Abstract
Aim: To investigate the associations between the expressions of three lysophosphatidic acid (LPA) receptors (LPA1-3) and the development of ovarian carcinoma (OC). Method: Ovarian tissue specimens, including normal ovarian epithelium tissues, benign ovarian tumor tissues and OC tissues were collected from patients who underwent surgical resections between March 2012 and December 2014. Immunohistochemical staining was used to detect LPA receptor expressions in ovarian tissues. Reverse transcription-polymerase chain reaction and Western blotting were used to detect mRNA and protein expression of LPA receptors, respectively. Association analysis between LPA receptors protein expression and clinical pathological characteristics was conducted. The value of LPA2 and LPA3 in discriminating OC was confirmed by receiver-operator characteristic (ROC) curves analysis. Results: The positive expression rates of LPA2 and LPA3 in OC group was obviously higher than normal control and benign groups. The LPA2 and LPA3 mRNA and protein levels in OC group were higher than in normal control and benign groups. LPA2 and LPA3 mRNA expression levels were positively correlated with LPA2 and LPA3 protein expression in OC group. ROC curve analysis revealed that LPA2 yield a specificity of 96.3% and a sensitivity of 97.9%, and LPA3 yield a specificity of 98.5% and a sensitivity of 97.9% for the detection of OC. Conclusion: LPA2 and LPA3 were highly expressed in OC tissues, which may be involved in the development of OC. Further, LPA2 and LPA3 had higher sensitivity and specificity in distinguishing the OC from benign ovarian tumors, which could be potential diagnostic indictors in OC.
Keywords: Ovarian carcinoma, lysophosphatidic acid receptors, pathological stages, pathological grades, pathological types, immunohistochemical staining, reverse transcription-polymerase chain reaction, ROC curve analysis
Introduction
Ovarian carcinoma (OC) is regarded as one of the most lethal tumors of the female reproductive organ and remains the fifth major cause of death related to gynecologic malignancy all around the world [1,2]. However, the mortality rate in epithelial OC, the most frequent type of OC, is at the highest rate among all types of gynecological tumors [3,4]. It has been reported that OC affects 22,240 women each year and approximately 14,000 women died of this disease in 2013 according to Surveillance, Epidemiology and End Results data [5]. The prognosis in OC may easily be the poorest among gynecological cancer with overall 5-year survival rate at 45%, steeply dropping down to 20-25% for stages III and IV due to lack of effective therapies for advance-stage OC [6,7]. Although the etiology of OC is not clearly identified, the development of OC may be caused by the interaction of environmental risk factors and genetic factors [8-10]. Further, greater lifetime ovulations, low parity, nulliparity, nulligravity, infertility, early menarche and late menopause appear to be leading risk factors for OC [11]. Additionally, evidence have showed that breast cancer susceptibility genes significantly enhance the lifetime risk of OC to 27%-44%, and the age and onset of OC is significantly earlier in women carrying breast cancer susceptibility gene mutations [11,12]. In recent years, various studies have focused on the serum biomarkers which could accurately identify in early stage of OC to improve the diagnostic accuracy of OC diagnosis [8,13,14].
Lysophosphatidic acid (LPA) is a water-soluble phospholipid signaling molecule, which has gained much attention in recent years for its wide-ranging effects in different target tissues [15,16]. LPA is a multifunctional lipid mediator known for its ability to stimulate cell proliferation, cell migration and survival, smooth muscle cell contraction, platelet aggregation and tumor cell invasion [17,18]. Evidence has revealed that LPA may be implicated in cell proliferation in various carcinoma cell lines, including OC and prostate cancer cells [19,20]. LPA is found at relatively low concentrations in plasma but higher concentrations are seen in ascites fluid from OC patients [21]. In addition, LPA also plays an important role in metastatic capacity and reduced susceptibility to apoptosis in OC cell lines treated with cisplatin [7]. Recent studies suggested that LPA is produced by malignant ovarian epithelium and exerts its influence by interacting with G-protein-coupled receptors, including all six LPA receptors (LPA1-6) [13,22]. Importantly, LPA binding to LPA receptors lead to downstream signaling leading to cell proliferation, differentiation, migration and morphogenesis [14]. These LPA receptors themselves may have different biological mechanisms that are context and tissue dependent, since LPA receptor expression and tissue distribution is diverse [23]. Aberrant expressions of LPA receptors (LPA1, LPA2 and LPA3) have been found in human ovarian tumors, and the LPA1 is mainly expressed in normal ovarian tissues, whereas LPA2 and LPA3 show high expression in OC tissues [24,25]. The underlying mechanism of the LPA receptors on the development of OC is still unclear; therefore, our study is aimed to investigate the expression levels of LPA (1-3) receptors in ovari an tissues to better understand its clinical significance related to the origin and progression of OC.
Materials and methods
Ethics statement
The study was carried out with the approval of the Institutional Review Board of Southern Medical University. Study subjects were enrolled in this study and tissue samples were collected after obtaining informed written consent. All the study procedures were in line with the Declaration of Helsinki [26].
Study subjects and tissue samples
Human ovarian tissue specimens were collected from patients who underwent surgical resection at the Department of Gynecology and Obsterics, Zhujiang Hospital of Southern Medical University between March 2012 and December 2014. All specimens were classified by two experienced pathologists based on histopathological evaluation. Fifty samples were confirmed as normal ovarian epithelium tissues obtained from hysteromyoma patients who underwent total hysterectomy with bilateral salpingo-oophorectomy, and were assigned as normal group. The average age of the patients in the normal group was 52.1 ± 7.3 years, ranging from 39 to 65 years. Forty-eight benign ovarian tumor tissues were assigned as benign group. The average age of patients in the benign group was 51.5 ± 10.4 years, ranging from 34 to 72 years. Totally 134 samples were confirmed as epithelial OC tissues and were assigned as OC group. The average age of patients in the OC group was 52.3 ± 9.5 years, ranging from 38 to 69 years. No significant differences were observed on the age among the three groups. The patients with complete clinical data and without any history of receiving radiotherapy and chemotherapy before operation were included in this study. Patients with OC were excluded if they had the following diseases: (1) other malignant neoplasms; (2) pelvic inflammatory diseases; (3) thrombotic diseases; and (4) diabetes, hypertension and coronary heart disease. All specimens were fresh tissue specimens that taken from surgical resections, avoiding the necrotic area and adipose tissues, and all specimens were frozen in liquid nitrogen and stored at -80°C for reserve. Of the 134 OC samples, 68 cases were confirmed as ovarian serous cystadenocarcinoma, 44 cases were ovarian mucinous cystadenocarcinoma, 12 cases were ovarian clear cell carcinoma and 10 cases were endometrioid ovarian carcinoma. On the basis of OC staging classification, 10 samples were in stage I, 34 in stage II, 84 in stage III and 6 in stage IV. Based on OC histological classification, 18 samples were in G1, 50 in G2 and 66 in G3. Both OC staging classification and OC histological classification are based on the International Federation of Gynaecology and Obstetrics (FIGO) criteria [27].
Immunohistochemical (IHC) staining
IHC staining was performed on sections from a selected block of each specimen. Sections were shaking in phosphate buffer saline (PBS) supplemented with 10% bovine serum albumin (BSA). Endogenous peroxides were eliminated with 3% hydrogen peroxide (H2O2) for 15 min. Sections were blocked with 10% normal goat serum and incubated for 10 min at room temperature. Subsequently, the sections were incubated in rabbit anti-human LPA1 polyclonal antibody (Chemicon), rabbit anti-human LPA2 polyclonal antibody (Chemicon) and rabbit anti-human LPA3 polyclonal antibody (Chemicon) for 1 hour at room temperature, rinsed (three times for 3~5 min each) in PBS. The sections were then incubated with a second biotinylated-conjugated anti-rabbit secondary antibody for 20 min at room temperature, and washed three times in PBS for 3~5 min each. After incubation with streptavidin-horseradish peroxidase (HRP) for 20 min at room temperature and washed three times in PBS for 3~5 min each, 3,3’-diaminobenzidine (DAB) color liquid was added and the sections were observed under the microscope to appropriately terminate the reaction. Finally, the sections were rinsed with tap water, counterstained using hematoxylin, dehydrated by gradient ethanol and mounted with neutral gum. IHC Streptavidin-Perosidase (SP) kit and DAB kit were purchased from Shanghai-Tian Cheng (Shanghai, China).
The protein expressions of LPAs were observed under the light microscope. Brownish yellow staining of cytoplasm or cytomembrane was recorded positive [28]. One-hundred cells were calculated from each 10 arbitrarily selected high-power fields. The proportion of positive cells was counted in 10 high-power fields and the mean values were calculated. The proportion score described the estimated fraction of positively stained cells (0, no visible reaction, < 5%; 1, 6%-25%; 2, 26%-50%; 3, 51%-75%; 4, > 75% of positive cells stained). The intensity score represented the estimated staining intensity (0, no staining; 1, light yellow; 2, yellow; 3, brownish yellow). Evaluation score of the reactive cells was calculated by the intensity score and its proportion score. Based on evaluation score, the expression level of LPA was classified into: 0 point, negative (-); 1~4 points, weakly positive (+); 5~8 points, moderate positive (++); 9~12 points, strongly positive (+++). The “+”, “++”, “+++” were regarded as positive signals with observable increase in staining intensity.
RT-PCR for LPA (1-3) receptors
Tissue samples were immersed in Trizol (100 mg/mL) (Sino-American Biotechnology., Ltd.) and then pulverized with a mortar and pestle under ice-bath. Tissue samples were maintained in 1.5 mL EP tube for 5~10 min for the extraction of total RNA. The integrity of RNA was identified by 1.5% agarose gel electrophoresis. The absorbance (OD value) at 260 nm and 280 nm were read with ultraviolet (UV) spectrophotometer for measuring the purity and concentration of total RNA. The transcriptional levels of LPA1, LPA2 and LPA3 were detected by applying reverse transcription polymerase chain reaction (RT-PCR). The β-actin was used as an endogenous reference for LPA1, LPA2 and LPA3. All primers were synthetized at Sangon Biotech Co., Ltd. (Shanghai, China), as listed in Table 1. The total volume of PCR reaction was 30 μL. The amplification conditions of LPA1 and LPA3 were as follows: an initial denaturation at 94°C for 5 min, and 35 cycles of denaturation at 95°C for 15 s, followed by annealing at 57°C for 1 min and extension steps at 72°C for 30 s; and with 1 cycle of final extension at 72°C for 7 min. The PCR procedures of LPA2 were as follows: an initial denaturation at 94°C for 5 min, and 35 cycles of denaturation at 95°C for 15 s, followed by annealing at 58°C for 60 s and extension steps at 72°C for 30 s; and with 1 cycle of final extension at 72°C for 7 min. The PCR amplification products (5.5 μL) mixed with 2.5 μL blue bromine phenol loading buffer and were run on 1.5% agarose gel electrophoresis (80 V, 40 min) with proper DNA size markers. Gel images were scanned with Gel Analyzer (Biosure, Greece). The specific PCR bands on the gel were positive. Semi-quantitative RT-PCR analysis was used to measure relative expression of LPA receptors which are expressed as the ratio of the band intensity of the target PCR product to that of β-actin.
Table 1.
Primers sequences for LPA1, LPA2, LPA3 and β-actin
| Genes | Primers sequences | Product length (bp) | |
|---|---|---|---|
| LPA1 | Forward: | 5’-ATCGGGATACCATGATGAGTC-3’ | 342 |
| Reward: | 5’-TCCGTTCTAAACCACAGAGTG-3’ | ||
| LPA2 | Forward: | 5’-GCTACCGAGAGACCACGCTC-3’ | 299 |
| Reward: | 5’-CTGGGCAGAGGATGTATAGTG-3’ | ||
| LPA3 | Forward: | 5’-ACACCCATGAAGCTAATGAAG-3’ | 379 |
| Reward: | 5’-AGGCATCCAGAGTTTAGGAAG-3 | ||
| β-actin | Forward: | 5’-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3’ | 838 |
| Reward: | 5’-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3’ |
LPA: lysophosphatidic acid; β-actin: endogenous reference.
Detection of the LPA protein expression by Western blotting
Western blotting analysis was used to detect LPA protein levels. Tissues were lysed in protein extraction buffer (100 mg/500 μL) and the quantitative verification of proteins was estimated by Coomassie blue staining method. Total protein (50 μg) was separated by 12% so-dium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using constant voltage, followed by protein transfer to polyvinylidene fluoride (PVDF) membrane (Millipore, Belfor, MA, USA). Membranes were blocked with 5% non-fat dry milk for 1 hour at 37°C and subsequently, incubated with monoclonal antibodies of LPA1, LPA2 and LPA3 (1:1000; ZSGB-BIO, Beijing, China) in the above solution on an orbital shaker at 4°C overnight. Following primary antibody incubations, membranes were incubated with horseradish peroxidase-linked secondary antibodies (1:4000; ZSGB-BIO, Beijing, China) for 1 hour at room temperature, and visualized using DAB staining. The β-actin was used as an endogenous reference. The specific PCR bands on the membranes were positive. Each band was scanned by an image scanner (Uniscan A600, China) and the OD value of each band was detected by a quantitative image analysis system (Image ProPlus 6.0, USA). The relative expression of LPA receptors are expressed as the ratio of the OD value of the target PCR product to that of β-actin.
Statistics analysis
Data was analyzed using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). Quantitative data was presented with mean ± standard deviation (SD). Comparisons between two groups were made using t test and comparison among groups were made by One-Way ANOVA. Categorical data were expressed as numbers and percentages and were analyzed by χ2 test or Fisher’s exact probability test. Pearson’s linear correlation analysis was used to test the mRNA and protein expression of LPA. The diagnostic value of LPA1, LPA2 and LPA3 in discriminating OC was confirmed by receiver-operator characteristic (ROC) curves analysis. A P value of < 0.05 was considered as statistically significant.
Results
LPA receptors expression detected by IHC staining
The IHC staining results showed that the cytoplasm or cytomembrane of LPA1, LPA2 and LPA3 protein were brownish yellow staining and recorded positive, and the proportion score and intensity score of the OC cells were significantly higher than those in normal ovarian epithelium cells and benign ovarian tumor cells (Figure 1). There were no significant differences on the positive expression rate of LPA1 among the three groups (both P > 0.05). However, the positive expression rate of LPA2 in OC group was obviously higher than that in normal control group and benign group (both P < 0.05). Similar association was also observed on LPA3 protein (both P < 0.05). Further, no significant differences were observed on the positive expression rates of LPA2 and LPA3 between the benign group and normal control group (P > 0.05) (Table 2).
Figure 1.
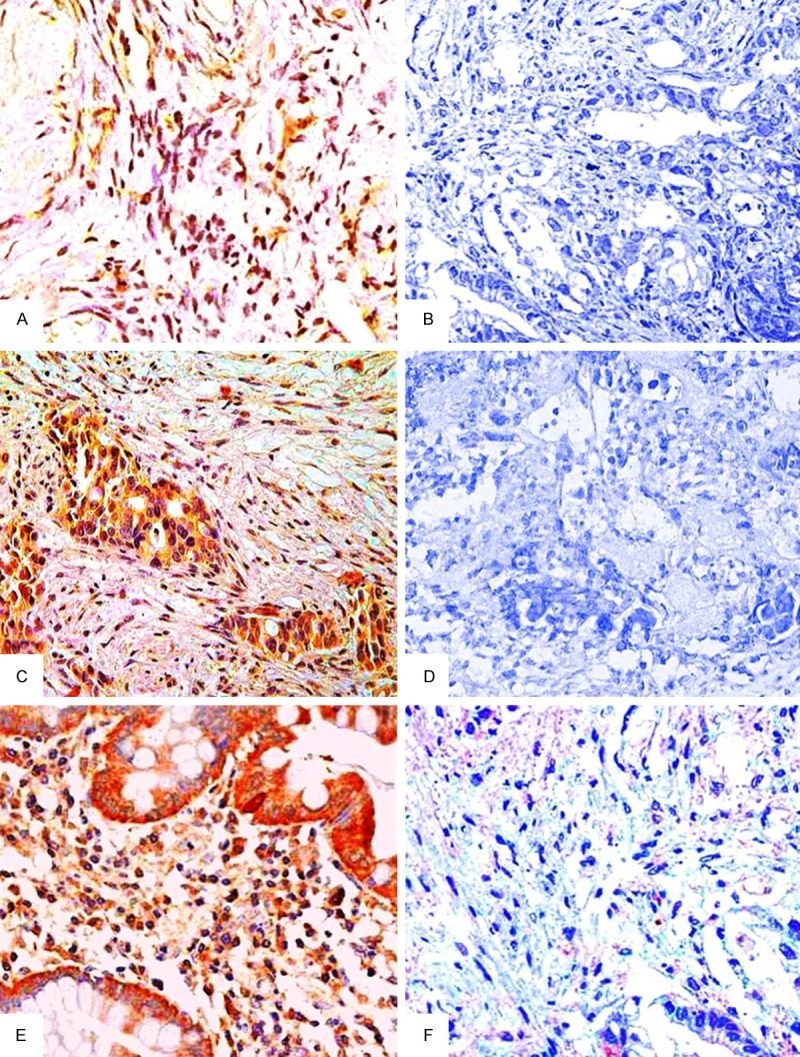
The expressions of LPA receptors in normal ovarian epithelium tissues, benign ovarian tumor tissues and epithelial ovarian carcinoma tissues by IHC staining (SP × 40). Note: A. Positive expression of LPA1 in benign ovarian tumor tissues; B. Negative expression of LPA1 in epithelial ovarian carcinoma tissues; C. Positive expression of LPA2 in epithelial ovarian carcinoma tissues; D. Negative expression of LPA2 in benign ovarian tumor tissues; E. Positive expression of LPA3 in epithelial ovarian carcinoma tissues; F. Negative expression of LPA3 in benign ovarian tumor tissues. LPA: lysophosphatidic acid; IHC staining: immunohistochemical staining.
Table 2.
Expression of LPA1, LPA2 and LPA3 protein in normal ovarian epithelium, benign ovarian tumor and epithelial ovarian carcinoma
| Groups | LPA1 | LPA2 | LPA3 |
|---|---|---|---|
| Normal control group (n = 50) | 49 (98.0%) | 16 (32.0%) | 20 (40.0%) |
| Benign group (n = 48) | 46 (95.8%) | 15 (31.3%) | 20 (41.7%) |
| OC group (n = 134) | 119 (88.8%) | 121 (90.3%)a,b | 119 (88.8%)a,b |
| χ2 | 5.392 | 86.240 | 60.480 |
| P | 0.068 | < 0.001 | < 0.001 |
LPA: lysophosphatidic acid; OC: ovarian carcinoma; Note:
compared with the normal control group, P < 0.05;
compared with the benign group, P < 0.05.
mRNA expression of LPA receptors detected by RT-PCR
The mRNA expression levels of LPA receptors in normal ovarian epithelium tissues, benign ovarian tumor tissues and epithelial OC tissues are shown in Figure 2. RT-PCR results showed that there were no significant differences on LPA1 mRNA expression among the normal control group, benign group and OC group (all P > 0.05). However, the mRNA expression of LPA2 in the OC group was significantly higher than that in normal control group and benign group, respectively (both P < 0.05). Similar associations were also observed on the LPA3 mRNA expression among the three groups (both P < 0.05). Further, no significant differences on LPA2 and LPA3 mRNA expression were observed between normal control group and benign group, respectively (both P > 0.05).
Figure 2.
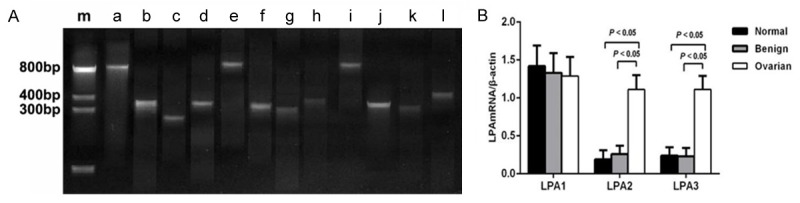
The mRNA expression levels of LPA receptors in normal ovarian epithelium, benign ovarian tumor and epithelial ovarian carcinoma. Note: A. Electrophoresis patterns for LPA receptor mRNA expressions analyzed by RT-PCR assay. m: Marker; a, e, i: β-actin; b, f, j: LPA1; c, g, k: LPA2; d, h, l: LPA3; b-d: Epithelial ovarian carcinoma tissues (OC group); f-h: Benign ovarian tumor tissues (benign group); j-l: Normal ovarian epithelium tissues (normal control group). B. Comparison of mRNA expressions of LPA1, LPA2 and LPA3 in normal control group, benign group and OC group. LPA: lysophosphatidic acid.
Protein expression of LPA receptors in normal ovarian, benign ovarian tumor and epithelial OC
LPA receptors protein expression profile in normal ovarian epithelium, benign ovarian tumor and epithelial OC was measured by Western blotting, and the results were illustrated in Figure 3. We found no significant differences in protein expression levels of LPA1 among the normal control group, benign group and OC group (all P > 0.05). Additionally, there were no significant differences on LPA2 and LPA3 protein expression levels in normal ovarian epithelium and benign ovarian tumor (all P > 0.05). However, LPA2 and LPA3 protein expression levels were significantly higher in OC group than those in normal control group and benign group, respectively (both P < 0.05).
Figure 3.
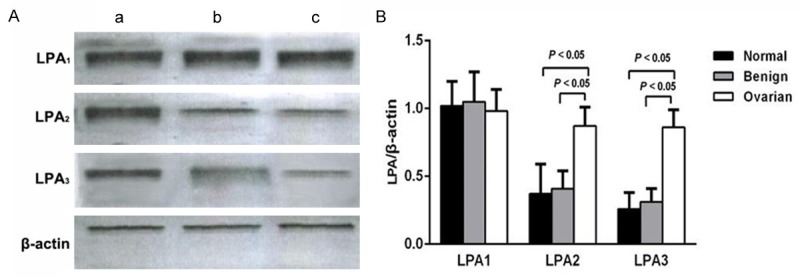
The protein expressions of LPA receptors in normal ovarian epithelium, benign ovarian tumor and epithelial ovarian carcinoma. Note: A. Western blotting results of the protein expressions of LPA1, LPA2, LPA3, and β-actin among three groups. a. Epithelial ovarian carcinoma tissues (OC group); b. Benign ovarian tumor tissues (benign group); c. Normal ovarian epithelium tissues (normal control group). B. Comparison of protein expressions of LPA1, LPA2 and LPA3 in normal control group, benign group and OC group. LPA: lysophosphatidic acid.
Pearson’s linear correlation analysis results
Pearson’s linear correlation analysis was used to assess the relationship between LPA receptors mRNA expression levels and protein expression levels of LPA receptors among the OC patients. The results revealed that the mRNA expression levels of LPA1, LPA2 and LPA3 were positively associated with the protein expression levels of LPA1, LPA2 and LPA3, respectively (LPA1: r = 0.962, P < 0.001; LPA2: r = 0.953, P < 0.001; LPA3: r = 0.977, P < 0.001).
Relationship between LPA receptors protein expression and clinical pathological characteristics
Table 3 shows the relationship between LPA receptors expression and clinical pathological characteristics in epithelial OC patients. LPA1 protein expression showed no significant difference on age, pathological type, pathological stage and pathological grading in epithelial OC patients (all P > 0.05). Similar associations were also observed on LPA2 and LPA3 protein expression (all P > 0.05).
Table 3.
Associations between LPA1-3 protein expression and clinical pathological characteristics in ovarian carcinoma tissues
| Groups | n | LPA1 | LPA2 | LPA3 | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Levels | P | Levels | P | Levels | P | ||
| Age | |||||||
| ≥ 50 | 74 | 0.96 ± 0.16 | 0.067 | 0.85 ± 0.12 | 0.089 | 0.86 ± 0.13 | 0.659 |
| < 50 | 60 | 1.01 ± 0.15 | 0.89 ± 0.15 | 0.87 ± 0.13 | |||
| Pathological type | |||||||
| Serous cystadenocarcinoma | 68 | 0.96 ± 0.16 | 0.225 | 0.87 ± 0.13 | 0.318 | 0.88 ± 0.12 | 0.333 |
| Mucinous cystadenocarcinoma | 44 | 1.02 ± 0.15 | 0.85 ± 0.16 | 0.85 ± 0.14 | |||
| Ovarian clear cell carcinoma | 12 | 1.00 ± 0.17 | 0.88 ± 0.13 | 0.81 ± 0.17 | |||
| Endometrioid ovarian carcinoma | 10 | 0.96 ± 0.10 | 0.94 ± 0.08 | 0.86 ± 0.14 | |||
| Pathological stage | |||||||
| I-II | 44 | 0.95 ± 0.16 | 0.079 | 0.88 ± 0.13 | 0.428 | 0.87 ± 0.13 | 0.677 |
| III-IV | 90 | 1.00 ± 0.15 | 0.86 ± 0.14 | 0.86 ± 0.13 | |||
| Pathological grading | |||||||
| G1 | 18 | 0.91 ± 0.12 | 0.095 | 0.89 ± 0.13 | 0.737 | 0.86 ± 0.14 | 0.137 |
| G2 | 50 | 0.98 ± 0.17 | 0.86 ± 0.13 | 0.89 ± 0.12 | |||
| G3 | 66 | 1.00 ± 0.15 | 0.87 ± 0.15 | 0.84 ± 0.14 | |||
LPA: lysophosphatidic acid.
ROC curve analysis of LPA2 and LPA3
ROC curve analysis was performed to assess sensitivity and specificity of the LPA2 and LPA3 for distinguishing the OC from benign ovarian tumors, as shown in Figure 4. The value of area under the curve (AUC) reveal ed the sensitivity and specificity of the LPA2 and LPA3. For distinguishing OC tissues from benign ovarian tumor tissues, the AUC of LPA2 was 0.992 (95% CI = 0.984-1.000, P < 0.001). When the cut-off value was set to the optimal point, 0.625, the specificity was 96.3% and the sensitivity was 97.9%. We also explored whether LPA3 could distinguish patients with OC from patients with benign ovarian tumors. The ROC results demonstrated that the AUC was 0.999 (95% CI = 0.997-1.000, P < 0.001). When the cut-off value was set to the optimal point, 0.565, the specificity was 98.5% and the sensitivity was 97.9%.
Figure 4.
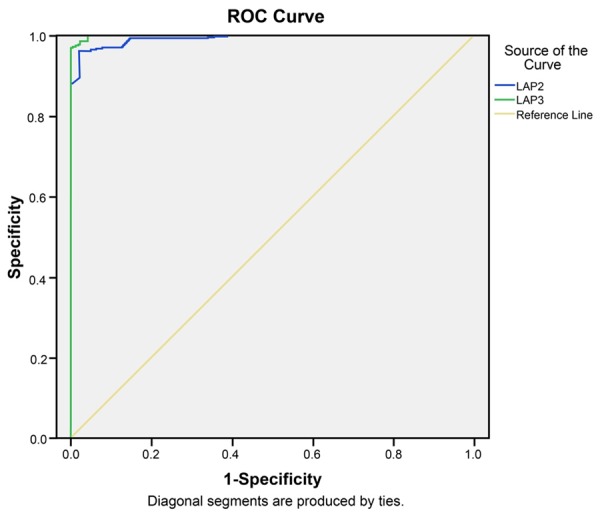
ROC curve analysis using LPA2 and LPA3 for discriminating the ovarian carcinoma from benign ovarian tumors. Note: LPA: lysophosphatidic acid; ROC: receiver-operator characteristic curves.
Discussion
In the present study, we intended to investigate the expression levels of LPA receptors in normal ovarian epithelium tissues, benign ovarian tumor tissues and epithelial OC tissues to better understand the potential mechanisms of LPA receptors in the development of OC. We found that the mRNA and protein expression of LPA2 and LPA3 in epithelial OC tissues were both significantly higher than those in normal ovarian epithelium tissues and benign ovarian tumor tissues, while no significant differences on LPA1 mRNA and protein expression were observed among the normal control group, benign group and OC group. These results suggested that increased mRNA and protein expression of LPA2 and LPA3 may be implicated in the development of OC. The biological function mediated by LPA in various cancers via LPA receptors is involved in cellular processes such as tumor cell growth, proliferation, differentiation, migration and apoptosis [17,29]. Further, LPA signaling via LPA receptors may contribute to the acquisition of malignant potency by some cancer cells, suggesting that LPA signaling may be a target molecule for the establishment of chemoprevention agents in clinical cancer approaches [30]. Acting as a general growth, survival and pro-angiogenic factor, LPA plays a critical role in the regulation of physiological and pathophysiological processes [15,31]. In addition, an early study has demonstrated that abnormalities in LPA metabolism and function in OC patients may contribute to the initiation and progression of the disease [32]. Pronounced LPA accumulation has been identified in the ascites and blood of patients with OC [33]. LPA can activate OC cells and may inhibit the apoptosis of OC cells, and it can also increase the expression levels of matrix metalloproteases (MMPs) and the urokinase plasminogen activator (uPA), which are crucial mediators of metastasis and invasion of cancer cells [34-36]. Meanwhile, LPA can accelerate the formation of focal adhesion and may enhance the migration of cancer cells via the cell signaling of Rho/ROCK/actomyosin and Ras/MEKK1 [37-39]. Notably, the LPA effects the development of OC is primarily mediated through LPA2 and LPA3 [35,38].
LPA-LPAR signaling has been reported to promote cell proliferation, migration and invasion suggesting that increased LPA2 and LPA3 expression may pose a significant risk in OC [40]. LPA2 and LPA3 overexpression correlated with the increased tumor size and metastatic potential, correlating with the aggressiveness in ovarian carcinogenesis [38]. Previous studies have shown that increased expression of LPA2 and LPA3 in OC tissues caused overproduction of vascular endothelial growth factor, accelerating angiogenesis and providing microenvironment for tumor cell proliferation, metastasis and invasion [41,42]. Jeong et al. have indicated that higher mRNA expression of LPA2 was observed in OC cells, and LPA2 may stimulate the expression of COX-2 and cell motility by regulating LPA2/Gi/Src/EGFR/ERK signaling cascade, which may be involved in the progression of OC [43]. Wang and his colleagues have also suggested that the LPA2 and LPA3 expression levels were obviously increased in OC tissues (92.6%) as compared to the benign tissues (45.5%) and normal ovarian tissue (43.8%) [44], which was in consistent with our study results. Previous studies have demonstrated that LPA1 may be a negative regulator in the development and progression of OC by inhibiting the cell proliferation and invasion through apoptosis and anoikis in OC [45-47]. However, such associations were not observed in the current study. We suspected that it may be attributed to ethnic differences or sample size. In this regard, further study will need to be confirmed in other populations.
Our findings also suggested that LPA2 and LPA3 had higher sensitivity and specificity in distinguishing the OC from benign ovarian tumors. The ROC curve analysis results showed that the value of AUC of LPA2 was up to 0.992, and when the cut-off value was set to the optimal point, 0.625, the specificity was 96.3% and the sensitivity was 97.9%. Additionally, the AUC of LPA3 was 0.999, and when the cut-off value was set to the optimal point, 0.565, the specificity was 98.5% and the sensitivity was 97.9%. These results implied that the expression of LPA2 and LPA3 could be used as potential diagnostic indictors in the development of OC. As one of the worst prognosis of gynecological malignancies, OC patients often relates to a poor prognosis, which resulted from the inability to detect ovarian tumors at an early stage, as well as from the lack of effective therapies for the disease in advanced stages [48]. The identification of effective biomarker for early cancer detection can improve survival rates of OC; in this regard, early detection of the development of OC needs to be conducted. It has been reported that increased LPA expression levels can be detected in 90% of patients with stage I OC, and the levels of LPA were higher in patients with OC than in women without ovarian pathology [7]. We suspected that the plasma LPA assay offers the possibility of earlier diagnosis of OC mainly because it is rarely detected in the early stages; hence, the elevated expression levels of LPA in the early stages of OC patients can be used as potential diagnostic indictors in the development of OC.
In summary, LPA and its receptors, especially elevated expression levels of LPA2 and LPA3, may play important roles in the initiation and progression of OC. Further, the LPA2 and LPA3 had higher sensitivity and specificity in distinguishing the OC from benign ovarian tumors, which could be potential diagnostic indictors in OC. Further studies with larger sample size are needed to confirm the findings in this study of the strong association of LPA receptors expression with OC risk.
Acknowledgements
We acknowledge the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Menon U. Ovarian cancer screening has no effect on disease-specific mortality. Evid Based Med. 2012;17:47–48. doi: 10.1136/ebm.2011.100163. [DOI] [PubMed] [Google Scholar]
- 2.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B, Crawford ED, Church TR, Andriole GL, Weissfeld JL, Fouad MN, Chia D, O’Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hartge P, Pinsky PF, Zhu CS, Izmirlian G, Kramer BS, Miller AB, Xu JL, Prorok PC, Gohagan JK, Berg CD, Team PP. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 3.Orfanelli T, Jeong JM, Doulaveris G, Holcomb K, Witkin SS. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int J Cancer. 2014;135:519–528. doi: 10.1002/ijc.28524. [DOI] [PubMed] [Google Scholar]
- 4.Bordon Y. Immunotherapy: Leukadherins get a grip on inflammation. Nat Rev Immunol. 2011;11:638. doi: 10.1038/nri3079. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CS, Maxwell GL, Darcy KM, Hamilton CA, McGuire WP. Current approaches and challenges in managing and monitoring treatment response in ovarian cancer. J Cancer. 2014;5:25–30. doi: 10.7150/jca.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, Bart J, Low PS, Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 7.Sedlakova I, Vavrova J, Tosner J, Hanousek L. Lysophosphatidic acid (LPA)-a perspective marker in ovarian cancer. Tumour Biol. 2011;32:311–316. doi: 10.1007/s13277-010-0123-8. [DOI] [PubMed] [Google Scholar]
- 8.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor P, Savill NJ, Hall D, Matthews KR. Transmission stages dominate trypanosome within-host dynamics during chronic infections. Cell Host Microbe. 2011;9:310–318. doi: 10.1016/j.chom.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, Wu SC, Lai YL. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12:900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 12.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A’Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB. Poly (ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 13.Dutta S, Wang FQ, Wu HS, Mukherjee TJ, Fishman DA. The NF-kappaB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC) Gynecol Oncol. 2011;123:129–137. doi: 10.1016/j.ygyno.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Saunders JA, Rogers LC, Klomsiri C, Poole LB, Daniel LW. Reactive oxygen species mediate lysophosphatidic acid induced signaling in ovarian cancer cells. Free Radic Biol Med. 2010;49:2058–2067. doi: 10.1016/j.freeradbiomed.2010.10.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer AE, Martens JJ, Kulik W, Rueff F, Kuiper EM, van Buuren HR, van Erpecum KJ, Kondrackiene J, Prieto J, Rust C, Geenes VL, Williamson C, Moolenaar WH, Beuers U, Oude Elferink RP. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018. 1018, e1. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Houben AJ, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 2011;30:557–565. doi: 10.1007/s10555-011-9319-7. [DOI] [PubMed] [Google Scholar]
- 18.Panupinthu N, Lee HY, Mills GB. Lysophosphatidic acid production and action: critical new players in breast cancer initiation and progression. Br J Cancer. 2010;102:941–946. doi: 10.1038/sj.bjc.6605588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG, Mills GB, Lee HY. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2011;30:1351–1359. doi: 10.1038/onc.2010.517. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Yue R, Wei B, Gao G, Du J, Pei G. Lysophosphatidic acid acts as a nutrient-derived developmental cue to regulate early hematopoiesis. EMBO J. 2014;33:1383–1396. doi: 10.15252/embj.201387594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snider AJ, Zhang Z, Xie Y, Meier KE. Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: roles for phospholipase D2 and receptor transactivation. Am J Physiol Cell Physiol. 2010;298:C163–170. doi: 10.1152/ajpcell.00001.2009. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Chun J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab. 2010;21:17–24. doi: 10.1016/j.tem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi M, Okabe K, Kato K, Okumura M, Fukui R, Fukushima N, Tsujiuchi T. Differential function of lysophosphatidic acid receptors in cell proliferation and migration of neuroblastoma cells. Cancer Lett. 2012;316:91–96. doi: 10.1016/j.canlet.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Shin SH, Kwon YW, Heo SC, Jeong GO, Kim BR, Seo EJ, Kim JH. Kruppel-like factor 4 mediates lysophosphatidic acid-stimulated migration and proliferation of PC3M prostate cancer cells. Exp Mol Med. 2014;46:e104. doi: 10.1038/emm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bese T, Barbaros M, Baykara E, Guralp O, Cengiz S, Demirkiran F, Sanioglu C, Arvas M. Comparison of total plasma lysophosphatidic acid and serum CA-125 as a tumor marker in the diagnosis and follow-up of patients with epithelial ovarian cancer. J Gynecol Oncol. 2010;21:248–254. doi: 10.3802/jgo.2010.21.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt GR. Declaration of Helsinki-the world’s document of conscience and responsibility. South Med J. 2014;107:407. doi: 10.14423/SMJ.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 27.Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133:401–404. doi: 10.1016/j.ygyno.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Brown RS, Wahl RL. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol. 2010;50:157–186. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 30.Tsujiuchi T, Araki M, Hirane M, Dong Y, Fukushima N. Lysophosphatidic acid receptors in cancer pathobiology. Histol Histopathol. 2014;29:313–321. doi: 10.14670/HH-29.313. [DOI] [PubMed] [Google Scholar]
- 31.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, Hasegawa Y, Tanyi JL, LaPushin R, Eder A, Jaffe R, Erickson J, Mills GB. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 33.Yoon HR, Kim H, Cho SH. Quantitative analysis of acyl-lysophosphatidic acid in plasma using negative ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788:85–92. doi: 10.1016/s1570-0232(02)01031-0. [DOI] [PubMed] [Google Scholar]
- 34.Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- 35.Wang GL, Wen ZQ, Xu WP, Wang ZY, Du XL, Wang F. Inhibition of lysophosphatidic acid receptor-2 expression by RNA interference decreases lysophosphatidic acid-induced urokinase plasminogen activator activation, cell invasion, and migration in ovarian cancer SKOV-3 cells. Croat Med J. 2008;49:175–181. doi: 10.3325/cmj.2008.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do TV, Symowicz JC, Berman DM, Liotta LA, Petricoin EF 3rd, Stack MS, Fishman DA. Lysophosphatidic acid down-regulates stress fibers and up-regulates pro-matrix metalloproteinase-2 activation in ovarian cancer cells. Mol Cancer Res. 2007;5:121–131. doi: 10.1158/1541-7786.MCR-06-0319. [DOI] [PubMed] [Google Scholar]
- 37.Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, Sun P, Huang S. Lysophosphatidic Acid Stimulates Ovarian Cancer Cell Migration via a Ras-MEK Kinase 1 Pathway. Cancer Res. 2004;64:4209–4217. doi: 10.1158/0008-5472.CAN-04-0060. [DOI] [PubMed] [Google Scholar]
- 38.Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, Stephens C, Fang X, Mills GB. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pua TL, Wang FQ, Fishman DA. Roles of LPA in ovarian cancer development and progression. Future Oncol. 2009;5:1659–1673. doi: 10.2217/fon.09.120. [DOI] [PubMed] [Google Scholar]
- 40.Ha JH, Ward JD, Varadarajalu L, Kim SG, Dhanasekaran DN. The gep proto-oncogene Galpha12 mediates LPA-stimulated activation of CREB in ovarian cancer cells. Cell Signal. 2014;26:122–132. doi: 10.1016/j.cellsig.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita T, Miyamoto S, Onoyama I, Sonoda K, Mekada E, Nakano H. Expression of lysophosphatidic acid receptors and vascular endothelial growth factor mediating lysophosphatidic acid in the development of human ovarian cancer. Cancer Lett. 2003;192:161–169. doi: 10.1016/s0304-3835(02)00713-9. [DOI] [PubMed] [Google Scholar]
- 42.Kitayoshi M, Kato K, Tanabe E, Yoshikawa K, Fukui R, Fukushima N, Tsujiuchi T. Enhancement of endothelial cell migration by constitutively active LPA(1)-expressing tumor cells. Biochem Biophys Res Commun. 2012;422:339–343. doi: 10.1016/j.bbrc.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Jeong KJ, Park SY, Seo JH, Lee KB, Choi WS, Han JW, Kang JK, Park CG, Kim YK, Lee HY. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp Mol Med. 2008;40:607–616. doi: 10.3858/emm.2008.40.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang P, Wu X, Chen W, Liu J, Wang X. The lysophosphatidic acid (LPA) receptors their expression and significance in epithelial ovarian neoplasms. Gynecol Oncol. 2007;104:714–720. doi: 10.1016/j.ygyno.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Murph MM, Nguyen GH, Radhakrishna H, Mills GB. Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta. 2008;1781:547–557. doi: 10.1016/j.bbalip.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato K, Yoshikawa K, Tanabe E, Kitayoshi M, Fukui R, Fukushima N, Tsujiuchi T. Opposite roles of LPA1 and LPA3 on cell motile and invasive activities of pancreatic cancer cells. Tumour Biol. 2012;33:1739–1744. doi: 10.1007/s13277-012-0433-0. [DOI] [PubMed] [Google Scholar]
- 47.Hirane M, Araki M, Dong Y, Honoki K, Fukushima N, Tsujiuchi T. Inhibitory effects of LPA1 on cell motile activities stimulated by hydrogen peroxide and 2,3-dimethoxy-1,4-naphthoquinone in fibroblast 3T3 cells. Biochem Biophys Res Commun. 2013;441:47–52. doi: 10.1016/j.bbrc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Oikonomopoulou K, Li L, Zheng Y, Simon I, Wolfert RL, Valik D, Nekulova M, Simickova M, Frgala T, Diamandis EP. Prediction of ovarian cancer prognosis and response to chemotherapy by a serum-based multiparametric biomarker panel. Br J Cancer. 2008;99:1103–1113. doi: 10.1038/sj.bjc.6604630. [DOI] [PMC free article] [PubMed] [Google Scholar]


