Abstract
Doxorubicin (DOX) is one of the widely used chemotherapeutic drugs for the treatment of human osteosarcoma (OS). However, acquisition of DOX resistance is common in patients with OS, leading to local and distant failure. In this study, we demonstrate that survivin expression is significantly upregulated in OS primary tumors compared to paired normal tissue. In addition, survivin expression was further increased in DOX resistant cells (MG63/DOX) as compared to its parent cells (MG63). Thus, we hypothesize that targeting of survivin in OS could reverse the DOX resistant phenotype in tumor cells thereby enhancing the therapeutic efficacy of DOX. We test the efficacy of YM155, a small molecule survivin inhibitor, either as a single agent or in combination with DOX in vitro and in vivo. We found that combination treatment of YM155 and DOX in DOX resistant cells (MG63/DOX) could significantly inhibited cell proliferation and colony formation, induce cell apoptosis and promoted caspase-3, -8, and -9 activity in vitro, and promoted tumor regression in established OS xenograft models. Taken together, the evidence presented here supports the favorable preclinical evaluation that YM155 could overcome DOX the resistance in tumor cells thereby enhancing the effectiveness of DOX in OS, suggesting that YM155 in combination with DOX has potential in the treatment of osteosarcoma.
Keywords: Osteosarcoma, YM155, doxorubicin, survivn
Introduction
Osteosarcoma (OS) is the most common histological form of primary bone cancer in the childhood and adolescent [1]. It arises from the malignant transformation of mesenchymal cells, often occurring during cell differentiation in the formation of osteoid and immature bone, leads to a high mortality rate [1,2]. Doxorubicin (DOX) well established to be used as chemotherapeutic drugs in osteosarcoma [3,4]. However, patients with late-stage cancer often develop resistance to DOX, and possess poor prognosis with 5~20% survival rate after surgery [5]. Therefore, it is urgent need to understand the molecular mechanisms that contribute to drug resistance of tumors, and to identify novel therapeutic targets in human OS.
Survivin, the smallest and structurally unique member of the inhibitors of apoptosis protein (IAP) family, plays crucial role in various cellular proliferation, including cell division, surveillance checkpoints and stress response [6]. Survivin protein is largely undetected or expressed at very low levels in normal tissues [7], whereas it is overexpressed in many malignancies including OS and has also been linked to poor patient survival of OS [8,9]. In addition, accumulating evidence suggest that survivin expression is associated with drug-resistance in cancer cells and cancer associated endothelial cells [10-12]. Therefore, we hypothesize that targeting of survivin in OS may enhance the therapeutic efficacy of DOX by inhibiting its expression.
To date, several strategies to modulate the expression/activation of surviving have been developed. YM155, a novel small molecule inhibitor of survivin, was identified by cell-based high-throughput screening [13]. YM155 has been to exhibit potent anti-tumor activity in non-small cell lung cancer [14], hepatoblastoma [15], melanomal [16], pancreatic cancer [17], prostate cancer [18]. In addition, in clinical settings, YM155 was shown to be tolerable in phase II studies with advanced cancer patients and showed antitumor activity in those with advanced refractory non-small cell lung carcinoma and unresectable melanoma [14,19]. Interesting, YM155 could enhance sensitive chemotherapeutic drugs to tumor cell [14,16,18,20]. For OS, our recently study showed that YM155 significantly inhibits OS cell proliferation, colony formation, migration and invasion, and induces cell apoptosis and increases caspase-3, -8 and -9 activities in vitro, and suppressed tumor growth in vivo [21]. However, whether downregulation of survivin in OS by YM155 could enhance the therapeutic efficacy of DOX remains unclear.
Here, we evaluated the therapeutic potential of YM155 alone and in combination with DOX in vitro and in vivo. We found that YM155 could overcome DOX the resistance in tumor cells thereby enhancing the effectiveness of DOX in OS, suggesting that YM155 in combination with DOX was effective treatment of osteosarcoma method.
Materials and method
Reagents and antibodies
The small-molecule survivin inhibitor YM155 was brought from Selleck Chemicals (Houston, TX, USA) and was dissolved and diluted in saline before its use in treatments in vitro and in vivo. DOX was brought from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and was dissolved and diluted in saline and stored at -20°C.
Patients and tissue samples
Twenty patients with osteosarcoma (12 male and 8 female) treated in the Department of Orthopedics, China-Japan Union Hospital of Jilin University, between June 2009 and August 2014, were enrolled retrospectively in this study. All human osteosarcoma biopsy specimens were obtained from primary lesions. After surgical resection, tumor tissues and adjacent normal tissues were collected and stored at -80°C until use. None of the patients had received chemotherapy or radiotherapy before surgery. Informed consent was obtained from all patients and the study was approved by the Research Ethics Committee of Jilin University (Changchun, China).
Cell culture
The human osteosarcoma cell lines MG-63 was obtained from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were all routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, GIBCO), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin in a 5% CO2 humidified atmosphere at 37°C.
Induction of doxorubicin resistance in OS cell lines
A DOX resistant cell line (MG-63/DOX) was established from its parental cell line MG-63 by gradually increasing the concentration of DOX to which the cells were exposed in a stepwise manner over a period of 6 months. In briefly, MG63 cells were initially cultured in DMEM containing 5 nM DOX and the cells that proliferated were repeatedly sub-cultured in DMEM containing increasing concentrations of DOX over a 6 month period. Cells that grew in 65 nM DOX were designated as MG63/DOX. The cells were incubated in drug-free medium for at least 1 week before use.
Quantitative real time PCR analysis
RNA from the OS tumors, adjacent normal controls, OS cell lines was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. M-MLV reverse transcriptase (Fermentas, USA) was used to create cDNA following the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (RT-PCR) assays were carried out using SYBR TAQ real-time kits (TaKaRa Biotechnology, Otsu, Japan) under ABI PRISM 7900HT (Applied Biosystems, Foster City, CA, USA). The PCR primers of survivin and GAPDH were design as previous described [22]. GAPDH was used as internal control. The 2-ΔΔCT method was used to calculate the relative abundance of target gene expression generated using Rotor-Gene Real-Time Analysis Software 6.1.81.
Immunohistochemistry
Sample were dewaxed in xylene, rehydrated in descending alcohols, and blocked for endogenous peroxidase and avidin/biotin activities. Sections were incubated with antibody against human survivin (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:5000 overnight at 4°C, followed incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibody for 2 h at room temperature. After washing with PBS three times, immunostaining was visualized by a streptavidin-peroxidase reaction system under an optical microscope.
Cell proliferation assay
Cell proliferation was measured using the MTT method. In briefly, the cell density of cells was adjusted to 5×104/ml, and cells were added to a 96-well plate (100 μl /well). After 24 h, in the blank controls, 100 μl of medium alone was added. At 24 h after culture, cells were treated with the indicated concentration of YM155 (0, 1, 10, 100 nM) or DOX (0, 1, 10, 50, 100, 200 nM), and cultured for 72 h. For combination treatment, cells were pretreated with YM155 for 6 hrs and then treated with DOX. At indicated time points, 20 μl methylthiazoletetrazolium (MTT) solution (5 mg/mL) was added into each well and cultured for 4 h, followed 200 μl of DMSO was added to each well for 10 min. Then absorbance was measured at 570 nm with a microplate reader (Molecular Devices Corp., Sunnyvale, CA, USA). The percentage cell growth inhibition for each treatment group was calculated by adjusting the untreated group to 100%. All experiments were performed in triplicate.
Colony formation assay
1.0×103 treated cells were plated in six-well plates in growth medium and were cultured for 14 days in drugs-free conditions, and the medium was replaced every 3 days. The colonies were fixed with 4% paraformaldehyde for 20 min and stained with 1% crystal violet for 10 min. The percentage colony formation was calculated by adjusting untreated cells to 100%.
Cell apoptosis assay
The effect of YM155 in combination with DOX on cell apoptosis was determined by flow cytometry. In briefly, 5.0×104 cells were plated in 60-mm dishes and treated with YM155 and DOX alone or combination for 48 h. Then cells were stained with AnnexinV (Molecular Probes) and propidium iodide (Sigma-Aldrich, USA) and analyzed by using flow cytometry (BD Biosciences, Mansfield, MA, USA).
In addition, caspase-3, -8 and -9 activity was detected as an additional indicator of apoptosis using caspases colorimetric protease assay kits (Millipore Corporation, Billerica, MA, USA) after treatment with YM155 and DOX alone or both, as previously described [23]. The relative caspase-3, -8 and -9 activity of the control blank group was referred as 100.
Western blot assay
Tissue sample and cultured cells were harvested and homogenized with RIPA lysis buffer (Sigma). Total protein concentration was detected using a bicinchoninic acid (BCA) protein assay kit (Boster, China). Each twenty micrograms of sample was f in separated by 8%-12% SDS-polyacrylamide gels, and the separated proteins were transferred to nitrocellulose membrane (Bio-Rad, Munich, Germany). The membranes were blocked with 5% non-fat dry milk for 2 h at room temperature and incubated with antibody against human survivin (Santa Cruz Biotechnology) and GAPDH (Santa Cruz Biotechnology) overnight at 4°C. After rinsing for three times, the membranes were incubated withhorseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit (Santa Cruz Biotechnology) for 2 h at room temperature. Protein bands were visualized with enhanced chemioluminescence reagent (ECL, Amersham, GE Healthcare, Velizy-Villacoublay, France). Protein loading was normalized by stripping the blots and then re-probing with anti-GAPDH antibody.
In vivo experiments
The in vivo antitumor activity of YM155 and DOX singly administered and in combination, was assessed on MG63/DOX cells xenotransplanted into five-week-old male BALB/c nude mice (Experiments Animal Center of Changchun Biological Institute, Changchun, China). Mice were maintained in laminar flow rooms keeping temperature and humidity constant and had free access to food and water. Experiments were approved by the Ethics Committee for Animal Experimentation of the Jilin University (Changchun, China).
2×106 exponentially growing MG63/DOX cells were subcutaneously injected into mouse right flank. When subcutaneous tumors reached a size of 100 mm3, xenografted animals were randomly divided randomly into control group (PBS), YM155, DOX and combination group. The control group received PBS. The other three groups were treated with YM155 (10 mg/kg body weight), DOX (10 mg/kg body weight), or YM155 plus DOX (2.5 mg/kg plus 2.5 mg/kg body weight, respectively) by subcutaneously administered as a 3-day continuous infusion per week for 4 weeks. Tumor growth was followed by weekly measurements of tumor diameters with a Vernier caliper according to the following formula: [π/6 × length × width × height]. 4 weeks after inoculation, mice were sacrificed, and tumors were striped and weighed. The efficacy of the drug treatment was assessed as tumor volume inhibition (TVI) percentage in treated vs. control mice, calculated as: TVI= (mean TV treated/mean TV control ×100). Part of tumor tissues was collected for analysis of the expression of survivin by western blot.
Statistical analysis
All data are expressed as mean ± SD (standard deviation) from at least three independent experiments. Statistical analysis between two samples was performed using Student t test and more than two groups was performed using one-way ANOVA followed by a Tukey post hoc test. Statistical analysis was performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). A P-value <0.05 was considered as statistically significant.
Results
Survivin is up-regulated in OS tissue
To identify the potential roles of survivin in the development and progression of OS, we detected survivin expression levels in tumor tissues and matched normal tissues from 20 patients with OS by real time quantitative RT-PCR (qRT-PCR) and immunohistochemistry. It was found that survivin expression on mRNA level was significantly up-regulated in OS tissues compared to in the matched adjacent normal tissues (Figure 1A, P<0.05). Consistent with qRT-PCR results, elevated levels of survivn protein were found in OS tissues compared with paired adjacent normal tissue from the same patients by immunochemical staining assay (Figure 1B).
Figure 1.
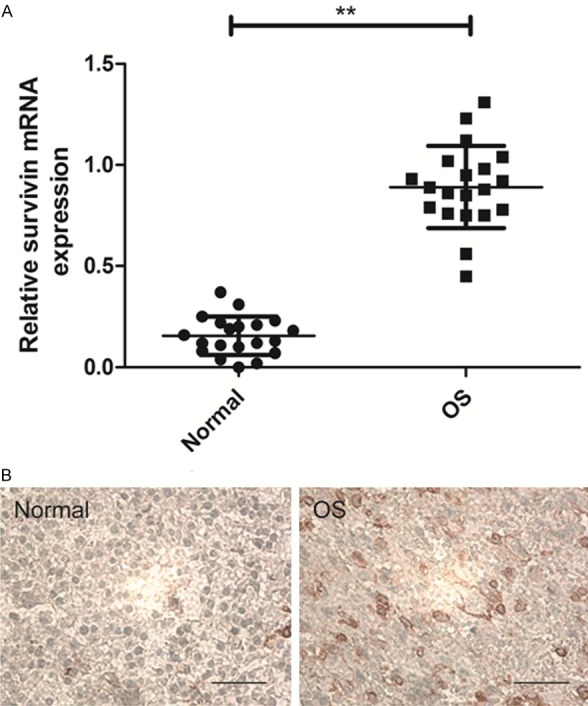
Levels of survivin are increased in OS clinical samples. A. mRNA expression level of survivin in 20 cases of OS tissue samples and adjacent normal tissue samples were determined by qRT-PCR. GAPDH was quantified as an internal standard. **P<0.01 compared to normal tissue. B. Survivin protein expression in OS tissue samples and matched adjacent non-tumoral tissue samples were detected by immunohistochemistry assay.
Survivin expression is markedly elevated in DOX resistant OS cell line and YM155 significantly down-regulates survivin expression in a dosed dependent manner
To examine the role of survivin in the acquisition of cisplatin resistance, we took a OS cell line (MG63) that is sensitive to DOX treatment (IC50 5.5 nM) and induced DOX resistance by increasing doses of DOX in MG63 cell over six months. This new cell line, designated MG63/DOX (IC50 11.9 nM) was found to be significantly more resistant to DOX treatment (>18.2 folds) as compared to its parental cell line (Figure 2A). Interestingly, MG63/DOX cells also showed a significant increase in survivin expression on mRNA level and protein level as compared to MG63 cells (Figure 2B, 2C). Next, we examined whether YM155 is effective at inhibiting survivin expression in MG63/DOX cell. We found that YM155 significantly decreased survivin protein expression in a dose-dependent manner (Figure 2D).
Figure 2.

Survivin expression is increased in DOX resistant cells and YM155 inhibits survivin expression in a dose dependent manner. (A) Cell proliferation in MG63/DOX cells and its parental cell line MG63 cells was assessed by MTT assay after treated with different concentrations of DOX. (B, C) Survivin expression in MG63/DOX and its parental cell line MG63 was examined by RT-PCR (B) and Western blotting (C). GAPDH was quantified as an internal standard. (D) Survivin expression was examined by Western Blotting in MG63/DOX cells after treated with different doses of YM155. GAPDH was quantified as an internal standard. *P<0.05, **P<0.01 compared to untreated group.
YM155 significantly reverses DOX resistance in OS cells
Recent studies have reported the role of survivin in the acquisition of drug-resistance in cancer cells [14,18,20]. We examined if YM155 could reverse the DOX resistance in OS cells and enhance its anti-tumor effects. Treatment of MG63/DOX cells with 70 nM DOX showed 50.5% inhibition of cell proliferation, whereas it completely inhibited cell proliferation in MG63 cells (Figure 3A). However, pretreatment of MG63/DOX cells with YM155 (10 nM) significantly reversed DOX resistance in DOX resistant cell line (Figure 3A). Then, we also investigated the effect of YM155 alone or in combination with DOX on MG63/DOX cell colony formation. MG63/DOX cells treated with YM155 and DOX alone or both had significantly inhibited colony formation compared to control group (Figure 3B), while YM155 and DOX combination treatment has strongest inhibition effect on colony formation compared to single YM155 treatment or DOX treatment. In addition, YM155 in combination with DOX in MG63/DOX cells could significantly induced cell apoptosis (Figure 3C), promoted caspase-3, -8 and -9 activities (Figure 3D-F). Taken together, these studies suggested that YM155 significantly reverses DOX resistance in vitro.
Figure 3.

YM155 significantly reverses DOX resistance in OS cells. A. Cell proliferation was assessed by MTT assay in MG63 and MG63/DOX cells after treated with YM155 or DOX alone or both. B. Cell colony formation was determined in MG63/DOX cells after treated with YM155 or DOX alone or both. C. Cell apoptosis was determined in MG63/DOX cells after treated with YM155 or DOX alone or both. D-F. Caspase-3, -8 and -9 activity in MG63/DOX were detected cells after treated with YM155 or DOX alone or both. *P<0.05, **P<0.01 compared to untreated group.
YM155 enhances the therapeutic efficacy of DOX in DOX resistant OS cells
Our in vitro data suggest that YM155 significantly reverses DOX resistance in MG63/DOX cells. To further validate our in vitro results, we assessed the in vivo therapeutic efficacy of YM155 and DOX alone or combination in male BALB mice bearing DOX resistant cells (MG63/DOX). We found that DOX treatment (10 mg/kg) of animal bearing MG63/DOX tumors did not significantly affect tumor growth compared to control group (Figure 4A-C, 15.4% inhibition at day 28), whereas YM155 (10 mg/kg) treatment of MG63/DOX tumors significantly inhibited tumor growth compared to control group (44.4% inhibition at day 28). YM155 in combination with DOX has strongest effect on tumor growth (73.6% inhibition at day 28), and can significantly decreased tumor volume (Figure 4B) and weight (Figure 4C) compared to other groups. In addition, we also detected survivin expression in tumor tissue by western blot. We found that survivin protein expression was decreased in YM155 treatment group and YM155 in combination with YM155 group compared to the untreated group and DOX group (Figure 4D). These findings suggested that YM155 enhances the therapeutic efficacy of DOX in DOX resistant OS by inhibiting survivin expression.
Figure 4.
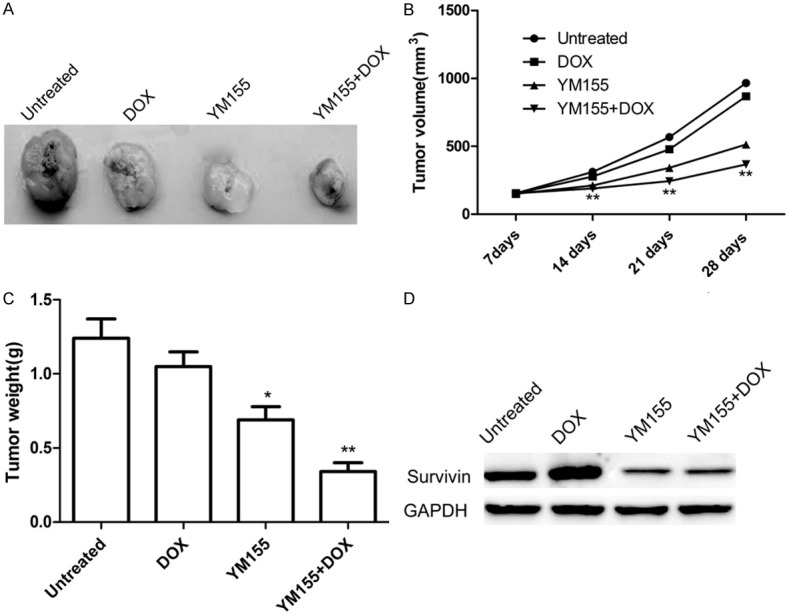
YM155 and DOX alone or combination inhibits tumor growth in nude model animals bearing MG63/DOX were treated with YM155 (10 mg/kg) or DOX (10 mg/kg) alone or in combination. A. Representative photomicrographs of tumors from untreated, DOX, YM155, or DOX in combination with YM155 treatment groups. B. Growth curves for tumor volumes in xenografts of nude mice from different group were established based on the tumor volume measured each week until four weeks. C. Tumor weights were measured from different groups. D. Survivin protein expression was determined in tumor tissue of different group by western blot assay. *P<0.05, **P<0.01 compared to untreated group.
Discussion
Chemoresistance is a therapeutic problem that severely limits successful treatment outcomes for OS since OS has high rate of local and distant failure, leading to the acquisition of chemo and radio-resistance [24,25]. Therefore, it is an urgent need to identify new therapeutic targets to improve the therapeutic efficacy and minimize the chemotherapeutic drugs toxic side effects for patients with OS. Survivin protein is one such therapeutic target for OS. Previous studies shown that survivin expression was highly expressed in most OS, and correlated with metastasis, poor survival and resistance against chemotherapy and radiotherapy [13-21]. In the present study, we also showed that survivin expression on mRNA level and protein level is significantly increased in primary tumors from OS patients as compared to adjacent normal tissue, which is in consistent with previous study. Of note, we found that survivin levels were further upregulated in DOX resistant cells as compared to their parental DOX sensitive cells. Therefore, we reasonable hypothesized that targeting of survivin in OS could reverse the DOX resistant phenotype in tumor cells thereby enhancing the therapeutic efficacy of DOX in OS treatment.
Growing evidence has demonstrated that inhibition of survivin expression using antisense oligonucleotides, small molecule antagonists, or small interfering RNA (siRNA) could suppress tumor cells proliferation and invasion, induce cell apoptosis, suppressed tumor growth, and increased chemotherapy sensitivity [13-18,20]. YM155, a novel small molecule inhibitor of survivin, was identified by using a survivin promoter luciferase assay [20]. Several reports have showed that YM155 could enhance sensitive chemotherapeutic drugs to tumor cell [14,16-18,21]. For example, YM155 potentiated chemosensitivity to gemcitabine in pancreatic cancer cells by suppressing survivin expression [17]. YM155 could reverse cisplatin resistance in head and neck cancers and enhance the therapeutic efficacy of cisplatin treatment by inhibiting tumor growth and tumor angiogenesis in head and neck cancers [26]. YM155 could overcome the rapamycin resistance in renal cancer cells (RCC) and enhancing the effectiveness of rapamycin therapy in RCC by decreasing survivin [27]. In addition, recently a study showed that YM155 in combination with cisplatin treatment showed antiproliferative effects and induced a greater rate of apoptosis than the sum of the single-treatment rates and promoted tumor regression in established OS xenograft models [28]. Here, our study showed that YM155 pretreatment significantly reversed acquired DOX resistance cell line (MG63/DOX). YM155 treatment also significantly downregulated survivin expression DOX resistant cell lines (MG63/DOX) in a dose dependent manner. In vivo, YM155 in combination with DOX significantly suppressed tumor growth of MG63/DOX xenograft mice. These results, collected with previous reports suggested that YM155 could reverse chemotherapeutic drugs resistant to tumor cells.
In summary, the results presented here demonstrate that YM155 could reverse DOX resistance in OS and enhance the therapeutic efficacy of DOX treatment. These results suggest a potentially novel strategy to reverse DOX resistance in OS.
Disclosure of conflict of interest
None.
References
- 1.Ottaviani G, Robert RS, Huh WW, Palla S, Jaffe N. Sociooccupational and physical outcomes more than 20 years after the diagnosis of osteosarcoma in children and adolescents: limb salvage versus amputation. Cancer. 2013;119:3727–3736. doi: 10.1002/cncr.28277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, Vernon P, Cao L, Tang D. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012;72:230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A, Jinks RC, Sydes MR, Uscinska B, Hook JM, van Glabbeke M, Bramwell V, Lewis IJ, Taminiau AH, Nooij MA, Hogendoorn PC, Gelderblom H, Whelan JS. Presence of chemotherapy-induced toxicity predicts improved survival in patients with localised extremity osteosarcoma treated with doxorubicin and cisplatin: a report from the European Osteosarcoma Intergroup. Eur J Cancer. 2012;48:703–712. doi: 10.1016/j.ejca.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Duan G, Feng S. MicroRNA-301a modulates doxorubicin resistance in osteosarcoma cells by targeting AMP-activated protein kinase alpha 1. Biochem Biophys Res Commun. 2015;459:367–373. doi: 10.1016/j.bbrc.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 6.Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013;332:225–228. doi: 10.1016/j.canlet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 8.Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Hemmi A, Sugitani M, Nemoto N, Ryu J. Survivin as a prognostic factor for osteosarcoma patients. Acta Histochem Cytochem. 2006;39:95–100. doi: 10.1267/ahc.06005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osaka E, Suzuki T, Osaka S, Yoshida Y, Sugita H, Asami S, Tabata K, Sugitani M, Nemoto N, Ryu J. Survivin expression levels as independent predictors of survival for osteosarcoma patients. J Orthop Res. 2007;25:116–121. doi: 10.1002/jor.20291. [DOI] [PubMed] [Google Scholar]
- 10.Asechi H, Hatano E, Nitta T, Tada M, Iwaisako K, Tamaki N, Nagata H, Narita M, Yanagida A, Ikai I, Uemoto S. Resistance to cisplatin-induced apoptosis via PI3K-dependent survivin expression in a rat hepatoma cell line. Int J Oncol. 2010;37:89–96. [PubMed] [Google Scholar]
- 11.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–25914. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, Pilotti S, Zunino F, Daidone MG. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I, Matsuhisa A, Kudoh M, Sasamata M. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 14.Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A, Shamsili S. Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J. Clin. Oncol. 2009;27:4481–4486. doi: 10.1200/JCO.2008.21.1862. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Zhao X, Zhang Y, Kang Y, Wang J, Liu Y. Antitumor activity of YM155, a selective survivin suppressant, in combination with cisplatin in hepatoblastoma. Oncol Rep. 2015;34:407–414. doi: 10.3892/or.2015.3947. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka K, Nakahara T, Yamauchi T, Kita A, Takeuchi M, Kiyonaga F, Kaneko N, Sasamata M. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17:5423–5431. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- 17.Yoon DH, Shin JS, Jin DH, Hong SW, Jung KA, Kim SM, Hong YS, Kim KP, Lee JL, Suh C, Lee JS, Kim TW. The survivin suppressant YM155 potentiates chemosensitivity to gemcitabine in the human pancreatic cancer cell line MiaPaCa-2. Anticancer Res. 2012;32:1681–1688. [PubMed] [Google Scholar]
- 18.Tolcher AW, Quinn DI, Ferrari A, Ahmann F, Giaccone G, Drake T, Keating A, de Bono JS. A phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer. Ann Oncol. 2012;23:968–973. doi: 10.1093/annonc/mdr353. [DOI] [PubMed] [Google Scholar]
- 19.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, Lawson D, Whitman E, Gonzalez R. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2011;29:161–166. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Pise-Masison CA, Shih JH, Morris JC, Janik JE, Conlon KC, Keating A, Waldmann TA. Markedly additive antitumor activity with the combination of a selective survivin suppressant YM155 and alemtuzumab in adult T-cell leukemia. Blood. 2013;121:2029–2037. doi: 10.1182/blood-2012-05-427773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Ma L, Wang J. YM155 exerts a growth inhibitory effect on human osteosarcoma in vitro and in vivo. Oncol Rep. 2015;34:1074–1080. doi: 10.3892/or.2015.4067. [DOI] [PubMed] [Google Scholar]
- 22.Zhang K, Li Y, Liu W, Gao X, Zhang K. Silencing survivin expression inhibits the tumor growth of non-small-cell lung cancer cells in vitro and in vivo. Mol Med Rep. 2015;11:639–644. doi: 10.3892/mmr.2014.2729. [DOI] [PubMed] [Google Scholar]
- 23.Yang Q, Zhang S, Kang M, Dong R, Zhao J. Synergistic growth inhibition by sorafenib and cisplatin in human osteosarcoma cells. Oncol Rep. 2015;33:2537–2544. doi: 10.3892/or.2015.3832. [DOI] [PubMed] [Google Scholar]
- 24.Bergman PJ. Mechanisms of anticancer drug resistance. Vet Clin North Am Small Anim Pract. 2003;33:651–667. doi: 10.1016/s0195-5616(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 25.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006;6:1075–1085. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 26.Kumar B, Yadav A, Lang JC, Cipolla MJ, Schmitt AC, Arradaza N, Teknos TN, Kumar P. YM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levels. Mol Cancer Ther. 2012;11:1988–1998. doi: 10.1158/1535-7163.MCT-12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koike H, Nitta T, Sekine Y, Arai S, Furuya Y, Nomura M, Matsui H, Shibata Y, Ito K, Oyama T, Suzuki K. YM155 reverses rapamycin resistance in renal cancer by decreasing survivin. J Cancer Res Clin Oncol. 2014;140:1705–1713. doi: 10.1007/s00432-014-1734-z. [DOI] [PubMed] [Google Scholar]
- 28.Gao JH, Chen FH, Wang L, Wei H, Meng SL. YM155 inhibits tumor growth and enhances chemosensitivity to cisplatin in osteosarcoma. Eur Rev Med Pharmacol Sci. 2015;19:2062–2069. [PubMed] [Google Scholar]


