Abstract
Diabetic retinopathy (DR) remains a prevalent complication of diabetes and one of the leading causes of blindness among working-age adults. However, the detailed molecular mechanism of the development of DR was still unclear by now. HMGB1 is a non-histone DNA-binding protein and serves as a structural component to facilitate the assembly of nucleoprotein complexes in the nucleus. In the present study, we examined the serum level of HMGB1 and VEGFA in the DR patients. Besides, we also detect the association between HMGB1 and VEGFA level. In the advanced in-vitro study, we detect the protective effect of HMGB1 on the RPE cells in high glucose condition. In this study, we demonstrated that HMGB1 and VEGFA expressions were upregulated in serum samples of DR patients. Advanced analysis showed that HMGB1 and VEGFA level was positively associated. In the in-vitro study, it was found that up-regulation of HMGB1 inhibited the RPE cell viability and induce the apoptosis. Besides, HMGB1 treatment would up-regulate the expression of VEGFA in the RPE cells in high glucose condition. In conclusion, we have demonstrated that HMGB1 and VEGFA are key players in the ability to suppress cell viability and induce apoptosis. The result of this current experiments shed light into the mechanism by which HMGB1 works. Besides, we also present the data of case control study data, our results showed that HMGB1 might be used as biomarkers of DR.
Keywords: HMGB1, diabetic retinopathy, in vitro, VEGF
Introduction
Diabetes mellitus (DM) is one of the most frequent chronic diseases, causing a high rate of morbidity and contributing to elevated rates of mortality [1]. As a global concern, DM affected over 360 million individuals worldwide [2]. This number of DM patients is expected to exceed half a billion by 2030. The main causes of morbidity were long-term microangiopathy complications of diabetes, which affects various organs and contributes to diseases such as diabetic retinopathy (DR), neuropathy, and nephropathy [3]. DR remains a prevalent complication of diabetes and one of the leading causes of blindness among working-age adults [4]. Although the major risk factors for DR (hyperglycemia, hypertension and dyslipidemia) have been examined in different epidemiologic studies and clinical trials, there is considerable variation in the consistency, pattern, and strength of these risk factors [5]. However, the detailed molecular mechanism of the development of DR was still unclear by now.
Nowadays the development of DR is believed to be the result of multiple factors synergies, mainly based on cellular apoptosis, non-aldehyde glycosylation of protein, free radicals and abnormal growth factor expression [6]. HMGB1 is a non-histone DNA-binding protein and serves as a structural component to facilitate the assembly of nucleoprotein complexes in the nucleus [7]. Chromatin-associated HMGB1 plays multiple roles in the regulation of genome replication, recombination, mRNA transcription, and DNA repair [8]. Besides, HMGBI, as a late inflammatory mediator, could directly stimulate the body’s innate immune response and the adaptive immune response [9]. The abnormal expression of HMGB1 can activate the release of other cytokines, breaking proinflammatory cytokines and body balance between anti-inflammatory cytokines, resulting in the occurrence and development of various autoimmune diseases.
Numerous biochemical changes have been observed in the vascular tissue of the retina, which are believed to be involved in diabetic retinopathy. Especially, vascular endothelial growth factor (VEGF) can trigger many of the retinal vascular changes caused by diabetes [10], including leukocyte adhesion to retinal capillaries and vascular leakage. VEGF was the crucial regulator of vasculogenesis, angiogenesis, lymphangiogenesis and vascular permeability in vertebrates. Extracellular HMGB1 functioned as a proinflammatory cytokine and exhibited antigenic effects [11]. HMGB1 was also reported to be associated with the up-regulation of VEGF. A study by Li et al showed that that HMGB1 upregulated VEGF mRNA and protein in a dose-dependent manner in HCT116 cells, and that this was mediated via NF-κB in an in-vitro study [12]. Another study was conducted by Park showed that HMGB1 could stimulate expression of vascular endothelial growth factor (VEGF) and inhibition of HIF-1α attenuated HMGB1-induced VEGF [13].
HMGB1 signals through the receptor for advanced glycation end products (RAGE), a member of the immunoglobulin superfamily of receptors, leading to activation of the transcription factor nuclear factor κB (NF-κB), and induces the expression of various leukocyte adhesion molecules and pro-inflammatory cytokines and chemokines Up-regulation of HMGB1 gene expression and protein activity in the retinal and vitreous samples were found in cases of DR [14,15]. Furthermore, it was demonstrated that diabetes induced significant upregulation of the expression of HMGB1 and RAGE in the retinas of rats and mice and that intravitreal administration of HMGB1 to normal rats induced activation of inflammatory signaling pathways in the retina and increased retinal vascular permeability [16]. In the present study, we examined the serum level of HMGB1 and VEGFA in the DR patients. Besides, we also detect the association between HMGB1 and VEGFA level. In the advanced in-vitro study, we detect the protective effect of HMGB1 on the RPE cells in high glucose condition.
Materials and methods
Study population
This is a case-control study with 60 DR cases and 65 age and gender matched DM controls. Serum samples were obtained from all participants from January 2014 to December 2014 at the Department of Ophthalmology, Xiehe Hospital of Fujian Medical University. The design and conduction of this study was approved by the Ethics Committee of Xiehe Hospital of Fujian Medical University. Clinical examinations were performed according to the principles in the Declaration of Helsinki. Additionally, serum samples of the all the cases. Written informed consents were obtained from all the participants before starting the study. As well, the data obtained from hematological and clinical examinations used for research purpose were also reported.
Detection of serum VEGF and HMGB1
Clinical venous blood samples of all participants were collected using the blood collection system. Venous blood was centrifuged for 10 min at 1700 × g and then the serum were isolated. The serum samples were stored frozen at -80°C until use. The concentrations of HMGB1 and VEGFA in each serum samples were determined using HMGB1 and VEGFA ELISA kit (Hamburg, Germany). The plate readings were done using FLUOstar Omega-Miroplate reader from BMG Labtech, Offenburg, Germany.
Besides, a series of biochemical indexes, including total cholesterol, LDL-cholesterol and HDL-cholesterol, were measured in this study. All the analyses were sent to the Inspection department of the Xiehe Hospital of Fujian Medical University. Serum levels of total cholesterol, LDL-cholesterol and HDL-cholesterol were measured by the related kit (Roche). All the procedures were performed according to the manufacturer’s instructions. All samples were assayed in duplicate and mean values was used for the analysis.
Cell culture and in-vitro model
Human retinal pigment epithelium cell line (ARPE-19) was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cells at 6 to 9 passages were used in this study. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY) and supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, USA) and a 1% antibiotic antimycotic solution containing 1×104 units penicillin, 10 mg streptomycin at 37°C in a humidified atmosphere of 5% CO2 95% air. For the in-vitro cell model, the ARPE-19 cells incubated in the presence of high glucose (5.5 g/L) culture condition for 24 hours were used as high-glucose cellular model. All the treatments were based on the in-vitro model described above.
Cell viability
The cellular viability was detected by MTT assay. The ARPE-19 cells in each group was treated with 20 μL of sterile MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye (Sigma-Aldrich, St. Louis, MO, USA) was added and incubated for another 4 h at 37°C. Then, 150 μL of dimethyl sulfoxide (DMSO) was added to each well and the plates were thoroughly mixed for 10 min. Spectrometric absorbance at a wavelength of 492 nm was measured on an enzyme immunoassay analyzer (model 680; Bio-Rad Laboratories, Hercules, CA, USA).
Apoptosis assay
To assess the apoptotic rate of ARPE-19 cells in each group, the cells were collected, rinsed and fixed overnight in 75% cold ethanol at -20°C. Then, cells were treated with Tris-HCl buffer (pH 7.4) supplemented with 100 lg/mL RNase A and stained with 25 lg/mL propidium iodide (BD Biosciences, San Diego, CA). The apoptotic rates were analyzed with flow cytometry (Flow-Count, Beckman Coulter, CA, USA) using the Annexin V-Fluorescein isothiocyanate (FITC)/Propidium iodide (PI) kit (Roche, Penzberg, Germany). All the operations were performed according to the manufacturers. All data were collected, stored, and analyzed by ModFit software.
Western blot analysis
Cells or tissues were harvested and lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 6.8, 100 mM 2-ME, 2% SDS, 10% glycerol). After centrifugation at 20000× g for 10 min at 4°C, proteins in the supernatants were quantified and separated by 10% SDS PAGE, transferred to NC membrane (Amersham Bioscience, Buckinghamshire, U.K.). Equal amount of protein (50 μg) were separated by SDS PAGE. The blots were then developed with SuperSignal-enhanced chemiluminescent substrate solution (Pierce Chemical Company, Rockford, USA). The primary antibody against VEGFA and α-actin (Santa Cruz, USA) were used in this study.
Statistical analysis
Data are expressed as the mean ± SD from at least three separate experiments. Differences between groups were analyzed using Student’s t-test. All statistical analyses were performed using SPSS 16.0 software (SPSS, Chicago, IL). Differences were considered significant if P < 0.05. All experiments were performed in triplicate and repeated at least three times.
Results
Characteristic of diabetes patients included in this study.
To detect the serum characters of the patients with and without DR, a total of 125 DM cases were included in this study. Among all the DM cases, a total of 60 DR patients were included. There were no significant differences between the age, gender, hypoglycemic agents use, smoking status and alcohol intake between patients with and without DR. However, the patients with DR showed a significant rate of insulin use, longer DM duration, higher HbA1c rate and total cholesterol contents. We also detect the levels of HMGB1 and VEGFA in all the DM cases. From the expression data, we found that HMGB1 and VEGFA were higher in the DR cases (HMGB1, 5.8±0.8 ng/mL vs 2.1±0.4 ng/mL, P < 0.001; VEGFA, 1109.1±119.2 ng/mL vs 493.1±108.7 ng/mL, P < 0.001). The detailed data were presented in Table 1.
Table 1.
Characteristic of diabetes patients included in this study
| Characters | Total (N = 125) | With DR (N = 60) | Without DR (N = 65) | P |
|---|---|---|---|---|
| Age (year) | 59.1±9.8 | 60.6±10.6 | 58.3±8.7 | 0.186 |
| Male | 67 | 33 | 34 | 0.859 |
| Diabetes duration (years) | 8.2±2.8 | 10.3±2.9 | 6.6±2.3 | < 0.001 |
| Insulin use | 57 | 35 | 22 | 0.007 |
| Hypoglycemic agents use | 123 | 60 | 63 | 0.497 |
| Ever Smoking | 57 | 28 | 29 | 0.859 |
| Current alcohol intake | 34 | 15 | 19 | 0.689 |
| HbA1c (%) | 8.1±1.6 | 9.2±1.8 | 7.4±1.5 | < 0.001 |
| Total cholesterol (mmol/L) | 98.3±16.6 | 101.3±17.2 | 95±14.9 | 0.032 |
| LDL-cholesterol (mmol/L) | 2.7±0.8 | 2.8±0.8 | 2.6±0.7 | 0.128 |
| HDL-cholesterol (mmol/L) | 1.7±0.7 | 1.6±0.6 | 1.8±0.7 | 0.119 |
| HMGB1 (ng/mL) | 3.9±0.7 | 5.8±0.8 | 2.1±0.4 | < 0.001 |
| VEGFA (ng/mL) | 818.8±112.5 | 1109.1±119.2 | 493.1±108.7 | < 0.001 |
Serum HMGB1 and VEGFA in DR cases
We found serum HMGB1 and VEGFA were higher in the patients with DR. In the In advance, we detect the expression of HMGB1 and VEGFA in DR cases in different group. It was found that the HMGB1 was high in both PDR and NPDR group compared with the control group. Besides, the HMGB1 was higher in the PDR group. Besides, the serum VEGFA was also higher in both PDR and NPDR group compared with the controls and PDR group demonstrated a higher expression level (Figure 1).
Figure 1.
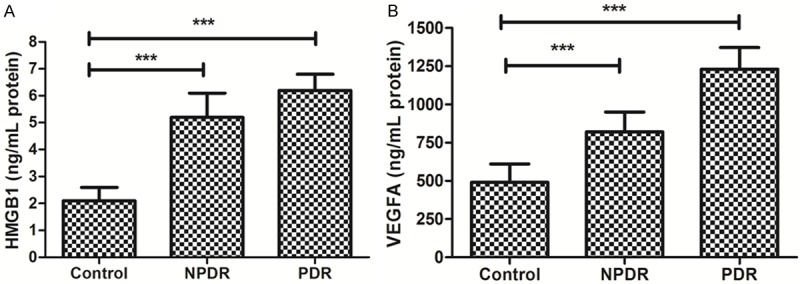
Expression of HMGB1 and VEGFA in serum in DR cases. A. Expression of HMGB1 in control, NPDR and PDR. B. Expression of VEGFA in control, NPDR and PDR.
Besides, we also conducted advanced studies to detect the possible relationship between the serum HMGB1 and VEGFA level in the DR cases. Both the PDR and NPDR samples were used in the detection of relationship between serum HMGB1 and VEGFA level. In the pearson correlation analyses, a significant association was detected (P < 0.001, R = 0.748). The scatterplot and trend line (in red) were presented in Figure 2.
Figure 2.
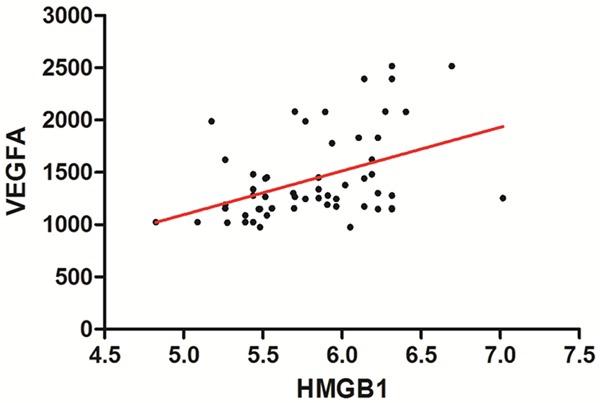
The association between HMGB1 and VEGFA in serum of DR cases.
The effects of HMGB1 on cell viability
In order to assess the effects of HMGB1 on ARPE-19 cell viability in high glucose condition, different concentrations of HMGB1 were adopted to test the cell viability of ARPE-19 cells. Ectogenic HMGB1 was used to treat with ARPE-19 cells at the concentrations of 1, 10, 100 and 1000 ng/mL for 72 hours. Cell viability was measured by MTT assay. Compared to negative control, HMGB1 significantly inhibited the growth of ARPE-19 cells in concentrations of 100 and 1000 ng/mL (P < 0.001) (Figure 3).
Figure 3.
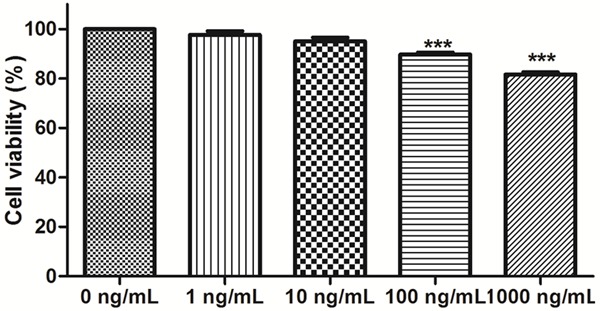
The effect of HMGB1 on the ARPE-19 cells.
HMGB1 induced apoptosis in ARPE-19 cells
We further investigated the effect of HMGB1 on apoptosis by flow cytometry. ARPE-19 cells in high glucose were treated with 100 ng/mL and 1000 ng/mL of HMGB1 for 72 hours. Flow cytometry analysis demonstrated that the percentage of apoptosis (100 ng/mL HMGB1, 16.3%±2.2% and 1000 ng/mL HMGB1, 35.6%±7.6%) in ARPE-19 cells treated with HMB1 were significantly higher than the control group (5.3%±1.1%). These results suggested that HMGB1 could act as an apoptosis inducer in ARPE-19 cells in vitro (Figure 4).
Figure 4.
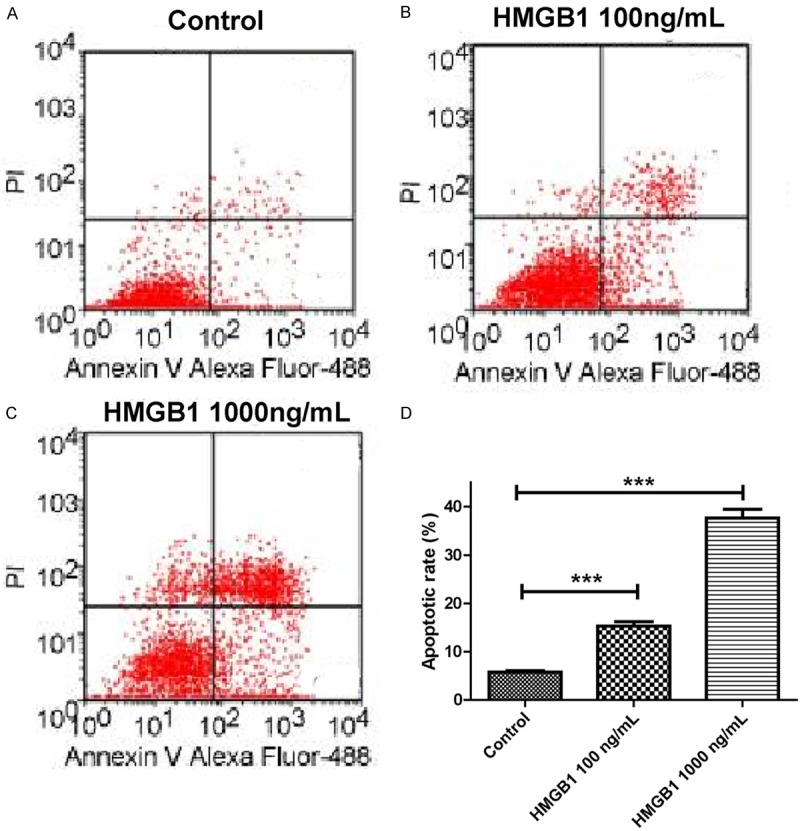
Modulation of apoptosis of RPE cells in high glucose by HMGB1. A. Control group. B. 100 ng/mL HMGB1; C. 1000 ng/mL HMGB1; D. The histogram of the above data.
HMGB1 positively regulated VEGFA protein expression
VEGFA protein expressions were measured by western blotting. In HMGB1 treated ARPE-19 cells in high glucose condition, the VEGFA protein expressions were significantly decreased compared with control group. Besides, higher concentrations (1000 ng/mL HMGB1) of HMGB1 induced a higher level of VEGFA than the lower HMGB1 (100 ng/mL HMGB1). Grayscale analysis showed that VEGFA protein expression was induced after treated with the HMGB1 (Figure 5).
Figure 5.
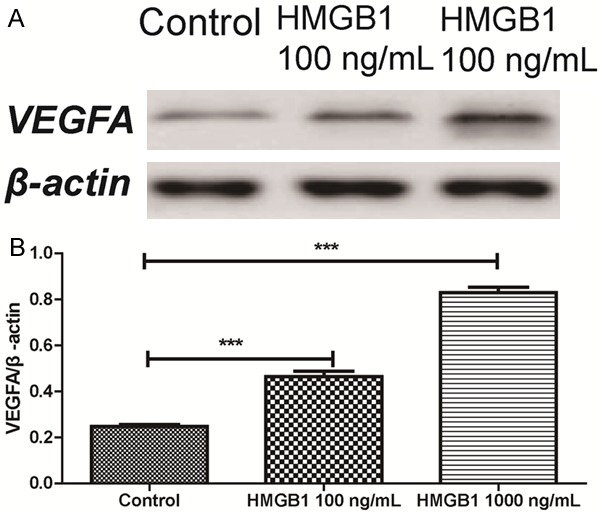
Effect of HMGB1 on the expression of VEGFA on RPE cells in high glucose.
Discussion
In this study, we demonstrated that HMGB1 and VEGFA expressions were upregulated in serum samples of DR patients. Advanced analysis showed that HMGB1 and VEGFA level was positively associated. In the in-vitro study, it was found that up-regulation of HMGB1 inhibited the RPE cell viability and induce the apoptosis. Besides, HMGB1 treatment would up-regulate the expression of VEGFA in the RPE cells in high glucose condition.
Previous studies have demonstrated the abnormal expression of HMGB1 in DR retina and vitreous body. As indicated in a previous study, the expression of HMGB1 is upregulated in epiretinal membranes and vitreous fluid from patients with proliferative diabetic retinopathy and in the diabetic retina [17]. Another study was conducted to determine the levels of the angiogenic and fibrogenic factors osteopontin (OPN), HMGB1, and connective tissue growth factor (CTGF) and the antiangiogenic and antifibrogenic pigment epithelium-derived factor (PEDF) in the vitreous fluid from patients with PDR and rhegmatogenous retinal detachment with no PDR [18]. Diabetes and intravitreal administration of HMGB1 induced significant upregulation of the expression of HMGB1, TBARS, and cleaved caspase-3, whereas the expression of BDNF and synaptophysin was significantly downregulated in rat retinas [19]. An study based on the rat animal showed that mean HMGB1 levels in PDR patients with hemorrhage were significantly higher than those in PDR patients without hemorrhage and nondiabetic patients (P = 0.0111). There were significant correlations between levels of HMGB1 and levels of MCP-1 (r = 0.333, P = 0.025) and sICAM-1 (r = 0.548, P < 0.001). HMGB1 expression was also upregulated in the retinas of diabetic mice [20]. In our study, we found HMGB1 and VEGFA expressions were upregulated in serum samples of DR patients. Advanced analysis showed that HMGB1 and VEGFA level was positively associated. Different from the previous study, we analyzed the data from serum samples of DR cases. Compared with the tissue sample, the data in serum sample showed more significant convenience in the application as biomarkers [21]. However, the application of serum HMGB1 as diagnostic and prognostic biomarkers for DR still needs to be tested by more well-designed and large-sample studies.
HMGB1 plays multiple roles in the regulation of genome replication, recombination, mRNA transcription, and DNA repair. In general, HMGB1 was reported to mediate inflammation, breakdown of the blood-retinal barrier and apoptosis in the diabetic retina [15]. Diabetes and intravitreal injection of HMGB1 in normal rats induced significant upregulation of the mRNA levels of the chemokine stromal cell-derived factor-1 (SDF-1/CXCL12) receptor CXCR4 and protein levels of hypoxia-inducible factor-1α, early growth response-1, tyrosine kinase 2 and the CXCL12/CXCR4 chemokine axis. Constant glycyrrhizin intake from onset of diabetes did not affect the metabolic status of the diabetic rats, but it restored these increased mediators to control values. Besides, HMGB1 is an architectural chromatin-binding protein that can be released actively by activated cells or passively by dying cells and can serve as a DAMP molecule to drive the pathogenesis of inflammatory and angiogenic diseases. Through TLR4 and RAGE signaling pathways, HMGB1 could regulate vascular growth in vivo and in vitro through diverse mechanisms, including induction of proangiogenic cytokine release and activation of ECs, macrophages, EPCs, and mesoangioblasts, all of which could contribute to vessel formation [22]. In our study, we found that HMGB1 could inhibit the RPE cell viability and induce the apoptosis. Advanced study showed that the effect of HMGB1 might though induction of VEGFA.
In conclusion, given the increasing understanding of HMGB1 expression have potent role in the development of DR, it is important to better understand the mechanisms. Here, we have demonstrated that HMGB1 and VEGFA are key players in the ability to suppress cell viability and induce apoptosis. The result of this current experiments shed light into the mechanism by which HMGB1 works. Besides, we also present the data of case control study data, our results showed that HMGB1 might be used as biomarkers of DR.
Disclosure of conflict of interest
None.
References
- 1.Mekonnen D, Gebre-Selassie S, Fantaw S, Hunegnaw A, Mihret A. Prevalence of hepatitis B virus in patients with diabetes mellitus: a comparative cross sectional study at Woldiya General Hospital, Ethiopia. Pan Afr Med J. 2014;17:40. doi: 10.11604/pamj.2014.17.40.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierce BL. Why are diabetics at reduced risk for prostate cancer? A review of the epidemiologic evidence. Urol Oncol. 2012;30:735–743. doi: 10.1016/j.urolonc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Davis TM, Peters KE, Bruce DG, Davis WA. Prevalence, incidence, and prognosis of hepatobiliary disease in community-based patients with type 2 diabetes: the Fremantle Diabetes Study. J Clin Endocrinol Metab. 2012;97:1581–1588. doi: 10.1210/jc.2011-3232. [DOI] [PubMed] [Google Scholar]
- 4.Ramavat PR, Ramavat MR, Ghugare BW, Vaishnav RG, Joshi MU. Prevalence of Diabetic Retinopathy in Western Indian Type 2 Diabetic Population: A Hospital-based Cross-Sectional Study. J Clin Diagn Res. 2013;7:1387–1390. doi: 10.7860/JCDR/2013/5259.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12:346–354. doi: 10.1007/s11892-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 6.Man RE, Sasongko MB, Xie J, Best WJ, Noonan JE, Lo TC, Wang JJ, Luu CD, Lamoureux EL. Decreased retinal capillary flow is not a mediator of the protective myopia-diabetic retinopathy relationship. Invest Ophthalmol Vis Sci. 2014;55:6901–6907. doi: 10.1167/iovs.14-15137. [DOI] [PubMed] [Google Scholar]
- 7.Umahara T, Uchihara T, Koyama S, Hashimoto T, Akimoto J, Haraoka J, Iwamoto T. Local extension of HMGB1 in atherosclerotic lesions of human main cerebral and carotid arteries. Histol Histopathol. 2014;29:235–242. doi: 10.14670/HH-29.235. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SM, Whitehouse A. Kaposi’s sarcoma-associated herpesvirus (KSHV) Rta and cellular HMGB1 proteins synergistically transactivate the KSHV ORF50 promoter. FEBS Lett. 2008;582:3080–3084. doi: 10.1016/j.febslet.2008.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W, Ku S, Yoo H, Song K, Bae J. Andrographolide inhibits HMGB1-induced inflammatory responses in human umbilical vein endothelial cells and in murine polymicrobial sepsis. Acta Physiol (Oxf) 2014;211:176–187. doi: 10.1111/apha.12264. [DOI] [PubMed] [Google Scholar]
- 10.Lee NY, Kang YS. The effects of bisphosphonates on taurine transport in retinal capillary endothelial cells under high glucose conditions. Adv Exp Med Biol. 2013;776:59–66. doi: 10.1007/978-1-4614-6093-0_7. [DOI] [PubMed] [Google Scholar]
- 11.Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M, Abu El-Asrar AM. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp Eye Res. 2013;107:101–109. doi: 10.1016/j.exer.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu L, Huang H, Chen Y. HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem. 2015;405:63–71. doi: 10.1007/s11010-015-2396-0. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Lee SW, Kim HY, Lee WS, Hong KW, Kim CD. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1alpha activation. Eur J Immunol. 2015;45:1216–1227. doi: 10.1002/eji.201444908. [DOI] [PubMed] [Google Scholar]
- 14.Mohammad G, Alam K, Nawaz MI, Siddiquei MM, Mousa A, Abu El-Asrar AM. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015;71:359–72. doi: 10.1007/s13105-015-0416-x. [DOI] [PubMed] [Google Scholar]
- 15.Abu El-Asrar AM, Mohammad G, Nawaz MI, Siddiquei MM. High-Mobility Group Box-1 Modulates the Expression of Inflammatory and Angiogenic Signaling Pathways in Diabetic Retina. Curr Eye Res. 2015;40:1141–52. doi: 10.3109/02713683.2014.982829. [DOI] [PubMed] [Google Scholar]
- 16.Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, Mohammad G. The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm. 2014;2014:746415. doi: 10.1155/2014/746415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu El-Asrar AM, Nawaz MI, Siddiquei MM, Al-Kharashi AS, Kangave D, Mohammad G. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediators Inflamm. 2013;2013:863036. doi: 10.1155/2013/863036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu El-Asrar AM, Nawaz MI, Kangave D, Abouammoh M, Mohammad G. High-mobility group box-1 and endothelial cell angiogenic markers in the vitreous from patients with proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:697489. doi: 10.1155/2012/697489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu El-Asrar AM, Nawaz MI, Siddiquei MM, Al-Kharashi AS, Kangave D, Mohammad G. High-mobility group box-1 induces decreased brain-derived neurotrophic factor-mediated neuroprotection in the diabetic retina. Mediators Inflamm. 2013;2013:863036. doi: 10.1155/2013/863036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, Al-Shabrawey M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–1838. [PMC free article] [PubMed] [Google Scholar]
- 21.Dang NA, Janssen HG, Kolk AH. Rapid diagnosis of TB using GC-MS and chemometrics. Bioanalysis. 2013;5:3079–3097. doi: 10.4155/bio.13.288. [DOI] [PubMed] [Google Scholar]
- 22.Yang S, Xu L, Yang T, Wang F. High-mobility group box-1 and its role in angiogenesis. J Leukoc Biol. 2014;95:563–574. doi: 10.1189/jlb.0713412. [DOI] [PubMed] [Google Scholar]


