Abstract
Background: The relationship of vasectomy to prostate cancer has great public health significance. However, the results of observational studies were conflicting. To determine whether vasectomy is associated with the risk of prostate cancer, we performed a meta-analysis of cohort studies. Methods: A literature search was carried out using Pubmed, Embase, Cochrane Libraryl, and China National Knowledge Infrastructure (CNKI) between January 1966 and July 2013. Before meta-analysis, between-study heterogeneity and publication bias were assessed using adequate statistical tests. Fixed-effect and random-effect models were used to estimate summary relative risks (RR) and the corresponding 95% confidence intervals (CIs). Potential sources of heterogeneity were detected by meta-regression. Subgroup analyses and sensitivity analysis were also performed. Results: A total of nine cohort studies contributed to the analysis. There was heterogeneity among the studies but no publication bias. Pooled results indicated that vasectomy was not associated with a significant increase of total prostate cancer risk (RR = 1.07, 95% CI [0.79, 1.46]). When stratified the various studies by geographic location, we found a significant association between vasectomy and increased PCa risk among studies conducted in the USA (RR = 1.54, 95% CI [1.23, 1.93]), however, there was no significant association between vasectomy and PCa risk among studies conducted in non-USA countries (RR = 0.74, 95% CI [0.50, 1.09]). Furthermore, sensitivity analysis confirmed the stability of the results. Conclusions: In conclusion, the present meta-analysis of cohort studies suggested that vasectomy was not associated with increased risk of prostate cancer. More in-depth studies are warranted to report more detailed results, including stratified results by age at vasectomy, tumor grade, and tumor stage.
Keywords: Vasectomy, prostate cancer, risk, meta-analysis
Introduction
Prostate cancer (PCa) is the second-most frequently diagnosed cancer and the sixth-leading cause of cancer death in males worldwide [1,2]. The cause of PCa is not well known, but multiple risk factors have been identified, including age, race, and family history of PCa [3-5]. Many putative risk factors, including androgens, diet, physical activity, sexual factors, inflammation, and obesity, have been implicated, but their roles in PCa etiology remain unclear.
Vasectomy is an important method of birth control, with approximately 500,000 vasectomies performed annually in the United States alone [6]. About 12% of married men have had a vasectomy and are generally under the age of 40 years when the procedure is performed [7]. The relationship of vasectomy to PCa has great public health significance [8-10]. Vasectomy has been hypothesized to increase the risk of PCa by diminishing the secretion of prostatic fluid or by altering immune response to sperm antigens [11-13]. Several epidemiological studies have investigated the association of vasectomy with PCa, however, their results were conflicting. The previous meta-analysis by LK Dennis et al [14] in 2002 suggested that men with a prior vasectomy may be at an increased risk of PCa (RR = 1.37, 95% CI, 1.15-1.62). However, the majority of observational studies included in their meta-analysis were case-control studies, and there were only five cohort studies. Since 2002, more cohort studies are published. To further evaluate the effect of vasectomy on the risk of developing PCa, we now performed a meta-analysis of cohort studies.
Materials and methods
Systematic search strategy
The present meta-analysis was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA) [15], and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [16]. A literature search was carried out using Pubmed, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) between January 1966 and July 2013. Search terms included: “vasectomy” OR “deferentectomy” OR “gonangiectomy” OR “vas ligation” OR “vasoligation” OR “vas ligature” OR “vasoligature” OR “vas occlusion” AND “prostate cancer”. The reference lists of each comparative study included in this meta-analysis and previous reviews were manually examined to identify additional relevant studies.
Study selection
Two reviewers independently selected eligible cohort studies that investigated vasectomy and the risk of PCa. Disagreement between the two reviewers was settled by discussing with the third reviewer. Inclusion criteria were: (i) used a cohort study design; (ii) evaluated the association between vasectomy and PCa risk; (iii) presented odds ratio (OR), relative risk (RR), or hazard ratio (HR) estimates with its 95% confidence interval (CI). When there were multiple publications from the same population, only data from the most recent report was included in the meta-analysis and the remaining was excluded. Studies reporting different measures of RR like risk ratio, rate ratio, hazard ratio, and odds ratio were included in the meta-analysis. In practice, these measures of effect yield a similar estimate of RR, since the absolute risk of PCa is low.
Assessment of study quality, data extraction, and analysis
Newcastle-Ottawa scale (NOS) was used to assess the methodological quality of cohort studies [17]. The NOS contains eight items that are categorized three categories: selection (four items, one star each), comparability (one item, up to two stars), and outcome (three items, one star each). A “star” presents a “high-quality” choice of individual study. Two reviewers assessed the methodological quality independently. Disagreement between the two reviewers was settled by discussing with the third reviewer.
The following data was collected by two reviewers independently using a purpose-designed form: name of first author, publishing time, country of the population studied, study design, study period, follow-up time, number of PCa cases and subjects, the study-specific adjusted ORs, RRs, or HRs with their 95% CIs, confounding factors for matching or adjustments.
Heterogeneity was assessed using the Cochran Q and I2 statistics. For the Q statistic, a P value < 0.10 was considered statistically significant for heterogeneity; for the I2 statistic, heterogeneity was interpreted as absent (I2: 0%-25%), low (I2: 25.1%-50%), moderate (I2: 50.1%-75%), or high (I2: 75.1%-100%) [18]. The overall analysis including all eligible studies was performed first, and subgroup analyses were performed according to (i) study design (prospective cohort study and retrospective cohort study), (ii) Study location (USA and non-USA), (iii) publication year (before 2000 and after 2000), and (iv) control for confounding factors (n ≥ 4, and n ≤ 3) to examine the impact of these factors on the association. When substantial heterogeneity was detected, the summary estimate based on the random-effect model (DerSimonian-Laird method) [19] was reported, which assumes that the studies included in the meta-analysis had varying effect sizes. Otherwise, the summary estimate based on the fixed-effect model (the inverse variance method) was reported, which assumes that the studies included in the meta-analysis had the same effect size. To derive the relationship between time since vasectomy and risk for PCa, we carried out dose-response analysis. For the dose-response meta-analysis, methods proposed by Greenland [20] and Orsini [21] were used to estimate study-specific slopes. To test the robustness of association and characterize possible sources of statistical heterogeneity, sensitivity analysis were carried out by excluding studies one-by-one and analyzing the homogeneity and effect size for all of rest studies. To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed [22]. Publication bias was assessed using Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test [23,24]. All analyses were performed using Stata version 11.0 (Stata Corp, College Station, TX).
Results
Search results and reporting quality
Figure 1 illustrates the search process and the final selection of relevant studies. 590 records were identified through database searching. On the basis of the titles and abstracts, we identified nine full-text articles. After further evaluation, one study was excluded because it was from the same population. One study was identified from reference lists. At last, a total of nine eligible cohort studies published between 1991 and 2012 were identified [10,25-32] (Baseline data and other details of included studies are shown in Table 1). A total of 331,436 male subjects, including 1,245 PCa cases were involved. Of the nine included studies, three studies were conducted in Europe [10,28,29], four studies in the USA [25-27,30], and remaining two studies in other countries [31,32]. Only two studies [26,32] were prospective cohort studies, and the others were retrospective cohort studies. The NOS scores for the included cohort studies ranged from 5 to 8, with a median 6; about 60% (6/9) of included studies were deemed to be of a high quality (≥ 6) (shown in Table S1).
Figure 1.
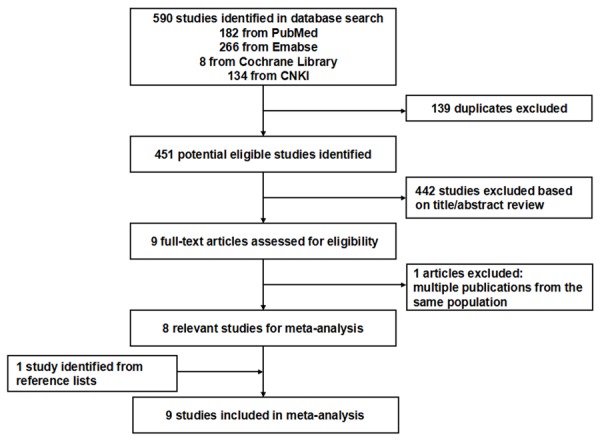
Flow diagram of screened, excluded, and analysed publications.
Table 1.
Characteristics of cohort studies included in the present meta-analysis
| Author | Year | Country | Study design | All male subjects | PCa cases | Age (y) | Study period | Follow-up (y) | Confounders for adjustment | Adjusted risk estimate, RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sidney S | 1991 | USA | Retrospective cohort study | 20,466 | 135 | 45.8 | 1977-1987 | 6.8 (mean) | age, race, marital status, date and location of multiphasic health exam | Total 1.1 (0.7-1.6) |
| Years Since Vasectomy | ||||||||||
| < 10 1.2 (0.5-2.6) | ||||||||||
| 10-20 1.2 (0.6-2.6) | ||||||||||
| > 20 1.2 (0.6-2.2) | ||||||||||
| Age at vasectomy | ||||||||||
| < 40 1.0 (0.5-1.9) | ||||||||||
| ≥ 40 1.4 (0.8-2.3) | ||||||||||
| Nienhuis H | 1992 | Britain | Retrospective cohort study | 35,442 | 6 | 25-49 | 1970-1986 | 7.5 (mean) | age | Total 0.44 (0.1-4.0) |
| Giovannucci E | 1993 | USA | Retrospective Cohort Study | 29,214 | 96 | 42 | 1976-1989 | NA | age | Total 1.56 (1.03-2.37) |
| Years Since Vasectomy | ||||||||||
| 1-9 1.11 (0.46-2.70) | ||||||||||
| 10-19 1.26 (0.75-2.10) | ||||||||||
| ≥ 20 1.89 (1.14-3.14) | ||||||||||
| Giovannucci E | 1993 | USA | Prospective Cohort Study | 47,855 | 300 | 40-75 | 1986-89 | NA | age, marital status, race, and geographicalregion | Total 1.66 (1.25-2.21) |
| Years Since Vasectomy | ||||||||||
| < 13 1.24 (0.61-2.55) | ||||||||||
| 13-21 1.39 (0.83-2.31) | ||||||||||
| ≥ 22 1.77 (1.18-2.64) | ||||||||||
| Møller H | 1994 | Denmark | Retrospective Cohort Study | 73,917 | 165 | NA | 1977-1989 | NA | age | Total 0.98 (0.84-1.14) |
| Lynge E | 2002 | Denmark | Retrospective Cohort Study | 115,862 | 93 | NA | 1977-1995 | 12.7 (mean) | none | Total 0.98 (0.73 -1.31) |
| Years Since Vasectomy | ||||||||||
| 0-4 0.95 (0.31-2.21) | ||||||||||
| 5-9 1.24 (0.71-2.01) | ||||||||||
| 10-14 1.12 (0.69-1.72) | ||||||||||
| ≥ 15 0.40 (0.11-1.02) | ||||||||||
| Age at vasectomy | ||||||||||
| ≤ 30 14.26 (1.73-51.57) | ||||||||||
| 30-39 0.84 (0.31-1.82) | ||||||||||
| 40-49 0.80 (0.48-1.25) | ||||||||||
| 50-59 1.06 (0.56-1.81) | ||||||||||
| ≥ 60 1.65 (0.61-3.60) | ||||||||||
| Rohrmann S | 2005 | USA | Retrospective Cohort Study | 3,373 | 78 | 54.8 | 1996-2004 | 8.0 (mean) | age | Total 2.03 (1.24-3.32) |
| Low-stage 1.47 (0.55-3.90) | ||||||||||
| High-stage 1.52 (0.46-5.06) | ||||||||||
| Low-grade 2.87 (1.49-5.54) | ||||||||||
| High-grade 0.99 (0.36-2.76) | ||||||||||
| Yong N | 2008 | China | Retrospective Cohort Study | 3,186 | 314 | NA | 1996-2005 | NA | none | Total 0.50 (0.37-0.67) |
| Years Since Vasectomy | ||||||||||
| 20-29 0.39 (0.14-1.09) | ||||||||||
| 30-39 0.31 (0.20-0.47) | ||||||||||
| ≥ 40 1.12 (0.75-1.68) | ||||||||||
| Romero FR | 2012 | Brazil | Prospective Cohort Study | 2,121 | 58 | ≥ 40 | 2006-2011 | 21.5 (mean) | none | Total 0.23 (0.03-1.70) |
NA, not available; RR, relative risk; CI, confidence interval.
Meta-analysis results
Because of significant heterogeneity was observed (I2 = 83.4%, P < 0.001), a random-effects model was chosen over a fixed-effects model and we found that vasectomy did not significantly affect the risk of PCa (RR = 1.07, 95% CI [0.79, 1.46]). Both multivariable adjusted RR estimates with 95% CIs of each study and combined RR is shown in Figure 2. In the present meta-analysis, no publication bias was observed among studies using Begg’s P value (P = 0.677); Egger’s (P = 0.966) test, which suggested there was no evidence of publication bias (Figure 3).
Figure 2.
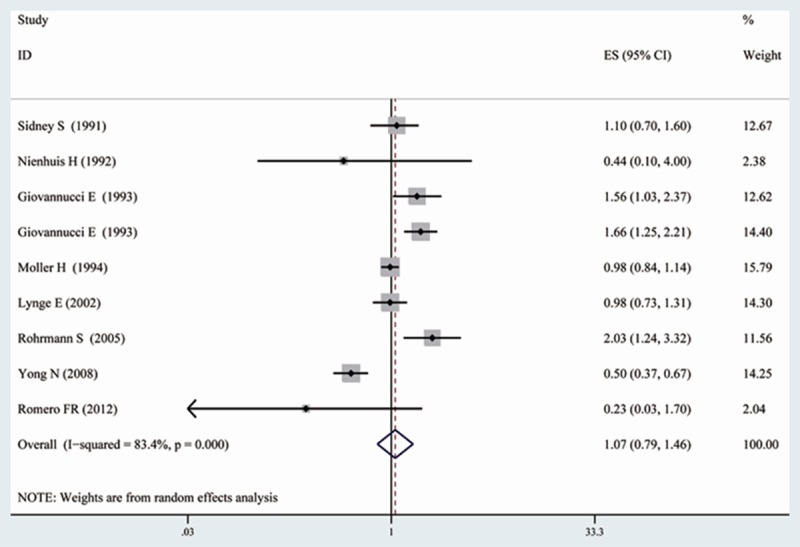
Forest plot: overall meta-analysis of vasectomy and prostate cancer risk. Squares indicated study-specific risk estimates (size of square reflects the study-statistical weight, i.e. inverse of variance); horizontal lines indicate 95% confidence intervals; diamond indicates summary relative risk estimate with its corresponding 95% confidence interval.
Figure 3.
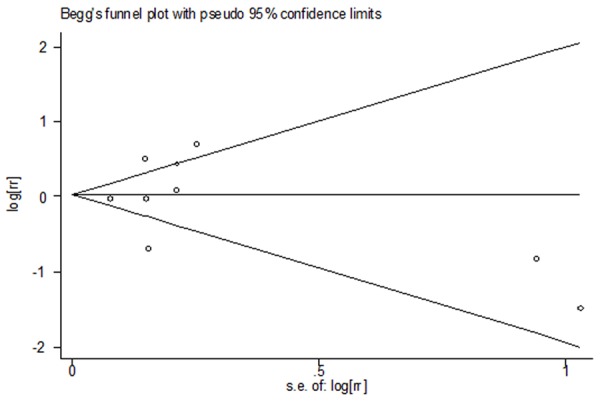
Funnel plot for publication bias in the studies investigating risk for prostate cancer associated with vasectomy.
Subgroup analyses, and sensitivity analysis
No significant association was detected between vasectomy and PCa risk among prospective cohort studies (RR = 0.80, 95% CI [0.12, 5.20]), as well as retrospective cohort studies (RR = 1.03, 95% CI [0.75, 1.42]), presented in Table 2. When stratified the various studies by geographic location, we found a significant association between vasectomy and increased PCa risk among studies conducted in the USA (RR = 1.54, 95% CI [1.23, 1.93]), however, there was no significant association between vasectomy and PCa risk among studies conducted in non-USA countries (RR = 0.74, 95% CI [0.50, 1.09]). When we examined whether the associations differed by publication year, no significant association was detected between vasectomy and PCa risk among studies published before 2000 (RR = 1.24, 95% CI [0.92, 1.67]), as well as studies published after 2000 (RR = 0.86, 95% CI [0.44, 1.70]). Further, we found that the association was not affected by the number of adjustment factors (see in Table 2).
Table 2.
Summary of pooled relative risks of prostate cancer in subgroups
| No. of studies | RR | 95% CI | P for heterogeneity | I2 (%) | |
|---|---|---|---|---|---|
| All | 9 | 1.07 | 0.79-1.46 | < 0.001 | 83.4 |
| Region | |||||
| USA | 4 | 1.54 | 1.23-1.93 | 0.259 | 25.4 |
| Non-USA | 5 | 0.74 | 0.50-1.09 | 0.001 | 78.4 |
| Study type | |||||
| Prospective cohort study | 2 | 0.80 | 0.12-5.20 | 0.057 | 72.3 |
| Retrospective cohort study | 7 | 1.03 | 0.75-1.42 | < 0.001 | 82.3 |
| Publication year | |||||
| Before 2000 | 5 | 1.24 | 0.92-1.67 | 0.008 | 70.7 |
| After 2000 | 4 | 0.86 | 0.44-1.70 | < 0.001 | 88.7 |
| Number of adjustment factors | |||||
| n ≥ 4 confounders | 2 | 1.39 | 0.93-2.07 | 0.108 | 61.3 |
| n ≤ 3 confounders | 7 | 0.98 | 0.68-1.41 | < 0.001 | 83.0 |
To test the robustness of association and characterize possible sources of statistical heterogeneity, sensitivity analyses were carried out by excluding studies one-by-one and analyzing the homogeneity and effect size for all of the rest studies. Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results.
Dose-response analysis and meta-regression analysis
We evaluated evidence for a dose-response relationship among the five studies [25-27,29,31] which reported information on time since vasectomy and PCa risk. However, there was no evidence of a linear association between time since vasectomy and PCa risk (P for linearity = 0.565; Figure 4). To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed. Study design, geographic area, publication year, and major confounders adjusted, which may be potential sources of heterogeneity, were tested by a meta-regression method. We found that only geographic area (P < 0.05) had statistical significance in a multivariate model.
Figure 4.
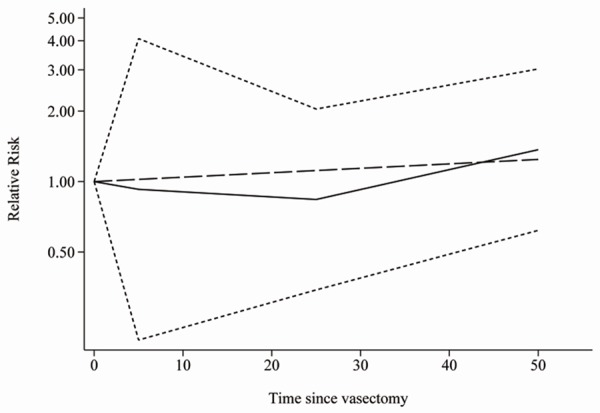
The dose-response analysis between time since vasectomy and prostate cancer risk obtained by the restricted cubic spline regression model. P for linearity = 0.565. The solid line and the short dash line represent the estimated RR and its 95% CI. Long dash line represents the linear relationship.
Discussion
Vasectomy is a common birth control method, and prostate cancer is the most frequently diagnosed solid tumor in men, so the relationship of vasectomy to PCa has great public health significance [8,33]. The present meta-analysis included nine cohort studies currently available (two prospective cohort studies and seven retrospective cohort studies), involving a total of 331,436 male subjects and 1,245 PCa cases. There was statistically significant heterogeneity among the nine included cohort studies, so a random-effects model was chosen over a fixed-effects model. Finally, we found that vasectomy did not significantly affect the risk of PCa. Further, there was no evidence of a linear association between time since vasectomy and PCa risk. Meta-regression analysis revealed that geographic area may be the source of heterogeneity. Sensitivity analysis indicated that an omission of any studies did not alter the magnitude of observed effect, suggesting a stability of our findings. Moreover, the results of Begg’s test and Egger’s test did not support the existence of major publication bias. In our subgroup analyses, the results were not substantially affected by study design, publication year, and confounder adjustment. Prospective cohort and retrospective cohort studies alone showed no significant association between vasectomy and the risk of PCa. However, we should notice that there were only two prospective cohort studies investigating the association between vasectomy and PCa risk. That number was rather low to draw firm conclusions. As we know, compared with retrospective cohort studies, prospective cohort studies are less susceptible to bias (e.g. recall bias, selection bias) due to their nature [34-36]. So, more prospective cohort studies are needed to confirm the associations found in the present meta-analysis. Although the association was not affected by the number of adjustment factors, we should notice that the number of adjustment factors was rather low among the included studies (shown in Table 1). There are a lot of factors which may affect the risk of PCa, such as age, race, family history of PCa, androgens, diet, physical activity, inflammation, and obesity. Further, sexual activity may also affect the association between vasectomy and PCa risk. Since an increased number of sexual partners and history of a sexually transmitted infection appear to be related to PCa but likely inversely related to having a vasectomy [37,38], this would cause negative confounding. The future studies should adjust as more confounders as possible [39]. We found a significant association between vasectomy and increased PCa risk among studies conducted in the USA but not non-USA countries. Explanations for the inconsistent findings between study location are not known. There are many possible reasons which will lead to the difference in association between different areas. The differences in genetic susceptibility, culture, and lifestyles may explain part of the inconsistency of the results [40].
Among the nine included studies, only two studies stratified the association by age at vasectomy. Sidney S et al [25] found that the RR of PCa associated with vasectomy increased with age at vasectomy (1.4 in men 40 or more years old and l.0 in men less than 40 years old), but the CIs around these RRs were wide and included one. In the study by Lynge E et al [29], no difference was seen in PCa risk by age at vasectomy. So whether age at vasectomy will affect PCa risk is unclear, and this topic should be further investigated in the future. Rohrmann S et al found that the risk of low-grade disease (HR = 2.87; 95% CI 1.49-5.54), but not high-grade disease (HR = 0.99; 95% CI 0.36-2.76), was higher in men who had had a vasectomy. No statistically significant associations were observed for low-stage or high-stage disease. Their findings should be confirmed by more cohort studies in the future.
The strength of the present meta-analysis lies in a large sample size (331,436 male subjects and 1,245 PCa cases) and no significant evidence of publication bias. Two investigators independently performed the article identification, data extraction, and verification and resolved all discrepancies. Furthermore, our findings were stable and robust in sensitivity analyses. However, several limitations to this meta-analysis should be noted. Firstly, as a meta-analysis of observational data, the possibility of recall and selection biases cannot be ruled out. Compared with retrospective cohort studies, prospective cohort studies are less susceptible to bias due to their nature. However, the present meta-analysis included only two prospective cohort studies, so more prospective cohort studies are need to confirm the associations in the future. Secondly, we did not search for unpublished studies, so only published studies were included in our meta-analysis. Therefore, publication bias may have occurred although no publication bias was indicated from both visualization of the funnel plot and Egger’s test. Thirdly, the number of adjustment factors was rather low among the included studies, so the future studies should adjust as more confounders as possible.
In conclusion, the present meta-analysis of cohort studies suggests that vasectomy is not associated with increased risk of PCa. More in-depth studies are warranted to report more detailed results, including stratified results by age at vasectomy, tumor grade, and tumor stage.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53:171–184. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 4.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 5.Haas GP, Sakr WA. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47:273–287. doi: 10.3322/canjclin.47.5.273. [DOI] [PubMed] [Google Scholar]
- 6.Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923–936. doi: 10.1016/s0015-0282(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 7.Massey FJ Jr, Bernstein GS, O’Fallon WM, Schuman LM, Coulson AH, Crozier R, Mandel JS, Benjamin RB, Berendes HW, Chang PC, et al. Vasectomy and health. Results from a large cohort study. JAMA. 1984;252:1023–1029. doi: 10.1001/jama.252.8.1023. [DOI] [PubMed] [Google Scholar]
- 8.Farley TM, Meirik O, Mehta S, Waites GM. The safety of vasectomy: recent concerns. Bull World Health Organ. 1993;71:413–419. [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald SW. Is vasectomy harmful to health? Br J Gen Pract. 1997;47:381–386. [PMC free article] [PubMed] [Google Scholar]
- 10.Nienhuis H, Goldacre M, Seagroatt V, Gill L, Vessey M. Incidence of disease after vasectomy: a record linkage retrospective cohort study. BMJ. 1992;304:743–746. doi: 10.1136/bmj.304.6829.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox B, Sneyd MJ, Paul C, Delahunt B, Skegg DC. Vasectomy and risk of prostate cancer. JAMA. 2002;287:3110–3115. doi: 10.1001/jama.287.23.3110. [DOI] [PubMed] [Google Scholar]
- 12.Kohler TS, Fazili AA, Brannigan RE. Putative health risks associated with vasectomy. Urol Clin North Am. 2009;36:337–345. doi: 10.1016/j.ucl.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Holt SK, Salinas CA, Stanford JL. Vasectomy and the risk of prostate cancer. J Urol. 2008;180:2565–2567. doi: 10.1016/j.juro.2008.08.042. discussion 2567-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis LK, Dawson DV, Resnick MI. Vasectomy and the risk of prostate cancer: A meta-analysis examining vasectomy status, age at vasectomy, and time since vasectomy. Prostate Cancer Prostatic Dis. 2002;5:193–203. doi: 10.1038/sj.pcan.4500586. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 21.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidney S, Quesenberry CP Jr, Sadler MC, Guess HA, Lydick EG, Cattolica EV. Vasectomy and the risk of prostate cancer in a cohort of multiphasic health-checkup examinees: Second report. Cancer Causes Control. 1991;2:113–116. doi: 10.1007/BF00053130. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. A prospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269:873–877. [PubMed] [Google Scholar]
- 27.Giovannucci E, Tosteson TD, Speizer FE, Ascherio A, Vessey MP, Colditz GA. A retrospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269:878–882. [PubMed] [Google Scholar]
- 28.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: A Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 29.Lynge E. Prostate cancer is not increased in men with vasectomy in denmark. J Urol. 2002;168:488–490. [PubMed] [Google Scholar]
- 30.Rohrmann S, Paltoo DN, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Association of vasectomy and prostate cancer among men in a Maryland cohort. Cancer Causes Control. 2005;16:1189–1194. doi: 10.1007/s10552-005-0304-8. [DOI] [PubMed] [Google Scholar]
- 31.Nie Y, Liu CR, Guo XK. Clinical study of long term effects of vasectomy on the incidence rate of prostate cancer. Journal of Clinical Urology. 2008;23:424–427. [Google Scholar]
- 32.Romero FR, Romero AW, Almeida RM, Oliveira FC Jr, Tambara Filho R. The significance of biological, environmental, and social risk factors for prostate cancer in a cohort study in Brazil. Int Braz J Urol. 2012;38:769–778. doi: 10.1590/1677-553820133806769. [DOI] [PubMed] [Google Scholar]
- 33.Stanford JL, Wicklund KG, McKnight B, Daling JR, Brawer MK. Vasectomy and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:881–886. [PubMed] [Google Scholar]
- 34.Junghans C, Jones M. Consent bias in research: how to avoid it. Heart. 2007;93:1024–1025. doi: 10.1136/hrt.2007.120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kho ME, Duffett M, Willison DJ, Cook DJ, Brouwers MC. Written informed consent and selection bias in observational studies using medical records: systematic review. BMJ. 2009;338:b866. doi: 10.1136/bmj.b866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Shahi R, Vousden C, Warlow C Scottish Intracranial Vascular Malformation Study (SIVMS) Steering Committee. Bias from requiring explicit consent from all participants in observational research: prospective, population based study. BMJ. 2005;331:942. doi: 10.1136/bmj.38624.397569.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarma AV, McLaughlin JC, Wallner LP, Dunn RL, Cooney KA, Schottenfeld D, Montie JE, Wei JT. Sexual behavior, sexually transmitted diseases and prostatitis: the risk of prostate cancer in black men. J Urol. 2006;176:1108–1113. doi: 10.1016/j.juro.2006.04.075. [DOI] [PubMed] [Google Scholar]
- 38.Sutcliffe S. Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses. Future Oncol. 2010;6:1289–1311. doi: 10.2217/fon.10.95. [DOI] [PubMed] [Google Scholar]
- 39.Toh S, Gagne JJ, Rassen JA, Fireman BH, Kulldorff M, Brown JS. Confounding adjustment in comparative effectiveness research conducted within distributed research networks. Med Care. 2013;51:S4–10. doi: 10.1097/MLR.0b013e31829b1bb1. [DOI] [PubMed] [Google Scholar]
- 40.Shariff-Marco S, Klassen AC, Bowie JV. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010;100:364–374. doi: 10.2105/AJPH.2009.163899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


