestly with increasing concentration of MPPAE, but the difference between the groups was not statistically significant (P>0.05) because the standard deviation was high (Table 3).
Table 3.
The impact of different concentrations of MPPAE on the transaminase activity of L-02 cell
| Group | N | CCl4 final Concentration (mmol/L) | MPPAE final Concentration (mg/L) | ALT (U/g Pr) | AST (U/g Pr) |
|---|---|---|---|---|---|
|
| |||||
| Group | n | CCl4 (mmol/L) | MPPAE (mg/L) | ALT (U/g Pr) | AST (U/g Pr) |
| Control | 4 | 0 | 0 | 1.25±0.35 | 11.25±0.35 |
| CCl4 Model | 4 | 40 | 0 | 2.50±0.71 | 13.75±7.42 |
| MPPAE Group I | 4 | 40 | 50 | 1.50±0.00 | 11.25±1.77 |
| Group II | 4 | 40 | 100 | 1.50±0.71 | 10.75±1.77 |
| Group III | 4 | 40 | 200 | 1.00±0.00 | 10.00±0.71 |
| Group IV | 4 | 40 | 400 | 0.75±0.35 | 11.75±0.35 |
Figure 3.
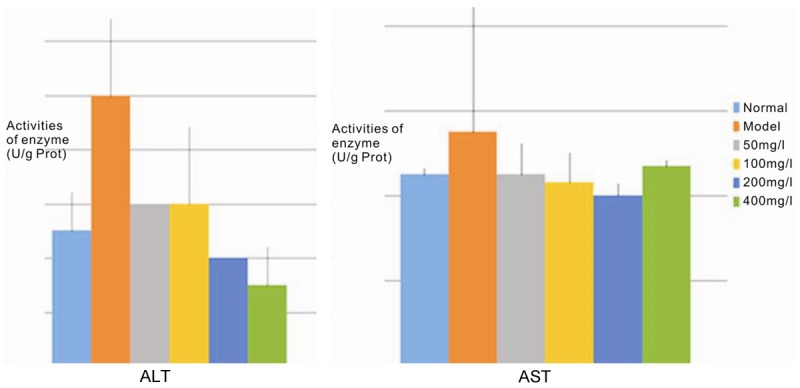
Histogram showing the aminotransferase activities of L-02 cell supernatants.
MPPAE’s influence on the antioxidant activity of liver cells
CCl4 induces liver injury primarily by inducing covalently bound lipid peroxidation and intracellular calcium homeostasis. CCl4 generates oxygen radicals when metabolized by the liver; secondary reactions of the radicals directly damage liver cells and leads to cell degeneration and fat necrosis [8]. Following CCl4 damage of L-02 cells, the supernatants showed a significant increase in SOD activity (3.9 times) and lipid peroxidation (6.5 times) (P<0.01; Table 4 and Figure 4, compared to control). MPPAE intervention significantly lowered the activity of SOD and content of MDA. MDA decreased by 34.8-87.5% and SOD activity decreased by as much as 68.4% (P<0.01), and both occurred in a dose-dependent manner (Table 4 and Figure 4, compared to model group). Importantly, the highest concentration of MPPAE restored SOD and MDA activities to normal levels.
Table 4.
Effect of different concentrations of MPPAE’s on SOD activity and MDA content in L-02 cells
| Group | n | CCl4 (mmol/L) | MPPAE (mg/L) | SOD (U/mg prot) | MDA (nmol/mg prot) |
|---|---|---|---|---|---|
| Control | 4 | 0 | 0 | 38.61±0.19a | 218.31±6.52a |
| CCl4 Model | 4 | 40 | 0 | 150.46±1.75d | 1422.21±139.8c |
| MPPAE Group I | 4 | 40 | 50 | 147.26±3.49d | 927.96±93.2b |
| Group II | 4 | 40 | 100 | 82.74±0.40c | 395.76±52.9a |
| Group III | 4 | 40 | 200 | 51.88±0.00b | 336.63±31.57a |
| Group IV | 4 | 40 | 400 | 47.58±2.25b | 177.38±7.52a |
Note: Different letters at the upper right corners indicated the difference between groups was statistically significant (P<0.05).
Figure 4.
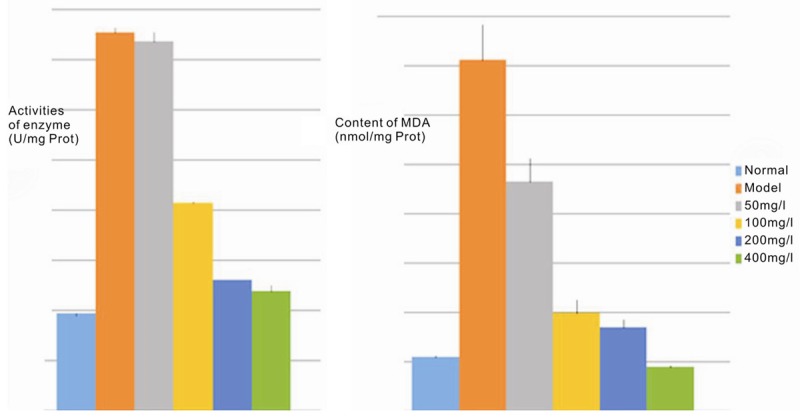
Histogram demonstrating the SOD and MDA levels in L-02 cell supernatants.
SOD is the primary substance in cells that eliminates free radicals. It can specifically block the damage of superoxide anion radicals and it can repair damaged cells. SOD activity is an important indicator of the antioxidant capacity of the human body [9]. The higher the enzyme activity, the stronger the antioxidant capacity. However, the results after CCl4 damage and MPPAE intervention were opposite to what we expected. This may be due to the fact that oxidative damage triggered a series of self-protection mechanisms that transiently enhanced the activity of SOD. When free radicals surge in the body an oxidative stress response occurs that affects regulation and response mechanisms, such as protein expression. With the induction of radicals and the body’s compensatory stress response, enhanced antioxidant capacity occurs and there is a transient increase in SOD level. However, with rapid consumption of SOD by large quantities of free radicals and the body’s decompensation, its biosynthetic capacity quickly drops to a low level forming a new dynamic equilibrium with a higher concentration of free radicals [8].
MDA is a membrane lipid peroxidation end product and its content reflects the level of oxygen free radicals and lipid peroxidation in cells [4]. Thus, SOD and MDA levels indicate the degree of cellular oxidative stress injury. Our results demonstrated that there was clearly a positive impact of MPPAE treatment on MDA elimination. MPPAE significantly reduced the MDA content (P<0.01; Table 4 and it is rich in polyphenols that remove free radicals, inhibit lipid peroxidation, integrate metal ions, and activate intracellular antioxidant defense systems [4,10,11]. Vitamin E is another well-known antioxidant found in MPPAE but the extraction process removes most of the vitamin E [12]. Thus, most of MPPAE’s antioxidant capacity is most likely from the polyphenols.
MPPAE’s effect on L-02 cell morphology
H&E staining showed normal liver cells with clear nuclear structure and liver cell cord. Cell growth was well organized with full cytoplasm. Following CCl4 injury, the nucleus and cytoplasm of L-02 cells shrunk and cell growth was in disorder (Figure 5, compare panels A and B). Cells looked irregular and nuclei were deeply stained. Following MPPAE intervention, the number of pyknotic nuclei was reduced (Figure 5, compare panel B with C and D), and with high concentrations of MPPAE, cell morphology was close to normal (Figure 5, compare panel A and D).
Figure 5.
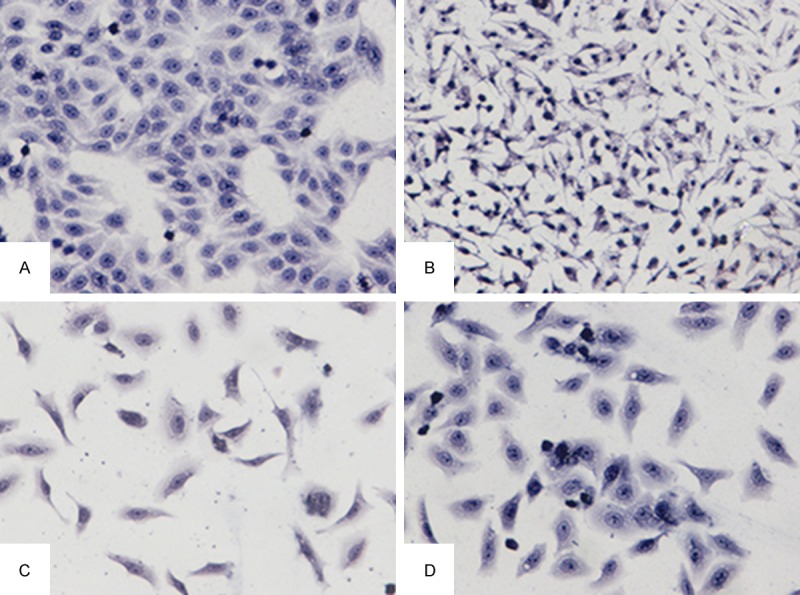
Light microscopy images showing L-02 hepatocyte morphology (200X. (A: Normal control group; B: Model Group; C: MPPAE 100 mg/L; D: MPPAE 400 mg/L).
Conclusions
In our in vitro model of hepatocyte oxidative damage, MPPAE did not protect the cells from decreased proliferation. However, it significantly improved hepatocyte morphology and inhibited the elevation of transaminase activities in a dose-dependent manner. Treatment of L-02 cells with CCl4 resulted in a significant increase in both SOD activity and MDA content due to oxidative stress and acute injury-induced SOD compensatory increase. MPPAE effectively suppressed the abnormal increase of SOD and MDA caused by CCl4 in a dose-dependent manner. The protective effects of MPPAE may be related to its polyphenolic compounds. In conclusion, MPPAE is an ideal component to inhibit fibrosis. Further studies will probe the impact of MPPAE on hepatic stellate cell morphology, function, and apoptosis.
Disclosure of conflict of interest
None.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L, Windisch W, Kirchgessner M. A study on the nutritive value of pollen from the Chinese Masson Pine (Pinus massoniana) and its effect on fecal characteristics in rats. Z Ernahrungswiss. 1996;35:341–347. doi: 10.1007/BF01610552. [DOI] [PubMed] [Google Scholar]
- 3.Tohamy AA, Abdella EM, Ahmed RR, Ahmed YK. Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts. Cytotechnology. 2014;66:283–297. doi: 10.1007/s10616-013-9568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KH, Kim AJ, Choi EM. Antioxidant and antiinflammatory activity of pine pollen extract in vitro. Phytother Res. 2009;23:41–48. doi: 10.1002/ptr.2525. [DOI] [PubMed] [Google Scholar]
- 5.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512–8. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar-Montes AM, Hernández-Ortega LD, Lucano-Landeros MS, Armendariz-Borunda J. New gene therapy strategies for hepatic fibrosis. World J Gastroenterol. 2015;21:3813–3825. doi: 10.3748/wjg.v21.i13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astegiano M, Sapone N, Demarchi B, Rossetti S, Bonardi R, Rizzetto M. Laboratory evaluation of the patient with liver disease. Eur Rev Med Pharmacol Sci. 2004;8:3–9. [PubMed] [Google Scholar]
- 8.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 9.Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Commons below Biological and physiological role of ROS-the good, the bad and the ugly. Acta Physiol (Oxf) 2015;214:329–48. doi: 10.1111/apha.12515. [DOI] [PubMed] [Google Scholar]
- 10.Lee KH, Kim AJ, Choi EM. Antioxidant and Antiinflammatory Activity of Pine Pollen Extract in Vitro. Phytother Res. 2009;23:41–48. doi: 10.1002/ptr.2525. [DOI] [PubMed] [Google Scholar]
- 11.Chu HL, Mao H, Feng W, Liu JW, Geng Y. Effects of sulfated polysaccharide from Masson pine (Pinus massoniana) pollen on the proliferation and cell cycle of HepG2 cells. Int J Biol Macromol. 2013;55:104–108. doi: 10.1016/j.ijbiomac.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Babitha S, Banji D, Banji OJ. Antioxidant and hepatoprotective effects of flower extract of Millingtonia hortensis Linn on carbon tetrachloride induced hepatotoxicity. J Pharm Bioallied Sci. 2012;4:307–312. doi: 10.4103/0975-7406.103258. [DOI] [PMC free article] [PubMed] [Google Scholar]


