Abstract
Objectives: Lymphovascular invasion (LVI) has been associated with a poor outcome in patients with breast cancer, but it is not included in international TNM staging system and molecular subtype criterion. The current studies have reported the relation between LVI and the tumor size (T), the status of axillary lymph node (ALN), age, histological grade in invasive breast cancer, but the results were debatable. So the meta-analysis was conducted to confirm the relation between LVI and the four clinicopathological factors. Methods: Literature was searched by entering the terms: breast AND (neoplasm OR cancer OR carcinoma) AND (lymphovascular OR “lymphatic vessel” OR “vascular vessel” OR “blood vessel” OR “lymph vessel”) AND (invasion OR “carcinoma embolus”) AND (lymph node OR grade OR size OR clinicopathological) in PubMed, The merged odds ratio (OR) and 95% confidence interval (CI) were estimated using fixed-effect or random-effect model, RevMan 5.3 was used to analyze the relation between LVI and tumor size, status of ALN, age, histological grade in invasive breast cancer respectively. The fail-safe number was used to estimate publication bias. Results: The analysis included 6 studies, LVI positive rate was significant lower in T≤2 cm, ALN negative, age >50 y and histological grade 1 groups statistically. The OR and 95% CI were 0.53 [0.46, 0.61], 0.23 [0.15, 0.35], 1.62 [1.42, 1.85], 0.36 [0.17, 0.77] respectively. Conclusions: LVI was significantly correlated with the expression status of the tumor size, status of ALN, age, histological grade in invasive breast cancer, and was consistent with adverse features of the four factors.
Keywords: Lymphovascular invasion, histological grade, axillary lymph node, clinicopathological factors, breast cancer
Introduction
Breast cancer is a common cancer and one of the leading causes of cancer death in female. It accounts for 29% of all female new cancers and 15% of all female deaths due to cancers [1]. LVI is a key step of tumor cells reaching lymph node, therefore, LVI has been known as an independent predictor of lymph node metastases. Lymph node positive breast cancer has a poor prognosis. In breast cancer, LVI has been as an independent predictor of disease-free survival (DFS) as well as overall survival (OS) [2,3]. The 2005 St. Gallen consensus guidelines suggested LVI was recognized as one of the factors upon which to base treatment plan decisions [4].
LVI is assessed in the carcinoma tissue on hematoxylin and eosin (H&E) stained sections, it is defined as carcinoma cells present within a definite endothelial-lined space (lymphatic or blood vessel). So LVI include lymphatic and blood vessel invasion. Routine assessment of LVI is now part of breast cancer pathology reporting.
The prognosis of breast cancer varies with different TNM stage, age, histological grade. The current studies have reported the relation between LVI and the four factors in invasive breast cancer, but the results were disputed [5-10]. So this meta-analysis was conducted to confirm the correlation between LVI and the tumor size, the status of ALN, age, and histological grade.
Materials and methods
Literature search strategy
Literature was searched by entering the terms: breast AND (neoplasm OR cancer OR carcinoma) AND (lymphovascular OR “lymphatic vessel” OR “vascular vessel” OR “blood vessel” OR “lymph vessel”) AND (invasion OR “carcinoma embolus”) AND (lymph node OR grade OR size OR clinicopathological) in PubMed. The publish time of literature was unlimited. Only literature written in English language was included.
Inclusion criteria
All of the following criteria had to be included in literature for this analysis: (1) Patients with breast cancer were not subjected to radiotherapy, hormone therapy and chemotherapy before the pathological specimen were extracted. (2) The stages of the disease were T1-4N0-3M0-1 or I-IV stages. (3) LVI was determined by H&E staining. (4) All literature was in English language.
Exclusion criteria
The literature in which the detection method of the LVI was not H&E, or from which the interested data could not be extracted was excluded.
Data extraction
The following information was extracted from each eligible literature: authors’ names, year of publication, the tumor size, the status of ALN, age, histological grade, case date, study location and LVI positive rate in each group.
Statistical analysis
RevMan 5.3 software was used to perform the meta-analysis. The OR and 95% CI were used to estimate the correlation of LVI and clinicopathological factors in the invasive breast cancer. The Mantel-Haenszel method was used to combine the ORs for the outcomes. The fixed-effect or random-effect model was used to calculate the pooled outcome according to heterogeneity. Each study was weighted according to the sample size. The heterogeneity among studies was defined significant when P<0.1 for χ2 test or I2>50%. Fail-safe number was used for detecting publication bias according to the formula Nfs0.05=(ΣZ/1.64)2-K.
Results
Eligible literatures
The searching deadline was Feb 12th, 2015. A total of 659 citations were identified from PubMed, 24 articles were remained after exclusion based on the titles and abstracts. 1 duplication, 3 original articles in Chinese language, 1 original article in Portuguese language, 5 articles that could not provide interested data, 5 articles in which cases were confided to special subjects and 3 original full texts that could not be obtained were removed. A total of 6 studies met the inclusion criteria for meta-analysis finally (Figure 1).
Figure 1.
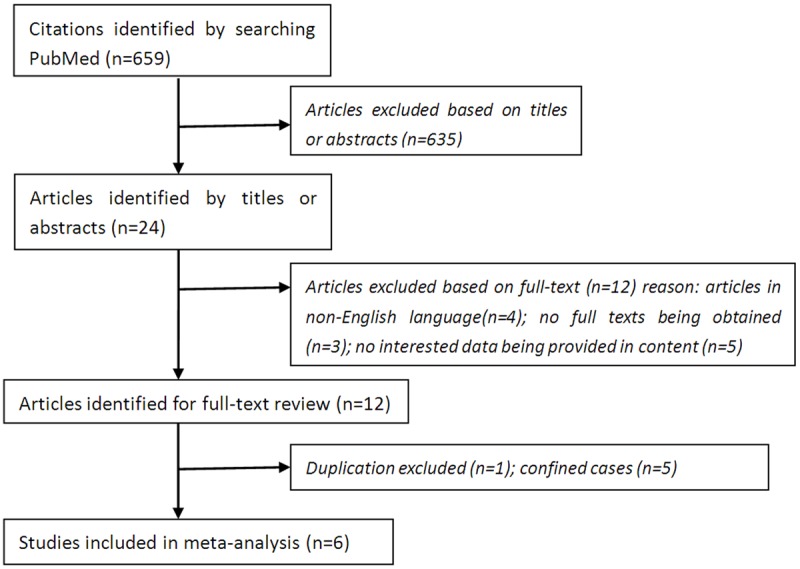
The flowchart of literature search.
Characteristic of included studies
The final 6 studies were published from 2007 to 2014 and appraised critically (Table 1). In the studies of [Rakha EA 2012] and [Gujam FJ 2014] the cases were divided into three classifications according to tumor size: T≤1 cm, T>1-2 cm, T=2-5 cm and T≤2 cm, T=2-5 cm, T≥5 cm respectively, while the cases were combined into two classifications in this analysis correspondingly: T≤2 cm and T>2 cm. In the study of [Aitken E 2010] and [Rakha EA 2012] the cases were divided into three classifications according to the status of ALN: ALN negative; 1-3 ALN positive and no less than 4 ALN positive, while the cases were combined into two classifications in this analysis correspondingly: ALN negative and ALN positive. In the study of [Lee JA 2011] the cases were divided into two classifications: age <50 y and age ≥50 y according to age, but the two classifications were modified or combined into age ≤50 y and age >50 y in this analysis. It was unknown how many cases with just 50 y were in the article, the modification maybe affect the result very little. In the study of [Lee JA 2011], [Rakha EA 2012] and [Gujam FJ 2014] the cases were divided into three classifications according to histological grade: 1, 2 and 3. The latter two classifications were combined into one classification in this analysis, that was grade 2/3.
Table 1.
The characteristic of cases in invasive breast cancer in included studies
| Characteristic of cases | LVI positive rate (no./cases) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Gujam FJ 2014 | Lee JA 2011 | Rakha EA 2012 | Tezuka K 2007 | Aitken E 2010 | Wu JL 2014 | |
| Tumor size (cm) | ||||||
| ≤2 | 38/185 | 1/41 | 559/1884 | 17/44 | ||
| >2 | 64/175 | 4/39 | 536/1232 | 50/88 | ||
| ALN status | ||||||
| negative | 41/206 | 0/41 | 443/2426 | 24/63 | 70/398 | 157/742 |
| positive | 61/154 | 5/39 | 683/1341 | 43/69 | 98/225 | 416/583 |
| Age (years) | ||||||
| ≤50 | 39/125 | 1/38 | 565/1572 | 26/42 | ||
| >50 | 63/235 | 4/42 | 563/2236 | 41/90 | ||
| Histological grade | ||||||
| 1 | 10/48 | 0/15 | 72/622 | |||
| 2/3 | 92/312 | 5/63 | 899/2633 | |||
| Date | 1995 to 1998 | 2005 to 2007 | 1989 to 2004 | 1997 to 2000 | 2003 to 2005 | 2004 to 2010 |
| Location | Royal Infirmary, Western Infirmary, Victoria or Stobhill Hospitals Glasgow | Korea University Anam Hospital Seoul | Nottingham City Hospital Nottingham | Osaka City University Hospital Osaka | Crosshouse Hospital, Kilmarnock Ayrshire & Arran | Changhua Christian Hospital Taiwan |
ALN: axillary lymph node; LVI: lymphovascular invasion.
Correlation of LVI with the tumor size, status of ALN, age, histological grade in invasive breast cancer
LVI positive rates were compared between T≤2 cm and T>2 cm groups in invasive breast cancer in 4 studies. There was no significant heterogeneity (I2=0, P=0.72). In the fixed-effect model there was statistical difference between T≤2 cm and T>2 cm groups (OR=0.50, 95% CI: 0.46-0.61, P<0.001) (Figure 2), which confirmed LVI positive rate was low in T≤2 cm group.
Figure 2.
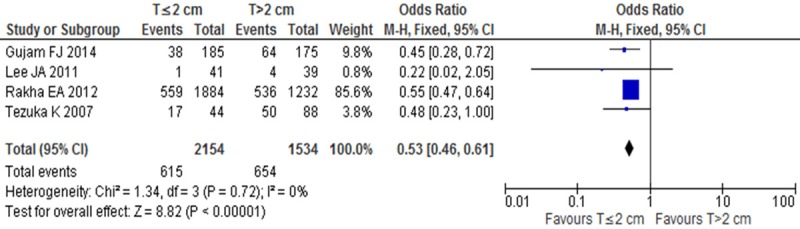
LVI positive rates between T≤2 cm and T>2 cm groups in invasive breast cancer.
LVI positive rates were compared between ALN negative and ALN positive groups in invasive breast cancer in 6 studies. There was significant heterogeneity (I2=87%, P<0.1), Nfs0.05=1037.62, so random-effect model was adopted, there was statistical difference between ALN negative and ALN positive groups (OR=0.23, 95% CI: 0.15-0.35, P<0.001) (Figure 3), which confirmed that LVI positive rate was low in ALN negative group.
Figure 3.
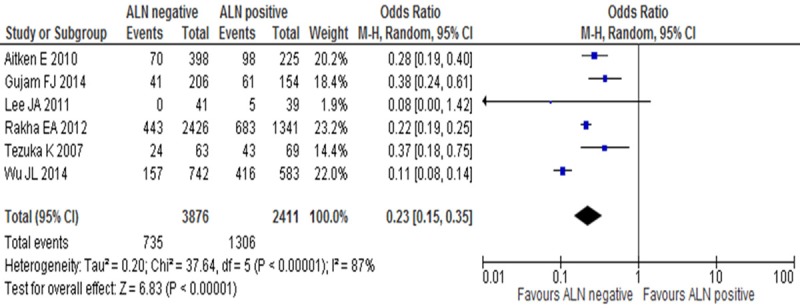
LVI positive rates between ALN negative and ALN positive groups in invasive breast cancer.
LVI positive rates were compared between age ≤50 y and age >50 y groups in invasive breast cancer in 4 studies. There was no significant heterogeneity (I2=29%, P=0.24). In the fixed-effect model there was statistical difference between age ≤50 y and age >50 y groups (OR=1.62, 95% CI: 1.42-1.85, P<0.001) (Figure 4), which suggested LVI positive rate was low in age >50 y group.
Figure 4.
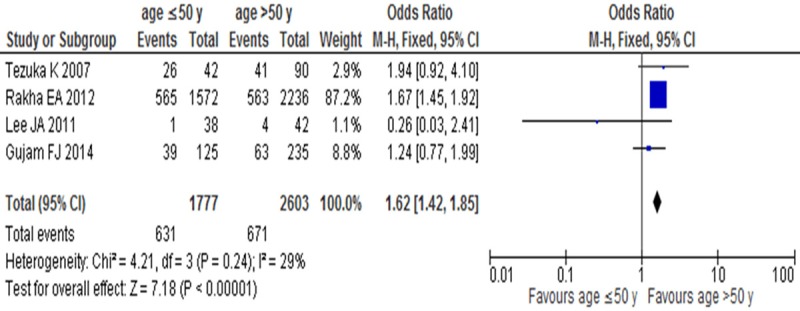
LVI positive rates between age ≤50 y and age >50 y groups in invasive breast cancer.
LVI positive rates were compared between grade 1 and grade 2/3 groups in invasive breast cancer in 3 studies. There was significant heterogeneity (I2=62%, P=0.07). Nfs0.05=53.95, so random-effect model was adopted, there was statistical difference between grade 1 and grade 2/3 groups (OR=0.36, 95% CI: 0.17-0.77, P=0.008) (Figure 5), which suggested LVI positive rate was low in grade 1 group.
Figure 5.
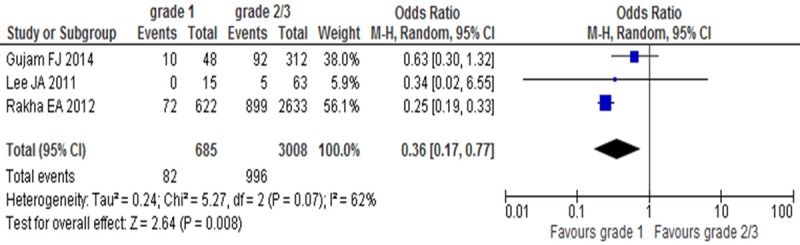
LVI positive rates between grade 1 and grade 2/3 groups in invasive breast cancer.
Evaluation of publication bias
As reports on LVI detected by H&E were rare in breast cancer, the publication bias was not visualized by funnel plot due to fewer studies, but the fail-safe number could demonstrate that the publication bias may not exist.
Discussion
LVI is a crucial step in the complex process of tumor metastasis and an important criterion for further therapy. So it is a significant prognostic factor in invasive breast cancer with respect to local and distance recurrence [8] and poorer survival [11]. It is also associated with other strong prognostic factors including tumor size, grade and regional lymph node involvement [12].
The combined outcomes indicated that LVI was correlated with the tumor size, status of ALN, age, histological grade respectively in invasive breast cancer, and could act as a predictor of poor prognosis for invasive breast cancer.
In breast cancer, tumor size is powerful predictor for local recurrence, regional and systemic spread, therefore for OS. The individual OR value of the 4 studies ranged from 0.02 to 2.05, which indicated that the studies were not consistent about the relation between LVI and tumor size. But the meta-analysis confirmed LVI positive rate was significant lower in T≤2 cm group than that in T>2 cm group statistically (P<0.001).
The status of ALN is the most powerful prognostic factor for breast cancer patients to date. The prognosis of breast cancer patients with the ALN positive is poor. Because LVI increased the chances of the ALN positive, it was one of the predictors of the ALN positive [5]. The relations between LVI and ALN were consistent in included 5 studies except [Lee JA 2011]. The individual OR value of the 5 studies ranged from 0 to 0.75, which indicated that the studies were consistent about the relation between LVI and ALN. [Rakha EA 2012] and [Lee JA 2011] were attributed to the high heterogeneity with the method of sensitivity analysis.The sample sizes in 2 studies were much bigger than those in the other 4 studies, so the high heterogeneity was ascribed to obvious difference of the sample sizes between the studies. The meta-analysis confirmed LVI positive rate was significant lower in ALN negative group than that in ALN positive group statistically (P<0.01). LVI offers an auxiliary method in the assessment of ALN status preoperatively, which may help the doctor in breast cancer counseling and decision-making for therapy.
A number of studies have demonstrated that younger age is a risk factor for ALN positive [5,13]. This has been attributed to biologically more aggressive tumors in this younger age group [14]. The average age of menopause is about 50 y, so most literature set 50 y as cut-off point in exploring the relation between age and clinicopathological factors in invasive breast cancer. The individual OR value of the 4 studies ranged from 0.03 to 4.10, which indicated that the studies were not consistent about the relation between LVI and age. But the meta-analysis confirmed LVI positive rate was significant higher in age ≤50 y group than that in age >50 y group statistically (P=0.003).
LVI had correlation with histological grade. High grade and fast growing tumor may produce more growth factors and offer a bigger clonal variety of tumor cells capable of invading lymphatic vessels compared with low grade and slow growing tumor. The individual OR value of the 3 studies ranged from 0.02 to 1.32, which indicated that the studies were not consistent about the relation between LVI and histological grade. But the meta-analysis confirmed LVI positive rate was significant lower in grade 1 group than that in grade 2/3 group statistically (P=0.008). There was significant heterogeneity due to too few included studies and obvious difference of sample sizes between the studies.
In summary, LVI has unfavorable pathological features, and is significantly correlated with the tumor size, status of ALN, age, histological grade in invasive breast cancer. It was consistent with adverse features of the four factors, and showed an aggressive predictor. The method of LVI detected with H&E staining is easy and cheap in almost of all departments of pathology, thus it is considerable to list LVI as a marker of clinical typing for breast cancer. Moreover, anti-LVI therapy may become new therapeutic target for breast cancer.
Acknowledgements
We acknowledge the support of the directors and pathologists involved in the collection and analysis of the data in these individuals (Xin Xu, Kang-Sheng Bei, Ri-Chang Du, Gao-Fang Xiao). Supported by National Natural Science Foundation of China No. 61427807; Fujian Province Introduction of Major Research and Development Institution Funding Project No. 2012I2004.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, Jakesz R, Horvat R Austrian Breast and Colorectal Cancer Study Group. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240:306–12. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vleugel MM, Bos R, van der Groep P, Greijer AE, Shvarts A, Stel HV, van der Wall E, van Diest PJ. Lack of lymphangiogenesis during breast carcinogenesis. J Clin Pathol. 2004;57:746–751. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ, Panel M. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 5.Aitken E, Osman M. Factors affecting nodal status in invasive breast cancer: a retrospective analysis of 623 patients. Breast J. 2010;16:271–278. doi: 10.1111/j.1524-4741.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- 6.Gujam FJ, Going JJ, Mohammed ZM, Orange C, Edwards J, McMillan DC. Immunohistochemical detection improves the prognostic value of lymphatic and blood vessel invasion in primary ductal breast cancer. BMC Cancer. 2014;14:676. doi: 10.1186/1471-2407-14-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JA, Bae JW, Woo SU, Kim H, Kim CH. D2-40, Podoplanin, and CD31 as a Prognostic Predictor in Invasive Ductal Carcinomas of the Breast. J Breast Cancer. 2011;14:104–111. doi: 10.4048/jbc.2011.14.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakha EA, Martin S, Lee AH, Morgan D, Pharoah PD, Hodi Z, Macmillan D, Ellis IO. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2012;118:3670–3680. doi: 10.1002/cncr.26711. [DOI] [PubMed] [Google Scholar]
- 9.Tezuka K, Onoda N, Takashima T, Takagaki K, Ishikawa T, Wakasa T, Wakasa K, Hirakawa K. Prognostic significance of lymphovascular invasion diagnosed by lymphatic endothelium immunostaining in breast cancer patients. Oncol Rep. 2007;17:997–1003. [PubMed] [Google Scholar]
- 10.Wu JL, Tseng HS, Yang LH, Wu HK, Kuo SJ, Chen ST, Chen DR. Prediction of axillary lymph node metastases in breast cancer patients based on pathologic information of the primary tumor. Med Sci Monit. 2014;20:577–581. doi: 10.12659/MSM.890345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AK, DeLellis RA, Silverman ML, Heatley GJ, Wolfe HJ. Prognostic significance of peritumoral lymphatic and blood vessel invasion in node-negative carcinoma of the breast. J. Clin. Oncol. 1990;8:1457–1465. doi: 10.1200/JCO.1990.8.9.1457. [DOI] [PubMed] [Google Scholar]
- 12.Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, Blamey RW. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42:357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Gill PG, Luke CG, Roder DM. Clinical and pathological factors predictive of lymph node status in women with screen-detected breast cancer. Breast. 2006;15:640–648. doi: 10.1016/j.breast.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Rivadeneira DE, Simmons RM, Christos PJ, Hanna K, Daly JM, Osborne MP. Predictive factors associated with axillary lymph node metastases in T1a and T1b breast carcinomas: analysis in more than 900 patients. J Am Coll Surg. 2000;191:1–6. doi: 10.1016/s1072-7515(00)00310-0. discussion 6-8. [DOI] [PubMed] [Google Scholar]


