Abstract
Background: Gallbladder cancer, with high aggressivity and extremely poor prognosis, is the most common malignancy of the bile duct. Thus, seeking targets gallbladder tumor cells is an attractive goal towards improving clinical treatment. Material and methods: In this study, we investigated the effects of pachymic acid (PA) on the tumorigenesis of human gallbladder cancer cells. Results: We found that PA significantly reduced cell growth in a dose- and time-dependent fashion. Meanwhile, cell cycle arrest at G0 phase was induced by PA. PA also significantly inhibited cancer cell migration, invasion in a dose-dependent manner. Interestingly, we demonstrated that cancer cell adhesion ability was suppressed dose-dependently, which may contribute to the inhibition of cell invasion. Finally, we showed that PA inhibited AKT and ERK signaling pathways. And oncoproteins, such as PCNA, ICAM-1 and RhoA which are involved intumorigenesis, were also downregulated by PA. Conclusion: Our study reveals that PA is able to inhibit gallbladder cancer tumorigenesis involving affection of AKT and ERK signaling pathways. Together, these results encourage further studies of PA as a promising candidate for gallbladder cancer therapy.
Keywords: Pachymic acid, growth, migration, invasion, adhesion, gallbladder cancer
Introduction
Gallbladder cancer, a very rare, highly-lethal disease, is the most common malignant tumor of the extrahepatic biliary tract and the seventh common gastrointestinal carcinoma [1]. Gallbladder cancer is highly invasive and aggressive and carries a dismal prognosis. The 5-year survival rate for all stages of gallbladder cancer is approximately 5% [2,3]. Most patients will die by one year following the surgery. Although gallbladder cancer is an uncommon disease, there is a very high incidence of gallbladder cancer in some regions, such as Chile, where gallbladder cancer has the highest mortality rate [4]. The incidence of gall bladder cancer is increasing in China as well as north central India [5]. Thus, it is important to study gallbladder cancer in relation to human health. In the absence of a cure, the search for new effectivechemopreventive and/or chemotherapeutic agentsagainst gallbladder cancer becomes exceedingly vital.
Natural products are one of the main sources for discovery of new drug compounds. Due to their diverse biological effects, cyclic triterpenoids such as ursolic acid and tubeimoside have attracted much attention in the field of cancer research [6,7]. Pachymic acid (PA) is a lanostrane-type triterpenoid from P. cocos, which is also called Fuling in Chinese medicine [8]. In addition to P. cocos, pachymic acid has also been reported to be isolated from the European fungus Fomitopsispinicola [9]. Meanwhile, PA alsopossesses anti-emetic, anti-inflammatory, and anti-cancer properties, PA is able to inhibit PMA-induced mouseskin tumor formation in mouse following initiation with 7, 12dimethylbenz [a] anthracene (DMBA), and Epstein-Barr virus early antigenactivation in Raji cells [10-14]. These findings suggest that PA serves as a potential anti-cancer agent.
Recently, many studies showed that PA is able to inhibit cancer growth, invasion or metastasis in different cancers, including lung, breast and pancreatic cancer [15-18]. However, there hasn’t been any research on effect of PA on tumorigenesis in gallbladder cancer. Therefore, we pay more attention to the effects of PA on gallbladder carcinogenesis. The objectives of this study were to assess the effects of PA on cell growth, migration and invasion using human gallbladder carcinoma cells. Our data suggest that PA is able to effectively inhibit gallbladder cancer development. These results provide compelling evidence that PA may be useful as anti-cancer agents for treatment and/or prevention of gallbladder cancer.
Materials and methods
Cell culture and treatment
The human gallbladder cell line, GBC-SD, was propagated at 37°C in a 5% CO2 humidifiedincubator in DMEMmedium containing 10% fetal bovine serum (FBS; HycloneLaboratories, Logan, UT), 10 mM HEPES (AppliChem, Darmstadt, Germany), and antibiotics (100 U/mL penicillin Gand 100 mg/mL of streptomycin; Invitrogen, Carlsbad, CA). PA was reconstituted in DMSO at a stock concentration of 20 mM and subsequently diluted tothe working concentrations for experimental procedures. Control treatments contained an equivalent concentration of DMSO (0.1%) for all experiments.
Cell viability assay
The antiproliferative effect of PA on GBC-SD cells was evaluated using a cell counting kit-8 (CCK-8, DojindoLaboratories, Kumamoto, Japan) following manufacturer’s protocol. Briefly, after indicated treatment, 10 µl of CCK-8 solution was added into each well, and following one hincubation, absorbance was measured at 450 nm using a microplate reader.
Cell cycle assay
After treatment, cells were trypsinized, harvested, and fixed in 1 ml 80% cold ethanol in test tubes and incubated at 4°C for 15 min. After incubation, cells were centrifuged at 1,500 rpm for 5 min and the cell pellets were resuspended in 500 μl propidium iodine (10 μg/ml) containing 300 μg/ml RNase (Sigma, MO, USA). Then cells were incubated on ice for 30 min and filtered with 53 μm nylon mesh. Cell cycle distribution was calculated from 10,000 cells with ModFit LTTM software (Becton Dickinson, CA, USA) using FACS caliber (Becton Dickinson, CA, USA).
Western blot analysis
Proteins were prepared with RIPA buffer. Each sample (30 μg) was subjected to SDS-polyacrylamide (10%) gel electrophoresis and electro-transferred onto a PVDF membrane. After blocked, the membranes were incubated with appropriate primary antibodies in blocking buffer overnight at 4°C. Then the membranes were incubated with secondary antibody. At last, the membranes were visualized with ECL Plus reagent (GE Healthcare) and developed onto X-ray film (Kodak). The antibodies used as follow: total AKT (1:1000), pAKT (1:1000), total ERK1/2 (1:1000), pERK1/2 (1:1000) and GAPDH (1:1500) are from Cell Signaling Technology (USA), PCNA (1:10000), ICAM-1 (1:200) and RhoA (1:1000) were from Abcam (USA).
Migration and invasion assay
Equal numbers of cells (1 × 105) in 0.5 ml DMEM complemented with 1% FBS were added to the upper compartment of the chamber and kept at 37°C for 16 hours. As a chemoattractant, the lower compartment contained DMEM supplemented with 10% FBS. At the end of the incubation period, cells from the upper surface of the filter were wiped off with a cotton swab. The lower surface of the filter was stained with crystalviolet. The number of cells having migrated to the bottom of the chamber was counted in the light microscope on ten randomly selected fields. The mean number of cells was calculated per field. Three sets of experiments were carried out, each in triplicate. For the matrigel assay, the filter was replaced by a BD BioCoatMatrigel invasion chamber (BD Bioscience).
Cell adhesion assay
12-well plates were coated with fibronectin overnight at 4°C. Wells were rinsed with 1X PBS the following day, preheated to 37°C, for surface neutralization. Remaining binding sites were blocked with 0.1% bovine serum albumin (BSA) in PBS for a period of 1 hr. Cells were plated with GBC-SD cells at 1 × 105 per well. After incubation for 60 minutes, cells were treated with percoll flotation medium and percoll fixative for 15 minutes, washed with PBS and treated with 0.5% crystal violet staining, washed again and allowed to dry. Then cells were counted.
Statistical analysis
Data are indicated as mean ± SEM of different determinations. Statistical significance between treatment and control groups was analyzed using a two-tailed Student’s t-test. Values of P < 0.05 were considered statistically significant.
Results
PA suppresses growth of human gallbladder carcinoma cells
To evaluate whether PA affects growth of human gallbladder carcinoma cells, we first investigated the effect of PA-treatment on growth of GBS-SD cells by CCK-8 assay. Cell growth was inhibited by 10 µg/ml PA 12 h after treatment, and a concentration of 30 µg/ml further reduced cell growth. And we also found that the growth of cells was suppressed in a time- and dose-dependent manner (Figure 1). After 48 h treatment, about 25%, 40% and 70% of the cell growth were inhibited by PA at concentration of 10 µg/ml, 20 µg/ml and 30 µg/ml, respectively. Our results suggest that PA also inhibits the growth of gallbladder carcinoma cells in a time-dependent and dose-dependent manner. To confirm these observations by an alternative method, we did cell cycle analysis using flow cytometry analysis. Our data showed that there was a dose-dependent increase of the cells in G1 phase and dose-dependent decrease of cells in S phase, which support that PA inhibit the growth of gallbladder carcinoma cells by inducing cell cycle arrest at G1 phase (Figure 2). However, the cells in G2/M phase were not affected by PA treatment. Our data strongly support that PA treatment inhibits the growth of gallbladder carcinoma cells.
Figure 1.
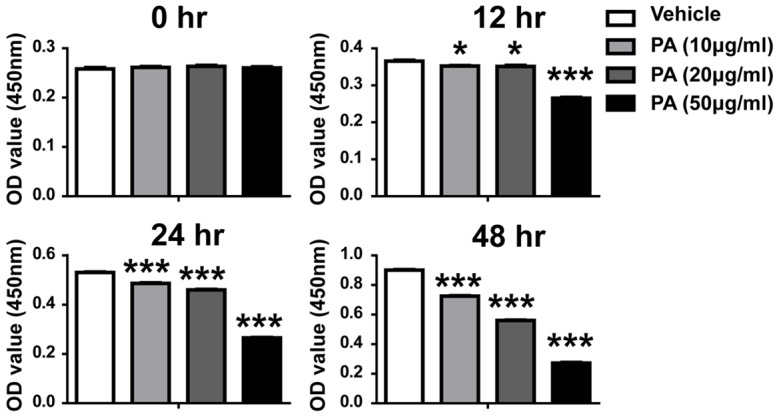
Effects of PA on growth and viability in GBC-SD cells. Each bar represents the mean ± SEM of three experiments. Similar results were obtained in three independent experiments. Statistical analysis by student t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2.
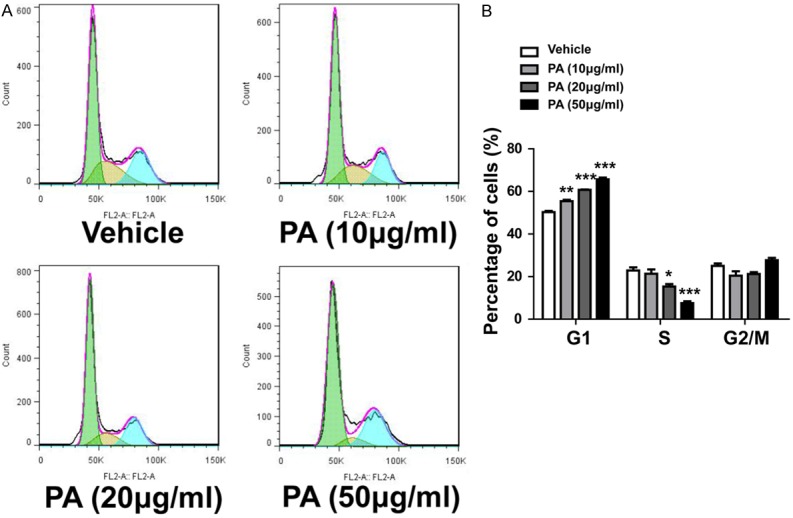
Effects of PA on cell cycle distribution. A. Representative flow plots in each group. B. Quantitative cell cycle distribution in each group. Statistical analysis by student t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
PA reduces gallbladder carcinoma cell migration and invasion
We next determined if cancer cell migration was affected by PA treatment in our cells. Normal transwell assay was conducted after PA treatment. As shown in Figure 3A, the migration ability of cells was inhibited in a dose-dependent manner by PA all the groups (10 µg/ml, 20 µg/ml and 50 µg/ml). Our qualification data demonstrated significantly reduces migrated cells in PA treated groups. To further confirm our finding, we did transwell assay with Matrigel in the small chamber, which detect the invasion ability of the cells. As shown in Figure 3B, the invasion ability of cells was also dramatically suppressed by PA treatment. Our data suggested that PA treatment can effective inhibit the migration and invasion ability of gallbladder carcinoma cells.
Figure 3.
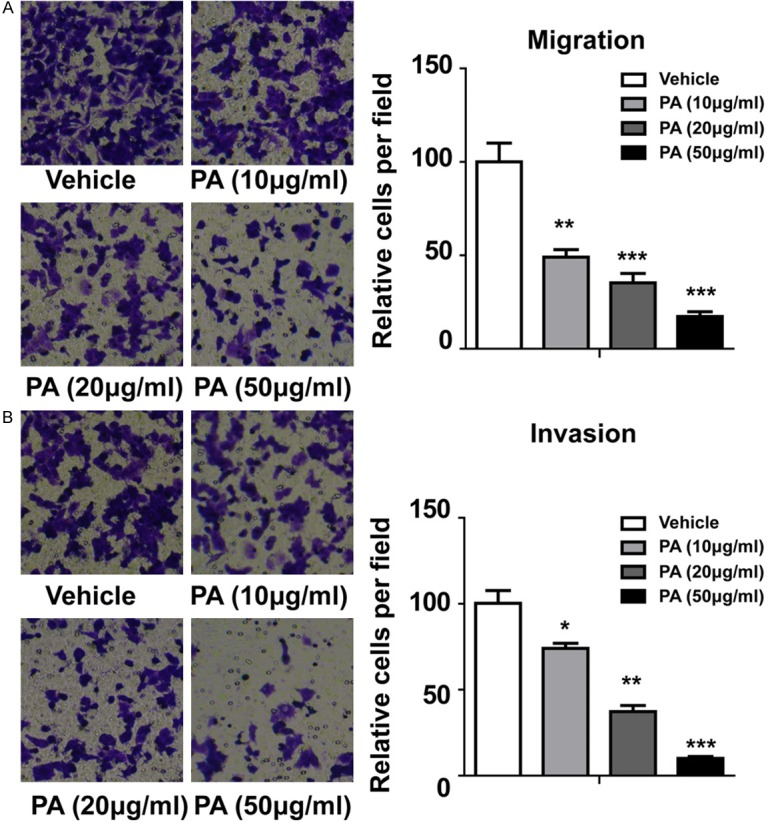
Effects of PA on migration and invasion in GBC-SD cells. A. Effect of PA on migration. B. Effect of PA on invasion. Statistical analysis by student t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
PA reduces gallbladder carcinoma cell adhesion
As adhesion molecules play a pivotal role in the development of recurrent, invasive, and distant metastasis, we further investigated whether cell adhesion ability was affected by PA treatment in gallbladder carcinoma cells. As shown in Figure 4, PA treatment at concentration of 10 µg/ml reduced the adhesion cells (P = 0.059). Relatively high concentration of PA treatment, such as 20 µg/ml and 30 µg/ml significantly inhibit cell adhesion ability. Thus, our data indicate the PA treatment can inhibit the adhesion of gallbladder carcinoma cells.
Figure 4.
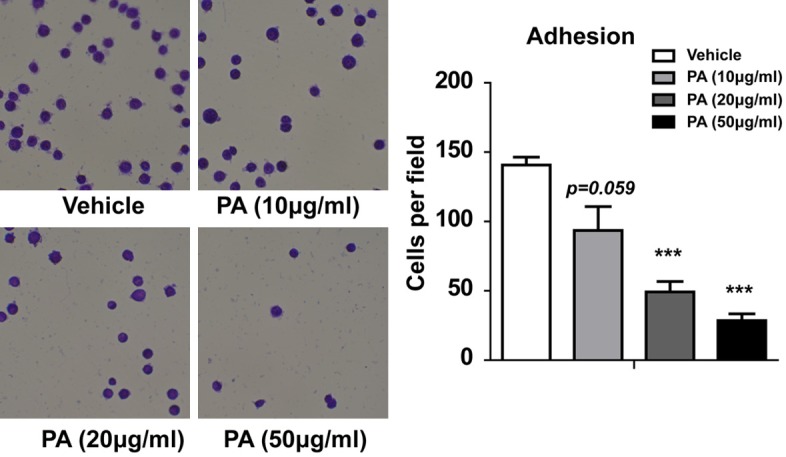
Effects of PA on adhesion in GBC-SD cells. Statistical analysis by student t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
PA inhibits many signaling pathways involved in cancer
To understanding the underlying molecular mechanism of PA treatment, we next try to find some downstream pathways that were affected by PA treatment. We detected many important proteins expression in our cells by western blot. We found that PA treatment can dose-dependently downregulated PCNA, ICAM-1, RhoA (Figure 5) [19-21], which are important proteins that affect cancer cell growth, adhesion and migration. Importantly, we also observed a dose-dependent inhibition of phosphorylated-AKT and phosphorylated-ERK1/2 without affect the total expression of AKT and ERK (Figure 5). Our data suggest that PA treatment significantly inhibits Rho A, AKT and ERK pathway in gallbladder carcinoma cells.
Figure 5.
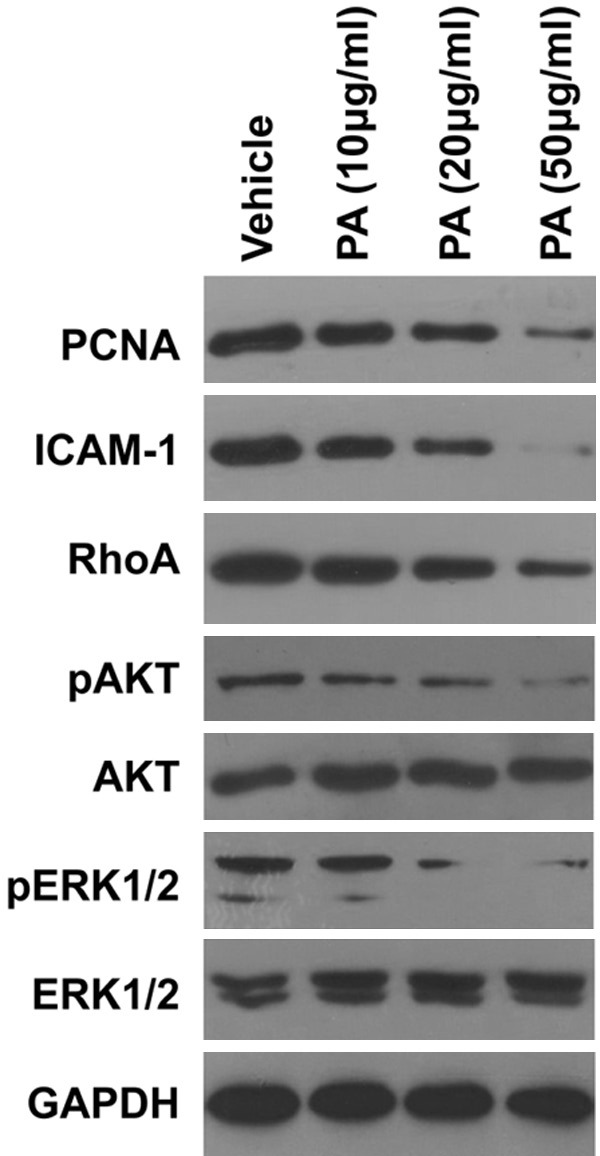
Effects of PA on the expression of PCNA, ICAM-1, RhoA, pAKT, pERK in GBC-SD cells. Similar results were obtained in at least two additional experiments.
Discussion
Gallbladder cancer is an aggressive neoplasia associated with late diagnosis, unsatisfactory treatment and poor prognosis [22,23]. An early diagnosis is rarely achieved because the signs and symptoms of GBC are not specific and often appear late in the clinical course of the disease. For this reason, the diagnosis is generally made when the cancer is already at an advanced stage [24]. Therefore, novel chemotherapy is urgently needed for improving the survival of patients with advanced GBC. In this study, we did a comprehensive study of the effects of PA in human gallbladder carcinoma cells, which strongly suggests that the PA is effective in inhibiting the carcinogenesis of gallbladder cancer.
As previous study showed that PA inhibits the growth, migration and invasion or induces apoptosis in different human cancers, including pancreatic cancer, breast cancer, lung cancer and prostate cancer et al [12,16,18,25], we aimed to investigate the role of PA treatment in gallbladder cancer cells. First we found that PA treatment can effectively inhibit the growth of gallbladder cancer cells, which is consistent with previous study. Then, we found the PA treatment mainly induced cell cycle arrest at G1 phase, which suggests the PA can inhibit the growth of gallbladder cells through inducing cell cycle arrest. To more compressively understand the role of PA treatment in gallbladder cancer, we further detected whether PA treatment can affect migration and invasion of the cells. As expected, PA treatment effectively inhibited the migration and invasion of the cells. This is consistent with recently report in breast cancer cells [16,17]. Interestingly, we also found that the adhesion ability of PA treated cells was significantly decreased, which may contribute to inhibition of the cell migration and invasion.
Then, we want to explore the molecular changes in gallbladder cells after PA treatment. We focus on some important proteins/pathways involved in cancer development. We found that PA treatment can dose-dependently downregulate PCNA, ICAM-1, RhoA, pAKT and pERK. PCNA is a popular oncoprotein that involved in cancer cell growth [26]. PCNA was originally described as an antigen for autoimmune disease in systemic lupus erythematosus patients, detected only in the proliferating-cell populations [27]. Later it was shown that expression levels of PCNA during cell cycle are differential and associated with proliferation or transformation [28,29]. The downregulation of PCNA supports our finding that PA induced cell cycle arrest. ICAM-1 is reported to be highly expressed in gallbladder cancer and contributes to cell adhesion [20]. The reduced expression of ICAM-1 is consistent with our data that PA inhibits cell adhesion in gallbladder cancer cells. RhoA is reported to be involved in cancer migration [21], which suggests that PA inhibits cell migration through downregulation of RgoAexpreesion. Therefore, these finding support our result that PA inhibits gallbladder cancer cell growth, migration, invasion and adhesion. As we know both AKT and ERK pathways play important role in the cancer cell growth, migration, invation and adhesion [30-32]. Our data also suggest that PA functions through AKT and ERK signaling pathways.
Taken together, we did a comprehensive study of the role PA treatment in gallbladder cancer cells for the first time. We found that PA can significantly inhibit cell growth, migration, invasion and adhesion. We also demonstrate the PA inhibits cell growth through inducing cell cycle arrest. Moreover, we find the PA is able to function through downregulation of AKT and ERK pathways. Therefore, we speculate that PA may be used in treatment of gallbladder cancer. Together, these results encourage further studies of PA as a promising candidate for gallbladder cancer therapy.
Disclosure of conflict of interest
None.
References
- 1.Donohue JH, Stewart AK, Menck HR. The National Cancer Data Base report on carcinoma of the gallbladder, 1989-1995. Cancer. 1998;83:2618–2628. doi: 10.1002/(sici)1097-0142(19981215)83:12<2618::aid-cncr29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, La Vecchia C. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20:146–159. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 5.Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J, Fraumeni JF Jr. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577–1582. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 7.Yu LJ, Ma RD, Wang YQ, Nishino H, Takayasu J, He WZ, Chang M, Zhen J, Liu WS, Fan SX. Potent anti-tumorigenic effect of tubeimoside 1 isolated from the bulb of Bolbostemma paniculatum (Maxim) Franquet. Int J Cancer. 1992;50:635–638. doi: 10.1002/ijc.2910500425. [DOI] [PubMed] [Google Scholar]
- 8.Tai T, Shingu T, Kikuchi T, Tezuka Y, Akahori A. Triterpenes from the Surface-Layer of Poria-Cocos. Phytochemistry. 1995;39:1165–1169. [Google Scholar]
- 9.Keller AC, Maillard MP, Hostettmann K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry. 1996;41:1041–1046. doi: 10.1016/0031-9422(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 10.Tai T, Akita Y, Kinoshita K, Koyama K, Takahashi K, Watanabe K. Anti-emetic principles of Poria cocos. Planta Med. 1995;61:527–530. doi: 10.1055/s-2006-959363. [DOI] [PubMed] [Google Scholar]
- 11.Kaminaga T, Yasukawa K, Kanno H, Tai T, Nunoura Y, Takido M. Inhibitory effects of lanostane-type triterpene acids, the components of Poria cocos, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology. 1996;53:382–385. doi: 10.1159/000227592. [DOI] [PubMed] [Google Scholar]
- 12.Gapter L, Wang Z, Glinski J, Ng KY. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun. 2005;332:1153–1161. doi: 10.1016/j.bbrc.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Xu ML, Lee CS, Woo MH, Chang HW, Son JK. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch Pharm Res. 2004;27:829–833. doi: 10.1007/BF02980174. [DOI] [PubMed] [Google Scholar]
- 14.Cuella MJ, Giner RM, Recio MC, Just MJ, Manez S, Rios JL. Two fungal lanostane derivatives as phospholipase A2 inhibitors. J Nat Prod. 1996;59:977–979. doi: 10.1021/np9604339. [DOI] [PubMed] [Google Scholar]
- 15.Ling H, Jia X, Zhang Y, Gapter LA, Lim YS, Agarwal R, Ng KY. Pachymic acid inhibits cell growth and modulates arachidonic acid metabolism in nonsmall cell lung cancer A549 cells. Mol Carcinog. 2010;49:271–282. doi: 10.1002/mc.20597. [DOI] [PubMed] [Google Scholar]
- 16.Ling H, Zhang Y, Ng KY, Chew EH. Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res Treat. 2011;126:609–620. doi: 10.1007/s10549-010-0929-5. [DOI] [PubMed] [Google Scholar]
- 17.Hong R, Shen MH, Xie XH, Ruan SM. Inhibition of breast cancer metastasis via PITPNM3 by pachymic acid. Asian Pac J Cancer Prev. 2012;13:1877–1880. doi: 10.7314/apjcp.2012.13.5.1877. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S, Eliaz I, Lin J, Thyagarajan-Sahu A, Sliva D. Triterpenes from Poria cocos suppress growth and invasiveness of pancreatic cancer cells through the downregulation of MMP-7. Int J Oncol. 2013;42:1869–1874. doi: 10.3892/ijo.2013.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol. 2005;11:2431–2437. doi: 10.3748/wjg.v11.i16.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YL, Xuan YH, Shin YK, Chae SW, Kook MC, Sung RH, Youn SJ, Choi JW, Kim SH. An immunohistochemical study of the expression of adhesion molecules in gallbladder lesions. J Histochem Cytochem. 2004;52:591–601. doi: 10.1177/002215540405200504. [DOI] [PubMed] [Google Scholar]
- 21.Sailland J, Tribollet V, Forcet C, Billon C, Barenton B, Carnesecchi J, Bachmann A, Gauthier KC, Yu S, Giguere V, Chan FL, Vanacker JM. Estrogen-related receptor alpha decreases RHOA stability to induce orientated cell migration. Proc Natl Acad Sci U S A. 2014;111:15108–13. doi: 10.1073/pnas.1402094111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 23.Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34:306–312. doi: 10.1016/j.ejso.2007.07.206. [DOI] [PubMed] [Google Scholar]
- 24.Donohue JH. Present status of the diagnosis and treatment of gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2001;8:530–534. doi: 10.1007/s005340100021. [DOI] [PubMed] [Google Scholar]
- 25.Ling H, Zhou L, Jia X, Gapter LA, Agarwal R, Ng KY. Polyporenic acid C induces caspase-8-mediated apoptosis in human lung cancer A549 cells. Mol Carcinog. 2009;48:498–507. doi: 10.1002/mc.20487. [DOI] [PubMed] [Google Scholar]
- 26.Dillehay KL, Lu S, Dong Z. Anti-tumor effects of a novel small molecule targeting PCNA chromatin association in prostate cancer. Mol Cancer Ther. 2014;13:2817–26. doi: 10.1158/1535-7163.MCT-14-0522. [DOI] [PubMed] [Google Scholar]
- 27.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–2234. [PubMed] [Google Scholar]
- 28.Bravo R, Fey SJ, Bellatin J, Larsen PM, Celis JE. Identification of a nuclear polypeptide (“cyclin”) whose relative proportion is sensitive to changes in the rate of cell proliferation and to transformation. Prog Clin Biol Res. 1982;85 Pt A:235–248. [PubMed] [Google Scholar]
- 29.Celis JE, Bravo R, Larsen PM, Fey SJ. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8:143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- 30.Lunardi A, Webster KA, Papa A, Padmani B, Clohessy JG, Bronson RT, Pandolfi PP. Role of aberrant PI3K pathway activation in gallbladder tumorigenesis. Oncotarget. 2014;5:894–900. doi: 10.18632/oncotarget.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Lu J, Zhang F, Li H, Zhang B, Wu X, Tan Z, Zhang L, Gao G, Mu J, Shu Y, Bao R, Ding Q, Wu W, Dong P, Gu J, Liu Y. Yes-associated protein 1 (YAP1) promotes human gallbladder tumor growth via activation of the AXL/MAPK pathway. Cancer Lett. 2014;355:201–9. doi: 10.1016/j.canlet.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Mishra V, Ansari KM, Khanna R, Das M. Role of ErbB2 mediated AKT and MAPK pathway in gall bladder cell proliferation induced by argemone oil and butter yellow. Argemone oil and butter yellow induced gall bladder cell proliferation. Cell Biol Toxicol. 2012;28:149–159. doi: 10.1007/s10565-011-9207-5. [DOI] [PubMed] [Google Scholar]


