Abstract
Cytokines and chemokines play an important role in defense against viral infection and modulating immune response. However, expression prolife of serum cytokines and chemokines, which were associated with the outcome of patients in response to anti-HCV treatment have not been fully elucidated. The current study aimed to determine the expression pattern of cytokines and chemokines in chronic HCV infection and their association with outcome in response to therapy. Seventy-two patients with HCV infection were enrolled, and fifty-one received peg-interferon α-2a and ribavirin therapy for 48 weeks. Thirty-nine cytokines and chemokines were analyzed by Luminex 200 and ELISA. In comparison to healthy individuals, production of IL-8 and IL-10 were increased in chronic hepatitis C patients. In contrast, IFN-γ, IL-7, and IL-15 were remarkably decreased, especially in HCV genotype 1b infection. HCV RNA load is closely associated with IL-10 and IL-15 expressions, and inhibition of HCV replication was accompanied by reduction in IL-10 and elevation in IL-7 and IL-15. Skewed cytokines and chemokines expression existed in chronic HCV infection, and might play an important role in persistent HCV infection. Exploiting the expression pattern of cytokines and chemokines may help to develop a better understanding of the pathogenesis of chronic HCV infection.
Keywords: Hepatitis C virus, cytokine, chemokine, antiviral therapy
Introduction
Hepatitis C virus (HCV) infection is world public health problem, with approximate 170 million chronic infections worldwide [1]. Chronic HCV infection lead to high risks for development of end-stage liver diseases, including decompensated liver cirrhosis, server hepatitis, and hepatocellular carcinoma [2,3]. Although HCV has been demonstrated as non-cytopathic, positive-stranded RNA virus, it can stimulate host immune response to induce variable extent of liver injury. Thus, the interaction between virus and host immune response may contribute to the outcome of chronic hepatitis C [4]. The use of direct antiviral agents has resulted in a remarkable improvement in the rate of sustained virological response than the combination therapy of pegylated interferon (PEG-IFN) and ribavirin [5], but they are still facing new issues such as viral mutation, relapse, and immune tolerance/exhaust [6]. Moreover, persistent hepatitis C virus infection often exhibited varied responses to therapy and immunological markers may associate with different clinical outcomes. Therefore, it is vital to identify the factors to further improve clinical management [7].
Cytokines and chemokines play an important role in initiating, maintaining, and regulating immunological homeostatic and inflammation in many pathological and physiological processes [8]. Recent studies revealed that dysregulation of cytokine and chemokine expressions might be involved in the pathogenesis of enterovirus 71 (EV71) infection and central nervous system complications of hand, foot and mouth disease [9]. Furthermore, certain cytokines and chemokines could recruit and active immune and inflammatory cells from peripheral blood to the liver in HBV infection [10,11]. Importantly, HCV is known to interfere and modulate cytokines and chemokines expression and escape immune response by altered leukocyte chemotaxis to establish persistent infection. This leads to the impaired viral clearance and recruitment of inflammatory infiltrates into hepatic environment by CXC chemokine ligand (CXCL)-9, -10, and -11, which results in sustained liver damage [12-14]. Hence, we hypothesized that the expression prolife of serum cytokines and chemokines might be associated with the outcome of patients in response to anti-HCV treatment. To test this possibility, we investigated the expression pattern of cytokines and chemokines in patients with chronic HCV infection, and thereby assess the relationship between cytokines/chemokines expression and effectiveness of IFN-α and ribavirin combination therapy.
Materials and methods
Subjects
A total of 72 patients with HCV infection were enrolled in this study. All patients were hospitalized or present for followed-up examinations in the Affiliated Hospital to Changchun University of Chinese Medicine from December 2012 to March 2014. Diagnoses were made in accordance with the standard of Chinese National Program for Prevention and Treatment of Viral Hepatitis. Twenty-one healthy individuals who matched for sex ratio and mean age were also enrolled for normal controls (NC). No subjects were co-infected with HIV or other hepatitis viruses. Those who received antiviral or immunomodulatory therapies within 6 months of baseline sampling were also excluded. The baseline characteristics of enrolled subjects were shown in Table 1. Fifty-one patients with HCV infection received peg-interferon α-2a (PEG-IFN-α2a, 40 KD, Roche, Shanghai, China) and ribavirin treatment for 48 weeks. Blood sampling were made on five different time points: baseline, 4 weeks, 12 weeks, 24 weeks, and 48 weeks after initiation of therapy. Based on the therapeutic response to antiviral treatment, those 51 patients could divide into three groups: rapid virological response (RVR, 16 patients), early virological response (EVR, 27 patients), and non-response (NR, 8 patients). RVR was defined as undetectable HCV RNA at 4 weeks of treatment. EVR was defined as detectable HCV RNA at 4 weeks but undetectable at 12 weeks after initiation of antiviral therapy. NR was defined as less than 2 log10 copies/mL decrease of HCV RNA at 12 weeks compared with baseline, and HCV RNA was still detectable at the end of standard therapy [15]. This study was approved by the ethics committee of the Affiliated Hospital to Changchun University of Chinese Medicine, and written informed consent was obtained from each subject.
Table 1.
Baseline clinical characteristics of enrolled subjects
| Group | Normal control | Chronic hepatitis C |
|---|---|---|
| Case | 21 | 72 |
| Sex (male/female) | 13/8* | 50/22* |
| Age (years) | 30 (22-38)# | 36 (18-52)# |
| ALT (U/L) | 24 (9-38)# | 38 (25-97)# |
| HCV RNA (log10 copies/mL) | N.D. | 4.61 (2.20-7.34) |
| HCV genotype (1b/2a) | N.D. | 43/29 |
Data are shown as median and range. ND: notdetermined.
P>0.05 Chi-squared test;
P>0.05 Mann-Whitney test.
Biochemical and virological assessments
Serum biochemical assessments (including aminotrasferase, albumin, bilirubin, urea nitrogen, and creatine) were tested by Hitachi 7600 automatic analyzer (Hitachi Ltd, Tokyo, Japan). Anti-HCV antibody was measured using commercial enzyme immunoassay kits (Jinhao Biotech, Beijing, China). HCV RNA was quantified using a commercial real-time PCR kits (PG Biotech, Shenzhen, China) with detection limit of 2 log10 copies/mL. HCV genotyping was performed by a second generation line probe assay (Inno-Lipa II, Innogenetics, Zwijndre, Belgium).
Cytokines and chemokines assay
Serum samples were collected by centrifugation of blood at 3,000 g for 10 min, and stored immediately to -80°C until use. Expression of epidermal growth factor (EGF), Eotaxin, fibroblast growth factors (FGF-2), fms like tyrosine kinase receptor 3 ligand (Flt-3L), Fractalkine, granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), growth-related oncogene (GRO), interferon (IFN)-α2, IFN-γ, interleukin (IL)-1Rα, IL-1α, IL-1β, IL-2, sIL-2Rα, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17A, IL-29, interferon-inducible protein-10 (IP-10), monocyte chemotactic protein (MCP)-1, MCP-3, macrophage inflammatory protein (MIP)-1α, MIP-1β, sCD40L, transforming growth factor (TGF)-α, tumor necrosis factor (TNF)-α, TNF-β, and vascular endothelial growth factor (VEGF), were measured by MILLIPLEX MAP Human Cytokine/Chemokine-Premixed 39 Plex (EMD Millipore, Billerica, MA, USA) using Luminex 200TM Multiplexing Instrument (EMD Millipore) according to the manufacturer’s instructions.
Enzyme linked immunosorbent assay (ELISA)
Concentrations of IFN-γ, IL-7, IL-8, IL-10, and IL-15 were measured using commercial ELISA kits (eBioscience, San Diego, CA, USA) according to the manufacture’s instruction.
Statistical analyses
Data were analyzed using SPSS version 17.0 for Windows (SPSS, Chicago, IL, USA). Mann-Whitney test was used for the comparison among groups. Pearson correlation tests were performed for correlation analysis. All tests were two-tailed and P values of <0.05 were considered to indicate a significant difference.
Results
Increase expression of IL-8 and IL-10, but decrease of IFN-γ, IL-7, and IL-15 in chronic hepatitis C patients
We examined the serum samples from 72 chronic HCV infected patients and 21 NC. A total of 39 cytokines and chemokines were tested. The expressions of 14 cytokines and chemokines (including Flt-3L, IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-9, IL-11, IL-12 (p40), IL-12 (p70), IL-29, M-CSF, TGF-α, and TNF-β) were below the limits of detection in the serum of both NC and patients with chronic hepatitis C. Among them, 22 (consisted of EGF, eotaxin, FGF-2, fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IL-R1α, IL-6, IL-13, IL-17, MCP-1, MCP-3, MIP-1α, MIP-1β, sCD40L, sIL-2Rα, TNF-α, and VEGF) could be detected. However, there were no remarkable differences in their serum concentrations between patients with chronic HCV infection and NC (data not shown). Interestingly, the expressions of IL-8 and IL-10 were significantly elevated in the serum of HCV-infected patients in comparison to that in NC (IL-8: 7.92 ± 3.01 pg/mL vs. 15.70 ± 14.07 pg/mL, P = 0.0312, Figure 1A; IL-10: 16.19 ± 5.89 pg/mL vs. 32.21 ± 24.85 pg/mL, P = 0.0035, Figure 1B). In contrast, serum levels of IFN-γ, IL-7, and IL-15 were markedly decreased in patients with HCV infection in comparison to levels in NC (IFN-γ: 43.28 ± 19.41 pg/mL vs. 28.04 ± 37.73 pg/mL, P = 0.0006, Figure 1C; IL-7: 31.43 ± 16.90 pg/mL vs. 18.64 ± 19.96 pg/mL, P = 0.0032, Figure 1D; IL-15: 28.66 ± 27.28 pg/mL vs. 14.89 ± 13.95 pg/mL, P=0.0148, Figure 1E).
Figure 1.
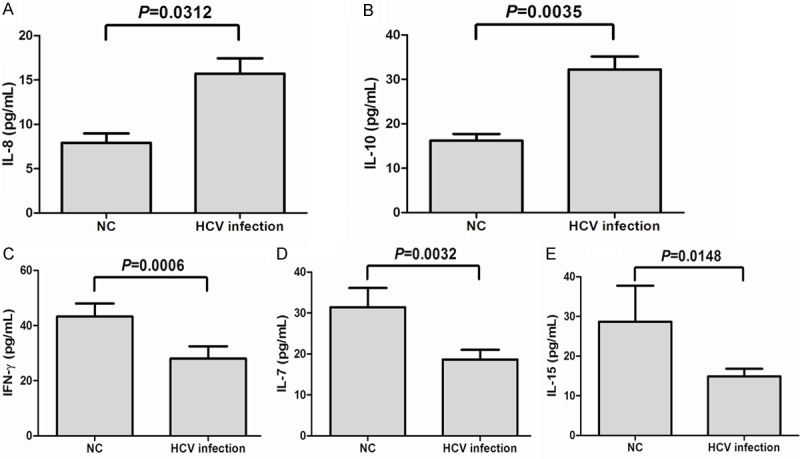
Serum cytokines and chemokines expression in patients with chronic hepatitis C and in controls. The concentrations of IL-8 (A) and IL-10 (B) were elevated in patients with chronic hepatitis C in comparison to the normal controls (NC). Expressions of IFN-γ (C), IL-7 (D), and IL-15 (E) was decreased in patients with chronic hepatitis C. All the samples were tested for baseline level.
Elevated IL-10 but decrease IL-7 and IL-15 in HCV genotype 1b patients
We further analyzed the cytokines and chemokines expression between the two different HCV genotypes. A total of 43 patients were demonstrated with HCV genotype 1b infection and 29 patients with HCV genotype 2a infection, respectively. There were no significant differences for IL-8 (15.28 ± 14.68 pg/mL vs. 16.58 ± 13.02 pg/mL, P = 0.3159, Figure 2A) and IL-7 (21.80 ± 23.14 pg/mL vs. 17.03 ± 18.18 pg/mL, P = 0.3438, Figure 2D) concentrations between the two genotypes infection. However, serum level of IL-10 was remarkably higher in HCV genotype 1b group (37.16 ± 27.42 pg/mL) compared with HCV genotype 2a (21.67 ± 13.49 pg/mL, P = 0.0079, Figure 2B). In contrast, IFN-γ and IL-15 concentrations were notably decreased in HCV genotype 1b group compared with genotype 2a group (IFN-γ: 20.40 ± 19.54 pg/mL vs. 38.74 ± 52.37 pg/mL, P = 0.0488, Figure 2C; IL-15: 7.592 ± 2.863 pg/mL vs. 19.31 ± 16.07 pg/mL, P < 0.0001, Figure 2E).
Figure 2.
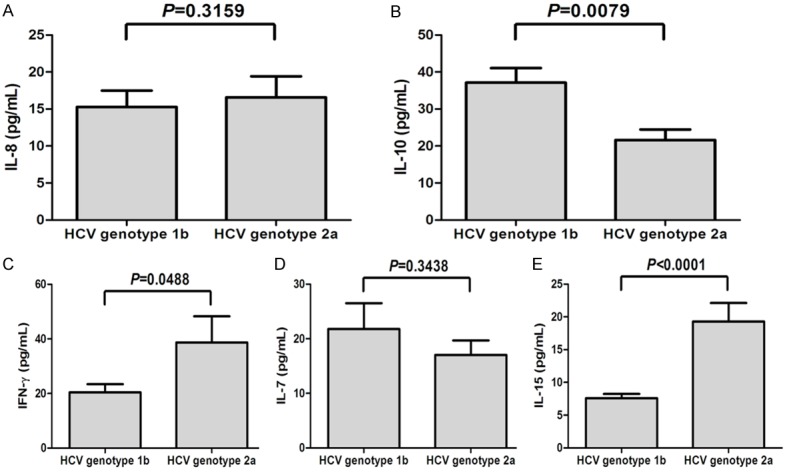
Serum cytokines and chemokines expression in patients with HCV genotype 1b and 2a infection. The levels corresponding to IL-8 (A), IL-10 (B), IFN-γ (C), IL-7 (D), and IL-15 (E) in patients with HCV genotype 1b and 2a infection. All the samples were tested for baseline levels.
Correlation between cytokine expression and HCV RNA level
We also investigated the relationship between cytokines/chemokines expression and HCV RNA levels. As shown in Figure 3, there was a positive correlation between IL-10 expression and HCV RNA (r = 0.2663, P = 0.0238, Figure 3A). Moreover, bivariate correlation revealed that IL-15 concentrations in the serum were directly and significantly associated with HCV RNA (r = -0.2667, P = 0.0362, Figure 3B). However, neither IL-7/IL-8 nor IFN-γ was notably associated with viral titre or liver inflammation (P > 0.05, data not shown).
Figure 3.
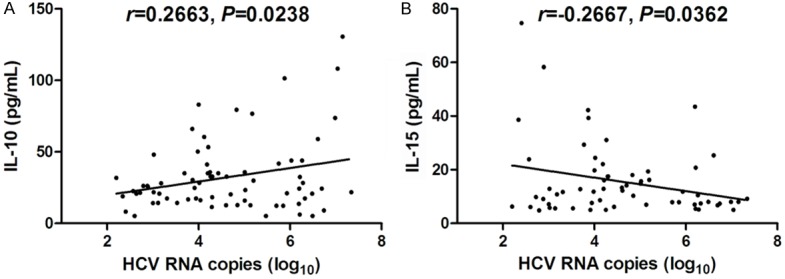
Correlation analysis of cytokines and chemokines with HCV RNA. A. IL-10 expression positively correlated with HCV RNA. B. IL-15 expression negatively correlated with HCV RNA. All the samples were tested for baseline levels. All patients types including RVR, EVR, and NR were enrolled into this analysis.
Kinetics of cytokines expression in response to anti-HCV therapy
Among the 72 patients with chronic HCV infection, 51 patients received PEG-IFN-α2a with ribavirin therapy. We tested the HCV RNA at baseline, 4, 12, 24, and 48 weeks post therapy, and found that 16 of the patients were RVR, 27 were EVR, and 8 were NR. To assess the effect of anti-HCV treatment in serum cytokines expression in patients with different response to therapy, we longitudinally determined the concentrations of serum IL-8, IL-10, IFN-γ, IL-7, and IL-15 by ELISA. The expressions of serum IL-8 and IFN-did not reveal significant changes in all patients in response to anti-HCV therapy (Figure 4A and 4C). The serum IL-10 concentration was reduced rapidly 4 weeks after initiation of antiviral therapy and continuously decreased during the observation period in chronic hepatitis C patients with RVR (Figure 4B). There was also a consistent trend of IL-10 reduction in EVR patients, but they still presented relatively high levels at both 4 weeks and 12 weeks (Figure 4B). However, there were no remarkable changes in NR patients in response to PEG-IFN-α2a and ribavirin treatment (Figure 4B). In contrast, IL-7 expressions showed an increase trend in response to antiviral therapy in both RVR and EVR patients, and IL-7 maintained at high levels after 12 weeks of therapy (Figure 4D). IL-15 concentrations revealed similar trends to IL-7. However, the levels of IL-15 were slowly increased in response to anti-HCV treatment, and were significantly elevated 24 weeks after initiation of therapy in patients with RVR and EVR (Figure 4E). Furthermore, NC patients revealed continuously low IL-7 and IL-15 levels during observation period (Figure 4D and 4E).
Figure 4.
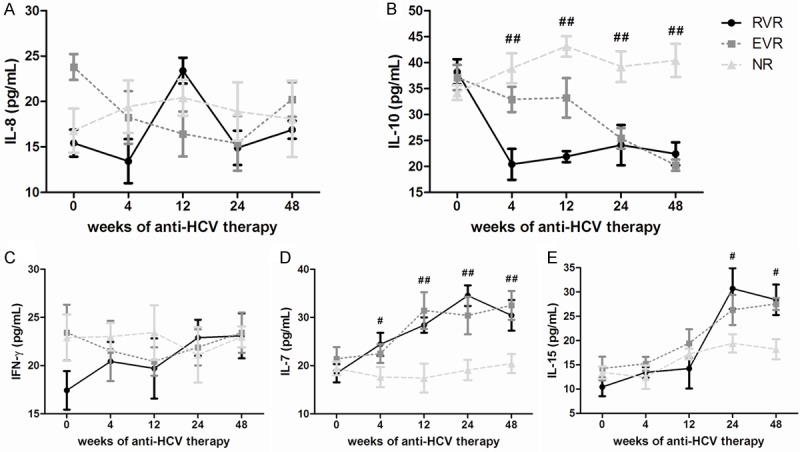
Serum cytokines and chemokines expression in response to pegylated interferon-a2a and ribavirin during the course of therapy. Levels of IL-8 (A), IL-10 (B), IFN-γ (C), IL-7 (D), and IL-15 (E) were observed from baseline to 48 weeks in rapid virological response (RVR), early virological response (EVR) and non-response (NR) patients. #P < 0.05 and ##P < 0.01 refers to the statistical differences among RVR, EVR and NR at each time-point.
Discussion
Viral and host factors are related to the progression of chronic viral hepatitis. Numerous studies have been focus on the molecules and immune cells in the ability to clear chronic HBV or HCV infection, and revealed that the profile of cytokines and chemokines involved in the immune response may contribute to the outcome of viral hepatitis. Lian et al. [16] demonstrated that augmented CXCR3-associated chemokines expressions contribute to liver inflammation, but MCP-3 and G-CSF were inhibited in chronic hepatitis B. Studies on hepatitis C also showed that cytokine profiles may predict the outcome of HCV infection, since chronic hepatitis C revealed increased expressions of IL-8 and CCL5, whereas elevated IL-1α, TNF-α, TGF-β, CCL-8, IL-13, and IL-15 were found in patients with spontaneously clearance of HCV [17]. Moreover, occult HCV infection exhibited a distinct immunoregulatory cytokine pattern that is shifted towards the Th2 arm [18]. In the present study, we screened the levels of 39 cytokines and chemokines in patients with chronic hepatitis C and analyzed their association with clinical indicators. We found that in comparison to NC, IL-8 and IL-10 levels were significantly elevated in patients with chronic hepatitis C, but only serum IL-10 was increased in HCV genotype 1b patients and had positive correlation with HCV RNA. In contrast, serum concentrations of IFN-γ, IL-7, and IL-15 were remarkably decreased in chronic hepatitis C. IFN-γ and IL-15 levels were associated with HCV genotype 1b infection, however, only IL-15 concentration was negatively correlated with HCV RNA. None of the changes in those cytokines and chemokines concentration had correlations with liver inflammation. Furthermore, we also longitudinally monitored the cytokine expressions in response to PEG-IFN-α2a and ribavirin induced suppression of HCV replication, and defined the relationship between HCV RNA and cytokine changes. Inhibition of viral replication was associated with decrease in IL-10 and increase in IL-7 and IL-15 but had little influence in IL-8 and IFN-γ, which was manifested by robust changes of IL-10, IL-7 and IL-15 in patients with virological response (RVR and EVR) but less change in NR patients. Hence, the imbalance expression profile of cytokines and chemokines might play an important role in persistent HCV infection.
IL-10 is known as an anti-inflammatory cytokine and a modulator of immune response, which is also has antifibrotic properties and play a role in the progression of liver diseases [19]. IL-10 gene polymorphisms might be a predictor of clinical outcome of hepatitis C and related liver disease, as well as the response to anti-HCV treatment [19,20]. Regulatory T cells (Tregs) secreted IL-10, which played a vital role in mediating Treg suppression in persistent viral infection [21]. HCV core protein induced the CD4+ primary T cells to express the markers of Tregs, increase IL-10 production, and decrease the secretion of IFN-γ in response to stimulation [22]. Furthermore, IL-10 could directly targeted to naïve HCV-specific CD8+ T cells and led to impaired differentiation of functional CD8+ T cells, which contributed to T cell failure and viral persistence [23]. We found that the increasing expression of IL-10 were closely associated with viral loads, and demonstrated a similar trend of changes with Tregs proportion in response to anti-viral therapy [7]. Concentrations of IL-10 were elevated in HCV infection especially in HCV genotype 1b patients, and significantly correlated with HCV RNA before treatment. IL-10 levels did not change remarkably in NR patients, whose HCV viral loads were consistently detectable during anti-HCV treatment. In contrast, IL-10 notably reduced in response to PEG-IFN-α2a and ribavirin therapy in RVR and EVR patients. Moreover, IL-10 production was also significantly lower in RVR than EVR at 4 weeks after initiation of therapy, when RVR patients have reached virological response while EVR patients still have detectable HCV RNA at that time point. Expression of IL-10 revealed similarly low levels 24 weeks after initiation of treatment in RVR and EVR patients when they reached complete inhibition of viral replication.
IL-7 and IL-15 are common γ chain cytokine family members. IL-7 is required for establishment and maintenance of memory CD4+ and CD8+ T cells. IL-15, which is secreted in response to viral infection, induces the proliferation of NK cells in the liver [24]. IL-7 immunotherapy reduced local and systemic inflammations in HIV-infected patients who had incomplete T cell reconstitution [25]. IL-7 also augmented HCV DNA vaccine-induced antibody and broad T cell response [26]. Moreover, IL-7 administration decreased the PD-1 expression in CD8+ T cells and transient T cell repertoire diversification, which will be benefit in treatment of HIV/HCV coinfection [27]. Elevated IL-15 induced the activation of CD4+ and CD8+ T cell in peripheral blood, as well as hepatic stellate cells in the liver in patients with HIV/HCV coinfection [28]. Importantly, recent study have demonstrated that common γ chain cytokines, including IL-2, IL-7, and IL-15, increased the antiviral efficacy of HCV-specific CD8+ T cells with CD127 expression, which is known as IL-7 receptor-α [29]. In this study, we found that both IL-7 and IL-15 were downregulated in chronic hepatitis C and IL-15 levels were negatively correlated with HCV RNA, which might indicate that HCV infection suppressed the secretion of IL-7 and IL-15. Furthermore, IL-7 represented an early response during anti-HCV therapy, as IL-7 productions were similar in patients with RVR and EVR at 4 weeks after initiation of treatment and maintained in relatively high levels in the following time points. In contrast, IL-15 represented an late response with sharply elevation of expression 24 weeks after treatment. Thus, IL-7 and IL-15 may contributed to the antiviral and immunomodulatory function in chronic HCV infection.
We also showed an increase in IL-8 production and a decrease in IFN-γ expression in chronic HCV infection. This was consistent with several previous studies, which revealed that elevated IL-8 associated with control of liver infection, especially in treatment non-responders of HCV infection [30,31]. However, no correlations were found between viral replication and expressions of these two cytokines. Moreover, there were stable IL-8 and IFN-γ levels during anti-HCV therapy. Ribavirin has been demonstrated to enhance the anti-HCV efficiency of IFN-α by augmenting IFN-γ response of NK cells [32] and activating IL-8 [33]. But ribavirin might only influence the function, but not expression of IL-8 and IFN-γ. Thus, based on the results from the current experiments, the involvement of IL-8 and IFN-γ in chronic HCV infection is not clear and needs further confirmation.
Conclusion
In conclusion, the current study highlights that the skewed cytokines and chemokines expression existed in chronic HCV infection. HCV RNA load is closely associated with IL-10, IL-7 and IL-15 productions during PEG-IFN-α2a and ribavirin therapy in patients with chronic hepatitis C, and these cytokines might be used as biomarker for the likelihood of treatment failure. More importantly, exploiting the expression pattern of cytokines and chemokines may help to develop a better understanding of the pathogenesis of chronic HCV infection.
Disclosure of conflict of interest
None.
References
- 1.Li HC, Lo SY. Hepatitis C virus: Virology, diagnosis and treatment. World J Hepatol. 2015;7:1377–1389. doi: 10.4254/wjh.v7.i10.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Martinez-Lopez E, Roman S, Panduro A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J Gastroenterol. 2014;20:3443–3456. doi: 10.3748/wjg.v20.i13.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Au JS, Pockros PJ. Novel therapeutic approaches for hepatitis C. Clin Pharmacol Ther. 2014;95:78–88. doi: 10.1038/clpt.2013.206. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, von Hahn T. Novel therapies for hepatitis C - one pill fits all? Nat Rev Drug Discov. 2013;12:595–610. doi: 10.1038/nrd4050. [DOI] [PubMed] [Google Scholar]
- 7.Hao C, Zhou Y, He Y, Fan C, Sun L, Wei X, Wang L, Peng M, Wang P, Lian J, Jia Z. Imbalance of regulatory T cells and T helper type 17 cells in patients with chronic hepatitis C. Immunology. 2014;143:531–538. doi: 10.1111/imm.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He D, Li M, Guo S, Zhu P, Huang H, Yan G, Wu Q, Tao S, Tan Z, Wang Y. Expression pattern of serum cytokines in hepatitis B virus infected patients with persistently normal alanine aminotransferase levels. J Clin Immunol. 2013;33:1240–1249. doi: 10.1007/s10875-013-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng M, Zheng X, Wei R, Zhang N, Zhu K, Xu B, Yang CH, Yang CF, Deng C, Pu D, Wang X, Altmeyer R, Leng Q. The cytokine and chemokine profiles in patients with hand, foot and mouth disease of different severities in shanghai, china, 2010. PLoS Negl Trop Dis. 2013;7:e2599. doi: 10.1371/journal.pntd.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakimi K, Lane TE, Wieland S, Asensio VC, Campbell IL, Chisari FV, Guidotti LG. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J Exp Med. 2001;194:1755–1766. doi: 10.1084/jem.194.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011;141:1897–1906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallahi P, Ferri C, Ferrari SM, Corrado A, Sansonno D, Antonelli A. Cytokines and HCV-related disorders. Clin Dev Immunol. 2012;2012:468107. doi: 10.1155/2012/468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brass A, Brenndorfer ED. The role of chemokines in hepatitis C virus-mediated liver disease. Int J Mol Sci. 2014;15:4747–4779. doi: 10.3390/ijms15034747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahey S, Dempsey E, Long A. The role of chemokines in acute and chronic hepatitis C infection. Cell Mol Immunol. 2014;11:25–40. doi: 10.1038/cmi.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for Study of Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Lian JQ, Yang XF, Zhao RR, Zhao YY, Li Y, Zhang Y, Huang CX. Expression profiles of circulating cytokines, chemokines and immune cells in patients with hepatitis B virus infection. Hepat Mon. 2014;14:e18892. doi: 10.5812/hepatmon.18892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Trujillo-Trujillo ME, Roman S, Trujillo-Ochoa JL, Panduro A. Spontaneous hepatitis C viral clearance and hepatitis C chronic infection are associated with distinct cytokine profiles in Mexican patients. Mem Inst Oswaldo Cruz. 2015;110:267–271. doi: 10.1590/0074-02760140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousa N, Eldars W, Eldegla H, Fouda O, Gad Y, Abousamra N, Elmasry E, Arafa M. Cytokine profiles and hepatic injury in occult hepatitis C versus chronic hepatitis C virus infection. Int J Immunopathol Pharmacol. 2014;27:87–96. doi: 10.1177/039463201402700111. [DOI] [PubMed] [Google Scholar]
- 19.Swiatek BJ. Is interleukin-10 gene polymorphism a predictive marker in HCV infection? Cytokine Growth Factor Rev. 2012;23:47–59. doi: 10.1016/j.cytogfr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Vasconcelos LR, Moura P, do Carmo RF, Pereira LB, Cavalcanti Mdo S, Aroucha DC, Dutra RA, Pereira LM. Low IL10 serum levels as key factor for predicting the sustained virological response to IFNalpha/ribavirin in Brazilian patients with HCV carrying IL28B CT/TT genotype. Hum Immunol. 2014;75:895–900. doi: 10.1016/j.humimm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Ponce C, Dominguez-Villar M, Aguado E, Garcia-Cozar F. CD4+ primary T cells expressing HCV-core protein upregulate Foxp3 and IL-10, suppressing CD4 and CD8 T cells. PLoS One. 2014;9:e85191. doi: 10.1371/journal.pone.0085191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niesen E, Schmidt J, Flecken T, Thimme R. Suppressive effect of interleukin 10 on priming of naive hepatitis C virus-specific CD8+ T cells. J Infect Dis. 2015;211:821–826. doi: 10.1093/infdis/jiu541. [DOI] [PubMed] [Google Scholar]
- 24.Golden-Mason L, Kelly AM, Doherty DG, Traynor O, McEntee G, Kelly J, Hegarty JE, O’Farrelly C. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clin Exp Immunol. 2004;138:94–101. doi: 10.1111/j.1365-2249.2004.02586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober A, Wilson EM, Natarajan V, Imamichi H, Boulassel MR, Lederman MM, Routy JP. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10:e1003890. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SH, Song MY, Nam HJ, Im SJ, Sung YC. Codelivery of IL-7 Augments Multigenic HCV DNA Vaccine-induced Antibody as well as Broad T Cell Responses in Cynomolgus Monkeys. Immune Netw. 2010;10:198–205. doi: 10.4110/in.2010.10.6.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker R, Dutrieux J, Beq S, Lemercier B, Rozlan S, Fabre-Mersseman V, Rancez M, Gommet C, Assouline B, Rance I, Lim A, Morre M, Cheynier R. Interleukin-7 treatment counteracts IFN-alpha therapy-induced lymphopenia and stimulates SIV-specific cytotoxic T lymphocyte responses in SIV-infected rhesus macaques. Blood. 2010;116:5589–5599. doi: 10.1182/blood-2010-03-276261. [DOI] [PubMed] [Google Scholar]
- 28.Allison RD, Katsounas A, Koziol DE, Kleiner DE, Alter HJ, Lempicki RA, Wood B, Yang J, Fullmer B, Cortez KJ, Polis MA, Kottilil S. Association of interleukin-15-induced peripheral immune activation with hepatic stellate cell activation in persons coinfected with hepatitis C virus and HIV. J Infect Dis. 2009;200:619–623. doi: 10.1086/600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seigel B, Bengsch B, Lohmann V, Bartenschlager R, Blum HE, Thimme R. Factors that determine the antiviral efficacy of HCV-specific CD8 (+) T cells ex vivo. Gastroenterology. 2013;144:426–436. doi: 10.1053/j.gastro.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 30.Sousa GM, Oliveira IS, Andrade LJ, Sousa-Atta ML, Parana R, Atta AM. Serum levels of Th17 associated cytokines in chronic hepatitis C virus infection. Cytokine. 2012;60:138–142. doi: 10.1016/j.cyto.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Pham TN, Lin DM, Mulrooney-Cousins PM, Churchill ND, Kowala-Piaskowska A, Mozer-Lisewska I, Machaj A, Pazgan-Simon M, Zalewska M, Simon K, King D, Reddy SB, Michalak TI. Hepatitis C virus load and expression of a unique subset of cellular genes in circulating lymphoid cells differentiate non-responders from responders to pegylated interferon alpha-ribavirin treatment. J Med Virol. 2013;85:441–448. doi: 10.1002/jmv.23481. [DOI] [PubMed] [Google Scholar]
- 32.Werner JM, Serti E, Chepa-Lotrea X, Stoltzfus J, Ahlenstiel G, Noureddin M, Feld JJ, Liang TJ, Rotman Y, Rehermann B. Ribavirin improves the IFN-gamma response of natural killer cells to IFN-based therapy of hepatitis C virus infection. Hepatology. 2014;60:1160–1169. doi: 10.1002/hep.27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokumoto Y, Hiasa Y, Uesugi K, Watanabe T, Mashiba T, Abe M, Kumagi T, Ikeda Y, Matsuura B, Onji M. Ribavirin regulates hepatitis C virus replication through enhancing interferon-stimulated genes and interleukin 8. J Infect Dis. 2012;205:1121–1130. doi: 10.1093/infdis/jis025. [DOI] [PubMed] [Google Scholar]


