Abstract
Aims: This study aims to investigate the effect of 5(S), 6(R)-7-trihydroxymethyl heptanoate (BML-111) on the Wnt5a/frizzled-5 (FZD5)/calcium/calmodulin-dependent protein kinase II (CaMKII) signaling pathway in septic mice, and to explore whether this pathway mediates the effect of BML-111 on inflammatory response in lipopolysaccharide (LPS)-induced RAW 264.7 cells. Methods: The cecal ligation and puncture-induced mouse model of sepsis was constructed, and the mice were pretreated with BML-111. In vitro, LPS-induced RAW 264.7 cells were incubated with various concentrations of BML-111. Activation of Wnt5a/FZD5/CaMKII signaling pathway was achieved by transfection of the Wnt5a overexpression plasmid. The levels of interleukin-1 beta (IL-1β), IL-6 and IL-8 in the mouse serum and cell supernatant were determined by ELISA assay. The expression of Wnt5a, FZD5 and CaMKIIδ was examined by western blot analysis. Results: The results from the in vivo studies revealed that BML-111 shows inhibitory effect on IL-1β, IL-6 and IL-8 expression in the serum of septic mice, and suppresses the expression of Wnt5a, FZD5 and CaMKIIδ protein. The in vitro studies demonstrated that BML-111 inhibits Wnt5a, FZD5 and CaMKIIδ proteins in a dose-dependent manner. BML-111 suppressed the levels of IL-1β, IL-6 and IL-8 in LPS-induced RAW 264.7 cells; however, this effect could be attenuated by transfection of the Wnt5a overexpression plasmid. Conclusion: This study firstly demonstrated that BML-111 suppresses Wnt5a/FZD5/CaMKII signaling pathway in sepsis, and Wnt5a/FZD5/CaMKII signaling pathway mediates the effect of BML-111 on inflammatory reactions. These findings provided a novel molecular basis for the potential effect of BML-111 in sepsis.
Keywords: BML-111, sepsis, Wnt5a/FZD5/CaMKII signaling pathway, cytokines
Introduction
Sepsis is a systemic inflammatory response to infection. It is the leading cause of death in people who have been hospitalized [1,2], and the incidence of sepsis is about 18 million cases per year worldwide [3].
Lipoxin A4 (LXA4) is a mediator of inflammation derived from arachidonic acid [4]. LXA4 binds to formyl peptide receptor 2/LXA4 receptor (FPR2/ALX) [5,6], which is a G protein-coupled receptor, and exerts its anti-inflammatory effects. 5(S), 6(R)-7-trihydroxymethyl heptanoate (BML-111) is a synthetic analogue of LXA4. Numerous studies demonstrated that BML-111 involves in inflammation and modulates the immune response [7,8], and it has been implicated that BML-111 may be a novel therapeutic agent for sepsis [9]. However, the molecular mechanisms underlying the effect of BML-111 on immune response in sepsis is not well understood.
The Wnt pathway is best known for its role in embryogenesis and development [10,11]. Some researchers highlight that the Wnt pathway is also involved in the regulation of inflammation and immunity [12,13]. Wnt5a is a member of the highly conserved wingless family. Liu reported that BML-111 inhibits the expression of toll-like receptor (TLR) 2 and TLR4 in the rat intestine following cecal ligation and puncture (CLP)-induced sepsis [9]. In addition, TLR mediated the response of Wnt5a to microbial stimulation [14,15].
The present study firstly investigated the effect of BML-111 on the Wnt5a/frizzled-5 (FZD5)/calcium/calmodulin-dependent protein kinase II (CaMKII) signaling pathway in septic mice, and explored whether this signaling pathway mediates the effect of BML-111 on inflammatory response in lipopolysaccharide (LPS)-induced RAW 264.7 cells.
Materials and methods
Experimental model of sepsis
Cecal ligation and puncture (CLP) induced sepsis model was established as described previously [16]. The animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Sichuan Medical University and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication no. 86-23, revised 1986). The mice were purchased from Shanghai Slac Laboratory Animal Company (Shanghai, China), and anaesthetized by injection of sodium pentobarbital (45 mg/kg) intraperitoneally. Following a midline abdominal incision, the cecum was exposed and punctured using the 18G needle (BD, USA). After that, the animals received 1 ml of saline subcutaneously, and were placed in a metabolic cage. Animals in the sham group underwent the same procedure without CLP. Animals in the treatment group received 1 mg/kg BML-111 (Cayman Chemical Company, Ann Arbor, Michigan, USA) intraperitoneally after rehydration. 24 h following CLP, the blood samples were collected and stored at -20°C for centrifugation.
Cell culture and cell transfection
The RAW 264.7 cells were purchased from American Type Culture Collection (Manassas, VA, USA), and cultured in DMEM (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (Invitrogen Life Technologies) at 37°C in 5% CO2. LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA), and dissolved in phosphate-buffer saline (PBS) to a final concentration of 2 μg/ml. Cells were treated with LPS for 12 h. BML-111 was diluted in PBS to the indicated final concentrations. Wnt5a-pcDNA3.1 plasmid was transfected into the RAW 264.7 cells using Lipofectamine™ 2000 (Invitrogen Life Technologies) according to the manufacturer’s instructions.
Measurement of cytokines
ELISA kits for mouse IL-1β, IL-6 were purchased from R&D Systems (Minneapolis, MN, USA), and IL-8 ELISA kit was from Cusabio (Wuhan, Hubei, China). Mouse IL-1β, IL-6 and IL-8 in the serum and culture supernatants were measured by the ELISA kits according to the manufacturer’s instructions.
Western blot analysis
Proteins in the serum and cells were extracted using Total Protein Extraction Kit (KeyGEN BioTECH, Nanjing, Jiangsu, China). Protein samples were separated by SDS-PAGE and were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). All the antibodies used were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). After blocking in 5% non-fat milk, the PVDF membranes were incubated overnight at 4°C with the specific antibodies, including goat polyclonal to Wnt5a (1:800), goat polyclonal to FZD5 (1:400), goat polyclonal to CaMKIIδ (1:800), rabbit polyclonal to β-actin (1:2000), followed by incubation with donkey anti-goat IgG-HRP or goat anti-rabbit IgG-HRP for 2 h at 37°C. A β-actin antibody was used to confirm equal loading. The blots were detected using an ECL western blotting kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Statistical analysis
All the data were analyzed by SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). The statistically significant difference between 2 groups was determined by Student’s t-test. A p-value less than 0.05 was considered significant.
Results
Effect of BML-111 on the expression of IL-1β, IL-6 and IL-8 in the serum of mice with sepsis
A CLP-induced sepsis model was established to investigate the effect on BML-111 on IL-1β, IL-6 and IL-8 expression. Compared with the sham group, the levels of IL-1β, IL-6 and IL-8 were significantly increased in the sepsis group. However, BML-111 showed inhibitory effect on IL-1β, IL-6 and IL-8 expression of mice with sepsis (Figure 1).
Figure 1.
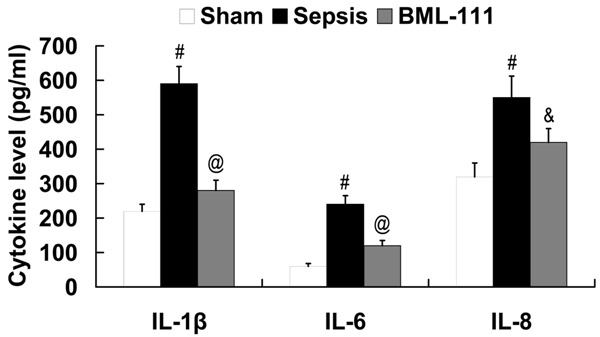
Effect of BML-111 on IL-1β, IL-6 and IL-8 expression in the serum of mice with sepsis. #P<0.01 compared with sham; &P<0.05, @P<0.01 compared with sepsis. BML-111, 5(S), 6(R)-7-trihydroxymethyl heptanoate; IL, interleukin.
Effect of BML-111 on the expression of Wnt5a, FZD5 and CaMKIIδ in the serum of mice with sepsis
To investigate the effect of BML-111 on Wnt5a/FZD5/CaMKII signaling in septic mice, the expression of Wnt5a, FZD5 and CaMKIIδ in the serum was examined by western blot analysis. As shown in Figure 2, Wnt5a/FZD5/CaMKII signaling was activated in septic mice, as the relative protein levels of Wnt5a, FZD5 and CaMKIIδ were upregulated in the sepsis group compared with the sham group. Treatment with BML-111 significantly downregulated Wnt5a, FZD5 and CaMKIIδ expression in the serum of mice with sepsis.
Figure 2.
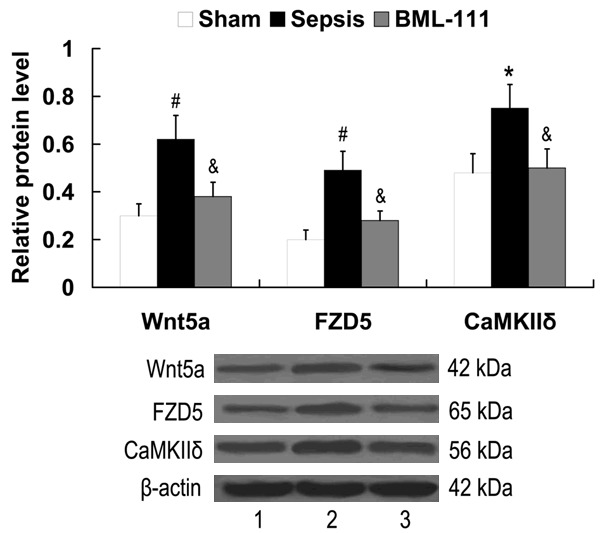
Effect of BML-111 on Wnt5a/FZD5/CaMKII signaling in septic mice. Lane 1, sham; lane 2, sepsis; lane 3, BML-111. #P<0.01, *P<0.05 compared with sham; &P<0.05 compared with sepsis. BML-111, 5(S), 6(R)-7-trihydroxymethyl heptanoate; FZD5, frizzled-5; CaMKIIδ, calcium/calmodulin-dependent protein kinase II delta.
Effect of BML-111 on the expression of Wnt5a, FZD5 and CaMKIIδ in LPS-stimulated RAW 264.7 cells
LPS-stimulated RAW 264.7 cells were treated with various concentrations of BML-111 for 24 h, the expression of Wnt5a, FZD5 and CaMKIIδ was then examined by western blot analysis. The results showed that compared with the control, the expression of Wnt5a, FZD5 and CaMKIIδ proteins were induced by LPS. BML-111 inhibited the expression of Wnt5a, FZD5 and CaMKIIδ proteins in a dose-dependent manner in LPS-stimulated RAW 264.7 cells, with the maximum effect at the concentration of 100 ng/ml (Figure 3).
Figure 3.
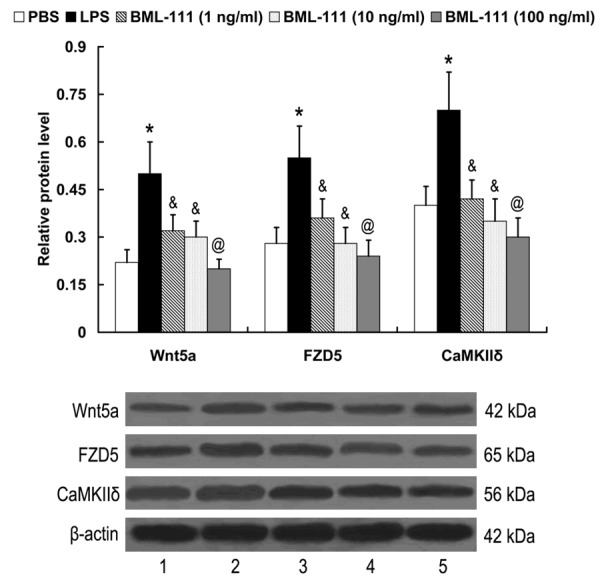
Effect of BML-111 on Wnt5a, FZD5 and CaMKIIδ expression in LPS-stimulated RAW 264.7 cells. Lane 1, PBS; lane 2, LPS; lane 3, BML-111 (1 ng/ml); lane 4, BML-111 (10 ng/ml); lane 5, BML-111 (100 ng/ml). *P<0.05 compared with PBS; &P<0.05, @P<0.01 compared with LPS. LPS, lipopolysaccharide; BML-111, 5(S), 6(R)-7-trihydroxymethyl heptanoate; FZD5, frizzled-5; CaMKIIδ, calcium/calmodulin-dependent protein kinase II delta.
Wnt5a/FZD5/CaMKII signaling mediates the effect of BML-111 on IL-1β, IL-6 and IL-8 expression in LPS-stimulated RAW 264.7 cells
To investigate the role of Wnt5a/FZD5/CaMKII signaling in mediating the effect of BML-111 on IL-1β, IL-6 and IL-8 expression, Wnt5a-pcDNA3.1 plasmid was transfected into the LPS-stimulated RAW 264.7 cells, and the cells were treated with 100 ng/ml BML-111 for 24 h. As demonstrated in Figure 4, BML-111 significantly downregulated Wnt5a, FZD5 and CaMKIIδ expression in LPS-stimulated RAW 264.7 cells. However, the expression of Wnt5a, FZD5 and CaMKIIδ proteins was significantly upregulated in the cells transfected with the Wnt5a-pcDNA3.1 plasmid.
Figure 4.
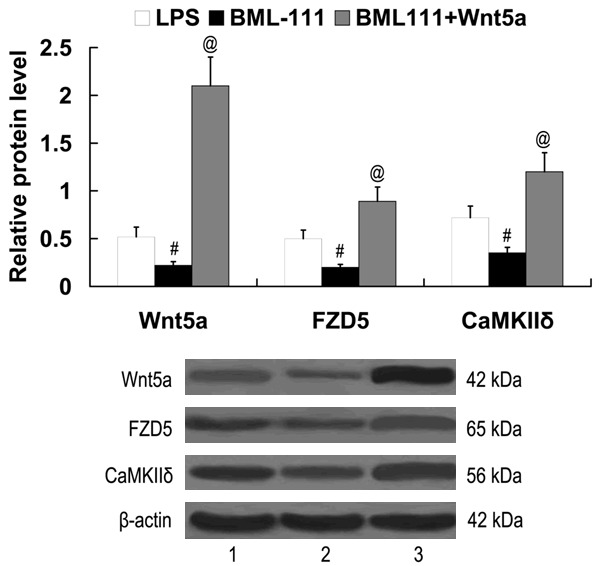
Expression of Wnt5a, FZD5 and CaMKIIδ in LPS-stimulated RAW 264.7 cells following treatment with BML-111 and transfection of Wnt5a-pcDNA3.1. Lane 1, LPS; lane 2, BML-111; lane 3, BML-111+Wnt5a. #P<0.01 compared with LPS; @P<0.01 compared with BML-111. LPS, lipopolysaccharide; BML-111, 5(S), 6(R)-7-trihydroxymethyl heptanoate; FZD5, frizzled-5; CaMKIIδ, calcium/calmodulin-dependent protein kinase II delta.
As shown in Figure 5, LPS induced the expression of IL-1β, IL-6 and IL-8 in RAW 264.7 cells. Furthermore, we found that the expression of IL-1β, IL-6 and IL-8 was significantly decreased in the LPS-stimulated RAW 264.7 cells by the treatment of BML-111; however, Wnt5a/FZD5/CaMKII signaling activation attenuated the effect of BML-111 on IL-1β, IL-6 and IL-8 expression.
Figure 5.
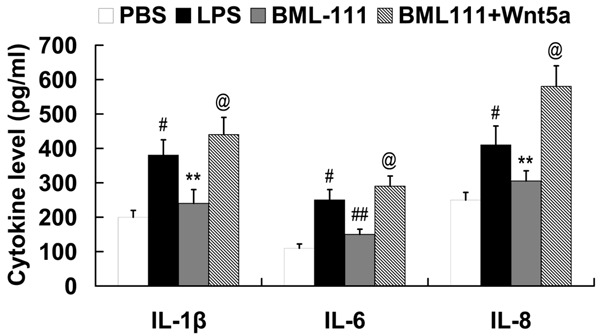
Wnt5a/FZD5/CaMKII signaling mediates the effect of BML-111 on IL-1β, IL-6 and IL-8 expression in LPS-stimulated RAW 264.7 cells. #P<0.01 compared with PBS; **P<0.01, ##P<0.01 compared with LPS; @P<0.01 compared with BML-111. LPS, lipopolysaccharide; BML-111, 5(S), 6(R)-7-trihydroxymethyl heptanoate; FZD5, frizzled-5; CaMKIIδ, calcium/calmodulin-dependent protein kinase II delta; IL, interleukin.
Discussion
BML-111 has been shown to regulate cytokines in some pathological processes. Wang et al reported that BML-111 shows anti-inflammatory effects on acute pancreatitis-associated lung injury induced by cerulein and LPS in mice. The serum levels of amylase, TNF-α, IL-1β were reduced, and the serum levels of IL-10 were markedly increased by BML-111 pretreatment [17]. In endotoxin exposed rats, BML-111 effectively alleviated morphologic damage of placenta and kidney, serum TNF-α and IL-8 levels, and LPS-reduced proliferation [18]. BML-111 also suppressed airway inflammation of ovalbumin-immunized mice. The levels of IL-1β, IL-4 and IL-8 in serum and bronchoalveolar lavage fluid were suppressed by BML-111 [19]. Recently, Liu et al suggested that BML-111 exerted protective effects on the intestinal mucosal barrier in sepsis by modulating cell apoptosis, upregulating rat defensin-5 mRNA expression levels, as well as regulating the expression of pro-inflammatory and anti-inflammatory cytokine in the intestine, and it may be a novel therapeutic agent for sepsis [9]. In the present study, we examined the expression of cytokines in the serum of mice with sepsis. The results demonstrated that BML-111 also shows anti-inflammatory effects on systemic immune response of mice with sepsis.
IL-1β, IL-6 and IL-8 are important pro-inflammatory cytokines that play vital roles in the pathogenesis of inflammatory [20-25]. LPS is the principal stimulator of inflammatory reactions [26,27]. Consistent with the results from our in vivo studies, the in vitro results revealed that BML-111 exerts anti-inflammatory effects in LPS-stimulated RAW 264.7 cells.
Some pathways have been implicated to be related to the action of BML-111, such as nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant responsive element (ARE) signaling pathway, mitogen-activated protein kinase (MAPK)/activator protein-1 signaling pathway [28-30]. Nevertheless, the molecular mechanisms involved in the function of BML-111 in sepsis and LPS-induced inflammatory reactions have not yet been clearly identified. The Wnt pathway plays a distinct role in inflammation and immunity [12,13]. It has been demonstrated that Wnt5a level is higher in patients with sepsis than in healthy individuals [31]. Wnt5a is induced in infectious and inflammatory diseases, and contributes to innate and adaptive immune responses by ligation to its receptor FZD5, which is independent of the canonical Wnt signaling pathway [32]. Pereira et al suggested that Wnt5a/FZD5/CaMKII signaling pathway was essential for inflammatory macrophage activation [33]. In this study, we investigated the effect of BML-111 on the expression of Wnt5a, FZD5 and CaMKIIδ. Both the in vivo and in vitro studies revealed that BML-111 shows inhibitory effect on Wnt5a/FZD5/CaMKII signaling pathway. Furthermore, the in vitro studies elucidated that Wnt5a/FZD5/CaMKII signaling pathway mediated the effect of BML-111 on inflammatory reactions.
In conclusion, this study firstly demonstrated that BML-111 suppresses Wnt5a/FZD5/CaMKII signaling pathway in sepsis, and Wnt5a/FZD5/CaMKII signaling pathway mediates the effect of BML-111 on inflammatory reactions. This study further expands our knowledge about the action of BML-111 in sepsis and provided a novel mechanism underlying its function in inflammation and immunity.
Disclosure of conflict of interest
None.
References
- 1.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyle NH, Pena OM, Boyd JH, Hancock RE. Barriers to the effective treatment of sepsis: antimicrobial agents, sepsis definitions, and host-directed therapies. Ann N Y Acad Sci. 2014;1323:101–114. doi: 10.1111/nyas.12444. [DOI] [PubMed] [Google Scholar]
- 4.Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:570–579. doi: 10.1038/ncprheum0616. [DOI] [PubMed] [Google Scholar]
- 5.Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 6.Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sordi R, Menezes-de-Lima O Jr, Horewicz V, Scheschowitsch K, Santos LF, Assreuy J. Dual role of lipoxin A4 in pneumosepsis pathogenesis. Int Immunopharmacol. 2013;17:283–292. doi: 10.1016/j.intimp.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, Li Y, Ye D, Chen B, Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflamm Res. 2008;57:157–162. doi: 10.1007/s00011-007-7141-z. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Liu Z, Zhao S, Sun C, Yang M. Effect of BML‑111 on the intestinal mucosal barrier in sepsis and its mechanism of action. Mol Med Rep. 2015;12:3101–3106. doi: 10.3892/mmr.2015.3746. [DOI] [PubMed] [Google Scholar]
- 10.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Ann Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 11.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 12.George SJ. Wnt pathway: A new role in regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 13.Pereira CP, Bachli EB, Schoedon G. The wnt pathway: A macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep. 2009;11:236–242. doi: 10.1007/s11883-009-0036-4. [DOI] [PubMed] [Google Scholar]
- 14.Gordon MD, Dionne MS, Schneider DS, Nusse R. WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature. 2005;437:746–749. doi: 10.1038/nature04073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 16.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock-a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang YZ, Zhang YC, Cheng JS, Ni Q, Li PJ, Wang SW, Han W, Zhang YL. BML-111, a lipoxin receptor agonist, ameliorates ‘two-hit’-induced acute pancreatitis-associated lung injury in mice by the upregulation of heme oxygenase-1. Artif Cells Nanomed Biotechnol. 2014;42:110–120. doi: 10.3109/21691401.2013.794355. [DOI] [PubMed] [Google Scholar]
- 18.Lin F, Zeng P, Xu Z, Ye D, Yu X, Wang N, Tang J, Zhou Y, Huang Y. Treatment of Lipoxin A(4) and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reprod Toxicol. 2012;34:677–685. doi: 10.1016/j.reprotox.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Kong X, Wu SH, Zhang L, Chen XQ. Roles of lipoxin A4 receptor activation and anti-interleukin-1β antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-κB pathway in airway inflammation induced by ovalbumin. Mol Med Rep. 2015;12:895–904. doi: 10.3892/mmr.2015.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarrett OD, Brady KE, Modur SP, Plants J, Landay AL, Ghassemi M, Golub ET, Spear GT, Novak RM. T. vaginalis Infection Is Associated with Increased IL-8 and TNFr1 Levels but with the Absence of CD38 and HLADR Activation in the Cervix of ESN. PLoS One. 2015;10:e0130146. doi: 10.1371/journal.pone.0130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palus M, Formanová P, Salát J, Žampachová E, Elsterová J, Růžek D. Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: identification of novel inflammatory markers with implications for pathogenesis. J Med Virol. 2015;87:885–892. doi: 10.1002/jmv.24140. [DOI] [PubMed] [Google Scholar]
- 22.Keyel PA. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine. 2014;69:136–145. doi: 10.1016/j.cyto.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhao R, Zhou H, Su SB. A critical role for interleukin-1β in the progression of autoimmune diseases. Int Immunopharmacol. 2013;17:658–669. doi: 10.1016/j.intimp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Lesina M, Wörmann SM, Neuhöfer P, Song L, Algül H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol. 2014;26:80–87. doi: 10.1016/j.smim.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12. doi: 10.1016/j.smim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unuma K, Aki T, Funakoshi T, Yoshida K, Uemura K. Cobalt protoporphyrin accelerates TFEB activation and lysosome reformation during LPS-induced septic insults in the rat heart. PLoS One. 2013;8:e56526. doi: 10.1371/journal.pone.0056526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YZ, Zhang YC, Cheng JS, Ni Q, Li PW, Han W, Zhang YL. Protective effects of BML-111 on cerulein-induced acute pancreatitis- associated lung injury via activation of Nrf2/ARE signaling pathway. Inflammation. 2014;37:1120–1133. doi: 10.1007/s10753-014-9836-y. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Wu Z, Feng D, Gong J, Yao C, Wang Y, Yuan S, Yao S, Shang Y. BML-111, a lipoxin receptor agonist, attenuates ventilator-induced lung injury in rats. Shock. 2014;41:311–316. doi: 10.1097/SHK.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 30.Li HB, Wang GZ, Gong J, Wu ZY, Guo S, Li B, Liu M, Ji YD, Tang M, Yuan SY, Shang Y, Yao SL. BML-111 attenuates hemorrhagic shock-induced acute lung injury through inhibiting activation of mitogen-activated protein kinase pathway in rats. J Surg Res. 2013;183:710–719. doi: 10.1016/j.jss.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Schulte DM, Kragelund D, Müller N, Hagen I, Elke G, Titz A, Schädler D, Schumacher J, Weiler N, Bewig B, Schreiber S, Laudes M. The wingless-related integration site-5a/secreted frizzled-related protein-5 system is dysregulated in human sepsis. Clin Exp Immunol. 2015;180:90–97. doi: 10.1111/cei.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong S, Wu C, Hu J, Wang Q, Chen S, Wang ZR, Xiong W. Wnt5a Promotes Cytokines Production and Cell Proliferation in Human Hepatic Stellate Cells Independent of Canonical Wnt Pathway. Clin Lab. 2015;61:537–547. doi: 10.7754/clin.lab.2014.141127. [DOI] [PubMed] [Google Scholar]
- 33.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]


