Abstract
Introduction: Whether the expression level of MMP-1, MMP-13 and TIMP-1 has association with the degeneration of lateral meniscus after posterior cruciate ligament (PCL) fracture is poorly understood. The aim of this study was to investigate the influence of PCL fracture on lateral meniscus, including morphological changes, histological changes and roles of matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-13 (MMP-13) and tissue inhibitor of metalloproteinase-1 (TIMP-1) expression level in the secondary injury. Materials and Methods: Sixty male rabbits were used as PCL transection models and randomized into the PCL-transection side, which underwent PCL transection surgery, and the control side, which underwent PCL exposure without transection. On 4, 8, 12, 16 and 24 weeks after PCL-transection, 12 rabbits were randomly killed for H&E staining to determine the histological changes of lateral meniscus. Immunohistochemical staining was undertaken to evaluate the expression level of MMP-1, MMP-13 and TIMP-1 in lateral meniscus. The results were statistically analyzed using SPSS 15.0. Results: The lateral meniscus of PCL-transection side presented abnormal morphology. Histological evaluation score of meniscal degeneration in PCL-transection group was higher than that in the control group with statistical difference (P < 0.05). The expression levels of MMP-1, MMP-13 and TIMP-1 were significantly elevated in meniscus of the PCL-transection group with statistical difference (P < 0.05). MMP-1 expression displayed an increasing trend firstly then kept stable after PCL transection; MMP-13 and TIMP-1 expression displayed high level firstly then decreased in advanced stage after PCL transection. Conclusions: PCL transaction may induce a coordinated response of degeneration of lateral meniscus in a time-dependent manner. The high expression level of MMP-1, MMP-13 and TIMP-1 would contribute to the degeneration of lateral meniscus after PCL transection.
Keywords: PCL-transection, lateral meniscus, MMP-1, MMP-13, TIMP-1
Introduction
Posterior cruciate ligament (PCL) is the strongest ligament in the knee and its total tear results in posterior translation of the tibia as well as increased strain on the medial femoral condyle and posterolateral structures [1]. The PCL of human knee plays an important role in controlling and stabilizing knee joint but has a poor healing after injury. It is reported that an isolated PCL injury accounts for approximately 17% of all knee injuries [2,3]. When PCL is transected, menisci have to compensate to maintain normal function of the knee, which is likely lead to menisci injury [4]. Moreover, PCL deficiency may result in knee instability, pain and even progressive degeneration of other intra-articular tissues like cartilage and meniscus, which are known risk factors for osteoarthritis development [5].
However, whether PCL injury would induce molecule changes in meniscal tissues is poorly understood. Tissue remodeling occurs continuously in both normal and injured tissues. In this process, old or damaged structures are degraded and replaced with newly synthesized molecules [6]. The balance between the degradation and biosynthesis of this process is controlled by the activities of matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs) [7]. Previous studies have established close relationship between connective tissue damage/degeneration and the expression of MMPs and TIMPs [8-10]. This research about the expression of MMPs and TIMPs in meniscus tissues induced by PCL transaction may improve our understanding of meniscus degeneration induced by PCL deficiency and development of osteoarthritis [11].
However, it is poorly understood whether the expression level of MMP-1, MMP-13 and TIMP-1 has association with lateral meniscus degeneration after PCL transection. In this study, we focused on the meniscus tissue structure changes of rabbit knee joint and the expression level of MMP-1, MMP-13 and TIMP-1 and the association between them. It may contribute to better understand pathological changes in the lateral meniscus following transection.
Methods
Animals
A total of 60 adult male rabbits (body weight, range 2.6 ± 0.4 kg) from the Animal Center of Central South University were used in the present study. All animal experiments were carried out in accordance with animal welfare guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of Central South University (Approval No. 20130028). Animals were caged with free access to food and water under a 12-h light-dark cycle, relative humidity of 55 ± 5% and temperature of 21 ± 3°C. The knee joints of each rabbit were randomized into PCL-transection side which subjected to PCL transection and the other side subjected to PCL exposure without transaction (29). Every twelve rabbits were killed on 4, 8, 12, 16 and 24 weeks after PCL transection for the experiments described.
PCL transection model
Rabbits were anesthetized with 3% pentobarbital (0.03 mg/kg) and placed on a sterilized tray. Preoperatively, the drawer test was performed to examine knee stability. A medial incision was made to expose the patella which was dislocated to access the PCL for transection. Push and dislocate the kneecap, and then transect the PCL with scissors. After PCL transection was confirmed by the drawer test and hemostasis, articular cavity was washed with 3% aquae hydrogen dioxide and normal saline alternatively. The incision was closed. As for the control knee, the surgery was performed without PCL transection. The weight and injury condition of all rabbits were recorded every day. Rabbits were administered with penicillin (800000 units, 1 time/day) through intramuscular injection for 7 days.
Morphological change
Rabbits were killed using air embolism method. The knees were exposed to observe the meniscal morphology including surface flatness, color, flexibility and intactness.
Histological examination
After the rabbit was killed, lateral meniscus was cut off with the surrounding soft tissue removed. The lateral meniscus was cleaned with normal saline and then was fixed in 4% paraformaldehyde for 24 h. Then it was processed with routine histological methods and embedded in paraffin blocks. Sections of 3 μm thickness were used for H&E staining and immunohistochemical staining. The specimens were treated with dimethyl benzene dewaxing, gradient ethanol dehydration, hematoxylin dyeing (5 min), tap water rinsing (1 min), 1% hydrochloric acid ethanol differentiation (30 s), tap water soaking (15 min), 0.5% eosin staining (3 min) and washing with distilled water. After ethanol and xylene dehydration, the piece was sealed for observation. The histological changes in the meniscus tissue sections were evaluated using light microscopy. The damage showed in the H&E staining images was evaluated with the scoring system [12] (Table 1).
Table 1.
Scoring system of lateralmeniscus histology
| Histological characteristics | Score | |
|---|---|---|
| Surface layer | Smooth | 0 |
| Rough | 1 | |
| Local dent | 2 | |
| Fissure or breakage | 3 | |
| Cartilage cells | Oval or fusiform, orderly arrangement, big nucleus | 0 |
| Oval or fusiform, less orderly arrangement with diffusivity, big nucleus | 1 | |
| Irregular shape, disorderly arrangement, big nucleus | 2 | |
| Disorderly arrangement, less cells, vacuolus cell | 3 | |
| Rare cells | 4 | |
| Collagen fibers | Thick, orderly arranged and compact | 0 |
| Thick, orderly arranged and loose | 1 | |
| Unevenly thick, orderly arranged and loose | 2 | |
| Unevenly thick, disorderly arranged and loose | 3 |
For immunohistochemical assay, the sections were dewaxed according to the above method following with H2O2 (3%) incubation at room temperature for 10 min. After washed with PBS and incubation for 15 min using goat serum, it was incubated with rabbit polyclonal antibody MMP1/MMP13/TIMP-1 at 4°C overnight. They were rinsed and incubated with rabbit IgG at 37°C for 15 min. The MMP-1, MMP13 and TIMP-1 expression intensity in paraffin-embedded tissue sections was determined using Motic Images System. The specimen was detected with light microscopy for the correction cell number. The results were expressed as positive cell rate (PCR, PCR = positive-staining cell count/total cell count × 100%).
All histomorphological and immunohistochemical assessments were evaluated by examining six non-overlapping meniscus sections and a minimum of 10 fields per side of each animal.
Statistical analysis
Statistical analysis was done using SPSS 15.0. The results were expressed as mean ± SD. Paired data were evaluated by paired t-test. If the mean of the sample met the homogeneity of variance, pairwise comparison was performed using SNK-q test (Student-Newman-Keuls test); if the mean of the sample didn’t meet the homogeneity of variance, Dunnett’s-T3 test was performed. Nonparametric test was performed with Nemenyi rank-sum test and Wilcoxon ran-sum test. P < 0.05 was considered to be statistical difference.
Results
Morphological degeneration of meniscus after PCL-transection
The morphology of meniscus in the PCL-transection group showed degenerative characteristics while it was normal in the control group (Table 2). It indicated that PCL-transection induced progressive degeneration of meniscus.
Table 2.
Morphological characteristics of lateral meniscus
| Control group | PCL-rapture group | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| All time points | 4 weeks | 8 weeks | 12 weeks | 16 weeks | 24 weeks | |
| Structural integrity | Integrated | Integrated | Integrated | Integrated | Worn free edge | Avulsion |
| Surface | Smooth | Smooth | Smooth | Not smooth | Rough | Rough |
| Color | Bright white | Gray-white | Gray-white | Faint yellow | Faint yellow | Yellow |
| Elasticity | Good | Good | Good | Slight slack | Slack | Slack |
Histological abnormalities of meniscus after PCL-transection
Compared with control group, the histological characteristics of meniscus showed some abnormities and deterioration in a time-dependent manner in the PCL transection group (Figure 1A-F).
Figure 1.
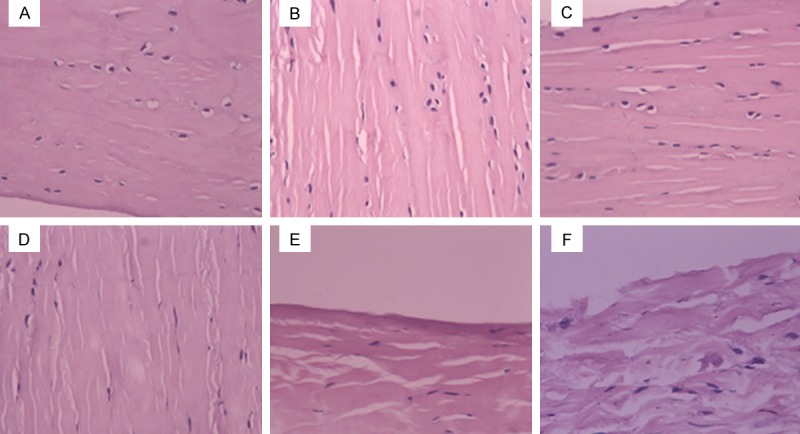
Histological characteristics of lateral meniscus by H&E staining. A. In the control side, collagen fibers and meniscal cell morphology were normal. Meniscal cells were oval or fusiform with big and round nucleus. Collagen fibers were thick and orderly aligned. B. On 4 weeks after PCL-rupture, the H&E staining showed integrate surface structure and thick, loose and orderly arranged collagen fibers. C. On 8 weeks after PCL-rupture, the H&E staining showed integrate surface structure and thick, loose and orderly arranged collagen fibers. D. On 12 weeks after PCL-rupture, H&E staining showed rough surface structure with dent, and loose, orderly aligned and unevenly thick collagen fibers. Meniscal cells appeared disorderly arrangement and decreased in number. E. On 16 weeks after PCL-rupture, H&E staining appeared rough surface structure with dent, and unevenly thick, disorderly arranged collagen fibers. Meniscus cells appeared disorderly arrangement and obvious decreased in number. F. On 24 weeks after PCL-rupture, H&E staining showed fracture surface, and loose, unevenly thick and disorderly arranged collagen fibers. Cartilage cells were rarely visible.
The histological scores of meniscus were 0-1 (within the normal range) in the control group (Table 3). The histological scores in the PCL-transection group were above 2.5 with statistical difference (P < 0.05) except for the comparison between the score on 4 weeks after PCL-transection and the score on 8 weeks after PCL-transection. There were statistical significance between the histological scores of control group and that of the PCL-transection group on all time points (Table 4). It suggested that PCL-transection induced the progressive degeneration of meniscus tissue.
Table 3.
Pairwise comparison of LMHS, MMP-1, MMP-13 and TIMP-1 (P value▲)
| Weeks | 4:8 | 4:12 | 4:16 | 4:24 | 8:12 | 8:16 | 8:24 | 12:16 | 12:24 | 16:24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LMHS | PCL-rupture group | > 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Control group | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| MMP-1 | PCL-rupture group | > 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | > 0.05 | > 0.05 | > 0.05 |
| Control group | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| MMP-13 | PCL-rupture group | > 0.05 | < 0.05 | < 0.05 | > 0.05 | < 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 | < 0.05 |
| Control group | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| TIMP-1 | PCL-rupture group | > 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 | > 0.05 | > 0.05 | < 0.05 | < 0.05 | > 0.05 |
| Control group | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
Dunnett-T3 test.
LMHS, lateral meniscus histological score; MMP-1, MMP-1 expression level in lateral meniscus; MMP-13, MMP-13 expression level in lateral meniscus; TIMP-1, TIMP-1 expression level in lateral meniscus.
Table 4.
Comparison of lateral meniscus histological score between PCL-rupture group and control group
| Weeks | 4 w | 8 w | 12 w | 16 w | 24 w | |
|---|---|---|---|---|---|---|
| LMHS | PCL-rupture group | 2.50 ± 0.67 | 2.58 ± 0.79 | 4.50 ± 1.00 | 7.25 ± 1.14 | 9.41 ± 1.00 |
| Control group | 0.67 ± 0.49 | 0.75 ± 0.45 | 0.42 ± 0.67 | 0.50 ± 0.52 | 0.33 ± 0.49 | |
| P value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | |
| MMP-1 | PCL-rupture group | 5.22 ± 0.77 | 5.14 ± 0.92 | 5.47 ± 1.09 | 5.67 ± 0.98 | 5.39 ± 0.83 |
| Control group | 10.43 ± 1.22 | 11.57 ± 2.59 | 57.14 ± 2.85 | 51.51 ± 6.80 | 59.21 ± 6.80 | |
| P value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | |
| MMP-13 | PCL-rupture group | 5.28 ± 0.79 | 5.14 ± 0.92 | 5.47 ± 1.09 | 5.66 ± 0.98 | 5.39 ± 0.83 |
| Control group | 10.47 ± 1.57 | 11.14 ± 2.09 | 51.43 ± 6.17 | 51.51 ± 6.80 | 11.21 ± 2.20 | |
| P value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 | |
| TIMP-1 | PCL-rupture group | 3.35 ± 0.95 | 3.39 ± 0.96 | 3.43 ± 1.00 | 4.08 ± 1.36 | 4.45 ± 1.30 |
| Control group | 6.86 ± 1.13 | 7.42 ± 1.22 | 52.38 ± 6.42 | 7.64 ± 1.28 | 6.89 ± 1.15 | |
| P value | < 0.05 | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
▲Wilcoxon ran-sum test. LMHS, lateral meniscus histological score; MMP-1, MMP-1 expression level in lateral meniscus; MMP-13, MMP-13 expression level in lateral meniscus; TIMP-1, TIMP-1 expression level in lateral meniscus.
Increased expression of MMP-1, MMP-13 and TIMP-1 in meniscus
MMP-1 didn’t express in control group, while there was an increasing trend of MMP-1 expression in cytoplasm and matrix in a time dependent manner in the PCL-transection group (Figure 2A-F; Table 3). MMP-1 expression level showed an increasing trend (P < 0.05) and then remained a relative level (P > 0.05) after PCL transection. The comparison between the MMP-1 expression level in the control group and that in the PCL-transection group showed statistical significance at all time-points (Table 4).
Figure 2.
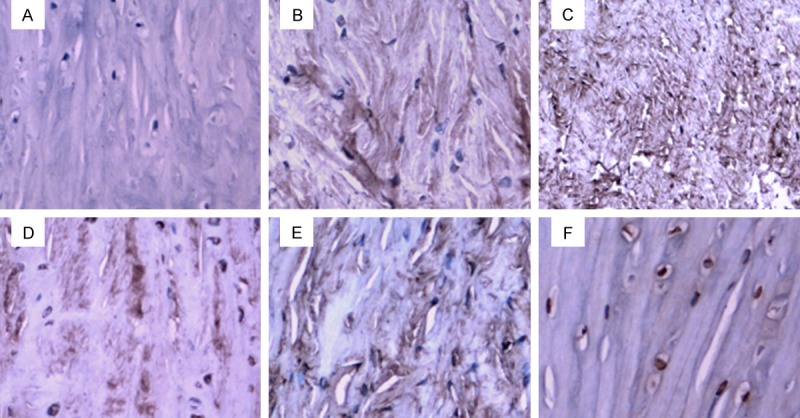
MMP-1 expression in the PCL-rupture side and the control side. A. In the control side, no MMP-1 positive-staining was observed. B. On 4 weeks after PCL-rupture, MMP-1 expression was detected in a small number of cells. C. On 8 weeks after PCL-rupture, MMP-1 expressed in a small number of cells with weakly positive staining in the image. D. On 12 weeks after PCL-rupture, more MMP-1 positive-staining cells were observed. The cytoplasm appeared strong-positive staining. E. On 16 weeks after PCL-rupture, strong-positive staining was observed in the image. F. On 24 weeks after PCL-rupture, strong-positive staining was observed in the image.
There was a small amount of MMP-13 expression in cytoplasm of control group. In the PCL-transection group there was an increasing trend of MMP-13 expression level in cytoplasm and matrix with time firstly and then a low expression level in advanced stage after PCL transaction (Figure 3A-F; Table 3). The expression level of MMP-13 in the PCL-transection group was higher than that in the control group with statistical significance (Table 4).
Figure 3.
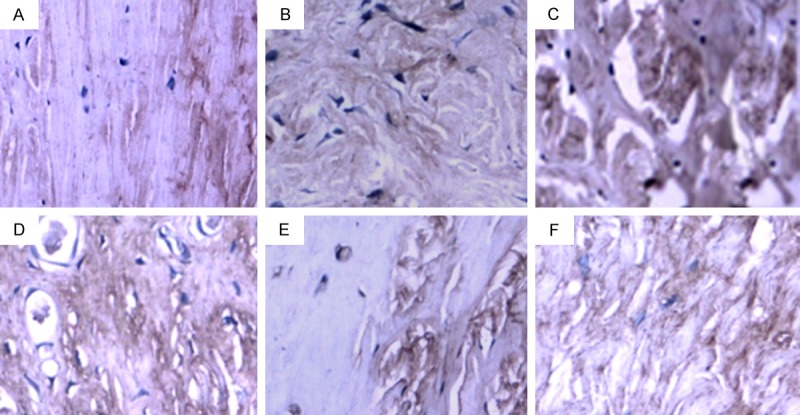
MMP-13 expression in the PCL-rupture side and the control side. A. In the control side, a very small number of MMP-13 positive-staining cells were observed. B. On 4 weeks after PCL-rupture, a small number of MMP-13 positive-staining cells were observed. C. On 8 weeks after PCL-rupture, a small number of MMP-13 positive-staining cells were observed. D. On 12 weeks after PCL-rupture, more MMP-13 positive-staining cells were observed with strongly-staining image. E. On 16 weeks after PCL-rupture, strongly-staining image was observed. F. On 24 weeks after PCL-rupture, MMP-13 positive-staining cells decreased.
As for TIMP-1 expression in the meniscus, it showed a very low level in the control group; it displayed an increasing trend in the early stage and reached the peak on 12 weeks after PCL-transection and then decreased in late stage in the PCL-transection group (Figure 4A-F; Table 3). The comparison of TIMP-1 expression level between the control group and the PCL-transection group showed statistical significance (Table 4).
Figure 4.
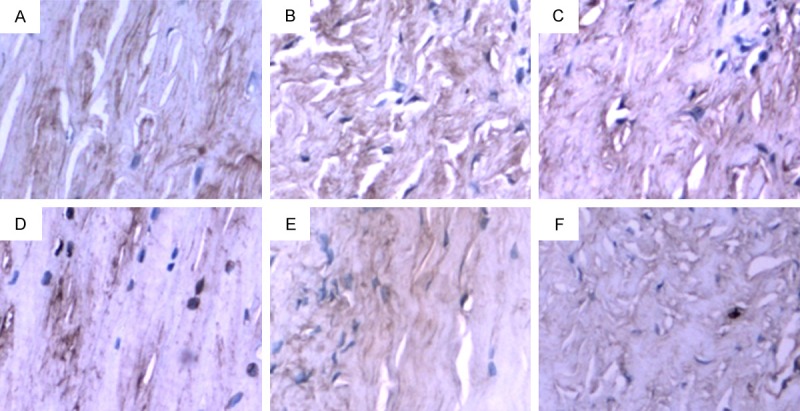
TIMP-1 expression in lateral meniscus of PCL-rupture side and the control side. A. In the control side, a very small number of TIMP-1 positive-staining cells were observed. B. On 4 weeks after PCL-rupture, a small number of TIMP-1 positive-staining cells were observed. C. On 8 weeks after PCL-rupture, a small number of TIMP-1 positive-staining cells were observed in weakly positive-staining image. D. On 12 weeks after PCL-rupture, more TIMP-1 positive-staining cells were observed. E. On 16 weeks after PCL-rupture, the image showed weak-staining. F. On 24 weeks after PCL-rupture, the image showed weak-staining and a few positive-staining cells.
Discussion
PCL injury may induce the degeneration of meniscus which further may result in osteoarthritis. MMPs and TIMPs have close association with connective tissue degeneration [13]. With a rabbit PCL transection model, we found the morphological degeneration of lateral meniscus with time. MMP-1 expression levels were significantly elevated in a time-dependent manner in the early and middle stage after PCL injury and remained a relative stable high expression level in the late stage. MMP-13 and TIMP-1 showed high expression level firstly then low expression in advanced stage after PCL rupture.
In the present study, the observation time points were determined according to the previous researches [14,15]. Wang et al. constructed rabbit PCL-transection model and found that on 12 weeks after PCL transection there were transections in cartilage, osteophyte formation and medial meniscus tear; on 26 weeks after PCL transection, cartilage degeneration and osteophyte were obvious [16]. In the present study, we used 4, 8, 12, 16, 24 weeks after PCL transection as the observation time for lateral meniscus tissue scoring and the immunohistochemical experiments. The morphological results demonstrated that the meniscus tissue degenerated with an increasing trend with time, which is in accordance with the result of Wang et al. study [16]. Therefore, we proposed that the degeneration of meniscus fibrouscartilage probably synchronized the degeneration of joint cartilage after PCL-transection.
In the present study, MMP-1, MMP13 and TIMP-1 were used as molecular markers for meniscus degeneration. Tissue remodeling occurs continuously in both normal and injured tissues. In this process, old or damaged structures are degraded and replaced with newly synthesized molecules [6]. The balance between the degradation and biosynthesis is controlled by MMPs and TIMPs [7]. MMP-1 can initiate the degradation of interstitial collagen, with the substrate collagen type I, II and III. Meniscus consists of 98% collagen type I and less than 2% collagen type II [4]. Previous studies have established close relationship between connective tissue damage/degeneration and the expression of MMPs [8-10]. TIMPs, as tissue inhibitors of metalloproteinases, include TIMP-1, TIMP-2, TIMP-3 and TIMP-4 and play an important role in osteoarthritis and meniscus injury after anterior cruciate ligament (ACL) transection. Among TIMPs, TIMP-1 is the most critical one as the inhibitor [17]. MMP-1, MMP13 and TIMP-1 were studied in the osteoarthritis pathology but, as far as we know, there is not any report about the association between the expression level of MMP-1, MMP13 and TIMP-1 and meniscus injury after PCL-transection. Therefore, in this study we focused on MMP-1, MMP13 and TIMP-1 to determine the early molecule response underlying the tissue damage and degeneration induced by PCL-transection, which is helpful for understanding the PCL-transection-induced knee degeneration as well as development of osteoarthritis.
The morphological change of meniscus was obvious on 12 weeks after PCL-transection and the histological score showed an increasing trend, which is in accordance with the previous study [16]. The present result showed that the expression level of MMP-1, MMP13 and TIMP-1 in the PCL-transection group was higher than that in the control group in all time points, indicating that the degeneration of lateral meniscus possibly has close relationship with the higher expression level of MMP-1, MMP13 and TIMP-1. In the PCL-transection group, the expression of MMP-1 and MMP13 obviously increased, which is in accordance with the initiating role of MMP-1 and meniscus morphological change [18]. In ACL-transection, the expression of MMP-1 and MMP-13 was obvious in the early stage (on 4, 8 weeks after ACL-transection) [15] while in the present study the expression of MMP-1 and MMP13 was unobvious until 12 weeks after PCL-transection. On 24 weeks after PCL-transection, the expression of MMP-13 decreased, while the expression of MMP-1 still remained the high level and was in accordance with the result of Fernandes’ study [19]. The different expression level and trend are possibly due to the different regulation pathways, ECM degradation, and chondrocyte apoptosis. It’s worth noting that the decreased expression level of MMP-13 did not mean the end of meniscus injury or apoptosis. Maybe MMP-13 plays an important role in the middle stage of degeneration rather than in the whole stage.
In ACL-transection model, the expression ofTIMP-1 obviously increased 4 weeks after transaction [15]. In the present study the expression of TIMP-1 obvious increased and reached the peak valueon 12 weeks after PCL-transection and decreased on 16 weeks after PCL-transection. This possibly is due to the compensatory repair characteristic of TIMP-1. Specifically, TIMP-1 binds with MMP-1 and MMP-13 in the ratio of 1:1. With the deterioration of meniscus and the high expression level of MMP-1 and MMP-13, TIMP-1 reached its limit and decreased. But this speculation should be further verified.
Conclusions
PCL rupture may induce a coordinated response of degeneration of lateral meniscus in a time-dependent manner. The high expression level of MMP-1, MMP-13 and TIMP-1 would contribute to the degeneration of lateral meniscus after PCL rupture.
Acknowledgements
The research was funded by Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC2014046) and Hunan Provincial Innovation Foundation for Postgraduates (CX2014B111).
Disclosure of conflict of interest
None.
Abbreviations
- PCL
Posterior cruciate ligament
- MMPs
Matrix metalloproteinases
- TIMPs
Tissue inhibitors of metalloproteinases
- MMP-1
Matrix metalloproteinase-1
- MMP-13
Matrix metalloproteinase-13
- TIMP-1
Tissue inhibitor of metalloproteinase-1
- ACL
Anterior cruciate ligament
References
- 1.Gao SG, Jiang W, Lei GH, Xu M, Yu F, Li KH. Effect of posterior cruciate ligament rupture on biomechanical features of the medial femoral condyle. Orthopaedic Surgery. 2011;3:205–210. doi: 10.1111/j.1757-7861.2011.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arøen A, Strand T, Mølster A. Primary suture of the posterior cruciate ligament. Tidsskr Nor Laegeforen. 1992;112:1582–1584. [PubMed] [Google Scholar]
- 3.Mair SD, Schlegel TF, Gill TJ, Hawkins RJ, Steadman JR. Incidence and location of bone bruises after acute posterior cruciate ligament injury. Am J Sports Med. 2004;32:1681–1687. doi: 10.1177/0363546504266481. [DOI] [PubMed] [Google Scholar]
- 4.Mcdermott ID, Masouros SD, Amis AA. Biomechanics of the menisci of the knee. Curr Orthopaed. 2008;22:193–201. [Google Scholar]
- 5.Aroen A, Sivertsen EA, Owesen C, Engebretsen L, Granan LP. An isolated rupture of the posterior cruciate ligament results in reduced preoperative knee function in comparison with an anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2013;21:1017–1022. doi: 10.1007/s00167-012-2132-1. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DR, Leco KJ, Beaudry PP, Atadja PW, Veillette C, Riabowol KT. Differential effects of transforming growth factor-β1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp Gerontol. 1996;31:207–223. doi: 10.1016/0531-5565(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 7.Everts V, Van Der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- 8.Hasaneen NA, Zucker S, Cao J, Chiarelli C, Panettieri RA, Foda HD. Cyclic mechanical strain-induced proliferation and migration of human airway smooth muscle cells: role of EMMPRIN and MMPs. Faseb J. 2005;19:1507–9. doi: 10.1096/fj.04-3350fje. [DOI] [PubMed] [Google Scholar]
- 9.Sun HB, Li Y, Fung DT, Majeska RJ, Schaffler MB, Flatow EL. Coordinate regulation of IL-1β and MMP-13 in rat tendons following subrupture fatigue damage. Clin Orthop Relat Res. 2008;466:1555–1561. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Lee HS, Villarreal F, Teng A, Lu E, Reynolds S, Qin C, Smith J, Sung KL. Differential MMP-2 activity of ligament cells under mechanical stretch injury: An in vitro study on human ACL and MCL fibroblasts. J Orthop Res. 2005;23:949–957. doi: 10.1016/j.orthres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Berg WB. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthritis Cartilage. 2011;19:338–341. doi: 10.1016/j.joca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Li K, Zhu Y, Li S, Zhang J. Histological changes of degenerated lateral meniscus after anterior cruciate ligament rupture in rabbits. J Clin Rehabilit Tissue Engineering Res. 2009;13:3873–6. [Google Scholar]
- 13.Tang Z, Yang L, Zhang J, Xue R, Wang Y, Chen PC, Sung KL. Coordinated expression of MMPs and TIMPs in rat knee intra-articular tissues after ACL injury. Connect Tissue Res. 2009;50:315–322. [PubMed] [Google Scholar]
- 14.Wang J, Ao Y. The histological and collagen phenotype changes of a semitendinosus autograft after of posterior cruciate ligament reconstruction in rabbit. Chin J Orthop. 2006;26:47. [Google Scholar]
- 15.Wu H, Du J, Zheng Q. Expression of MMP-1 in cartilage and synovium of experimentally induced rabbit ACLT traumatic osteoarthritis: immunohistochemical study. Rheumatol Int. 2008;29:31–36. doi: 10.1007/s00296-008-0636-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Ao Y. The effect of rupture and reconstruction of posterior cruciate ligament on the degeneration of articular cartilage in rabbit knee. Chin J Surg. 2005;43:1598–1601. [PubMed] [Google Scholar]
- 17.Ishiguro N, Ito T, Oguchi T, Kojima T, Iwata H, Ionescu M, Poole AR. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 2001;44:2503–11. doi: 10.1002/1529-0131(200111)44:11<2503::aid-art430>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Saffarian S, Collier IE, Marmer BL, Elson EL, Goldberg G. Interstitial collagenase is a Brownian ratchet driven by proteolysis of collagen. Science. 2004;306:108–11. doi: 10.1126/science.1099179. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes JC, Martel-Pelletier J, Lascau-Coman V, Moldovan F, Jovanovic D, Raynauld JP, Pelletier JP. Collagenase-1 and collagenase-3 synthesis in normal and early experimental osteoarthritic canine cartilage: an immunohistochemical study. J Rheumatol. 1998;25:1585–94. [PubMed] [Google Scholar]


