Abstract
Vitamin D deficiency during pregnancy is thought to play a role in the development of preeclampsia; however, the underlying mechanism is not fully understood. In this study, a randomized double-blind placebo-controlled clinical trial was performed among 60 pregnant women at risk for pre-eclampsia according to abnormal uterine artery Doppler waveform. Subjects were randomly divided into 2 groups to receive a daily dose of 2000 IU vitamin D3 supplements (n=30) or receive placebo (n=30) between gestational weeks 20-32 for a total of 12 consecutive weeks. Because vitamin D3 supplementation can induce anti-inflammatory cytokine signaling, peripheral blood monocytes were investigated by flow cytometry for expression of toll-like receptor 4 (TLR4), an important mediator of innate immune response. The pro-inflammatory cytokines secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1 from monocytes, which are typically upregulated in preeclampsia, was also assessed. The incidence of preeclampsia was significantly lower in patients treated with vitamin D3 compared to the placebo group. Both the mean fluorescence intensity and the positive percentage of monocytes TLR4 in the vitamin D group were significantly lower compared to the placebo group, as well as the concentrations of secreted TNF-α, IL-6, and IL-1, while the concentration of IL-10 was higher. In the placebo group, the positive frequency of monocytes TLR4 was negatively correlated with the concentration of serum 25-hydroxyvitamin D in preeclampsia patients. Based on these results, we conclude that vitamin D3 supplementation for patients at risk of preeclampsia leads to a decrease in the expression of peripheral blood monocytes TLR4 and a subsequent decrease in pro-inflammatory cytokine secretion. Therefore, inhibiting the expression of monocytes TLR4 through vitamin D3 supplement may be a new approach to preeclampsia prevention.
Keywords: Preeclampsia, vitamin D3, monocytes, Toll-like receptor 4
Introduction
Preeclampsia is a systemic disease involving multiple organ dysfunction that occurs exclusively during pregnancy. Preeclampsia is characterized by hypertension and proteinuria affecting 1.3-6.7% of all human pregnancies [1]. Maternal systemic inflammation has been implicated as a key determinant in the pathogenesis of preeclampsia [2]. During normal pregnancy, monocytes/macrophages distribute in the maternal/fetal interface, consisting of maternal decidua and placental trophoblast, and produce prostaglandin E2, indolamine dioxygenase, and interleukin 10 (IL-10) via interactions with trophoblast and regulatory T cells (Tregs). These immunosuppressant molecules help to establish maternal-fetal tolerance and participate in embryo implantation and placenta development.
If monocytes/macrophages are abnormally activated in the fetal and placental circulation under hypoxic conditions and may represent an important source of proinflammatory cytokines such as interleukins (IL)-β, IL-6, and IL-8. The differentiation of trophoblasts will be impeded, potentially leading to the increased vascular resistance and pregnancy failure and/or preeclampsia [3]. Monocytes display toll-like receptor 4 (TLR4) on their surface; this molecule is involved in the innate immune response. Activated TLR4 can form a complex with myeloid differentiation primary response gene 88 (MyD88) and interleukin-1 receptor associated kinases (IRAKs) to activate nuclear factor-κB (NF-κB) and promote the secretion of inflammatory factor, a key contributor in preeclampsia-related inflammation [4].
Many studies have focused on the regulation of monocytes TLR4 expression to reduce maternal inflammation. Vitamin D has a well established role in immunity [5] and its deficiency during pregnancy been shown to up-regulate maternal proinflammatory cytokines [6], leading to increased expression of tumor necrosis factor α, interleukin-6, and interfron-γ, and the onset of preeclampsia [7], making it a promising contender for targeting TLR4 signaling. We assessed the effects of vitamin D3 supplementation on patients at risk for developing preeclampsia and observed TLR4 expression in peripheral blood monocytes as a step toward identifying new targets for preeclampsia prevention.
Materials and methods
Study design
Sixty pregnant women at risk for preeclampsia were selected to participate in a randomized, double-blind controlled trial at the People’s Hospital of Binghai County from February to October 2014. All pregnant women were experiencing their first pregnancy, aged between 20 and 32 years, between 18 and 20 weeks gestation, and pregnant with a single fetus. Each pregnant woman selected for the study showed the following abnormalities on uterine artery Doppler, qualifying them as high-risk [8]: average resistance index (RI) > 0.67, pulsatility index (PI) > 1.65, and incisura at early diastole phase. Pregnant women were excluded from the study if they had taken warfarin or other drugs, or had any abnormalities of fetal development. No participants had a history of autoimmune disease or recent history of infection. There were no significant differences in age, body mass index, or gestational week between the two study groups. This study was approved by the Ethics Committee of the hospital (Ethics research 2014-03), and informed consent was obtained from all participants.
Pregnant women were randomized into two groups to take either cholecalciferol supplements (n=30) or placebo (n=30). Pregnant women in the vitamin D group received a daily dose of 2000 IU vitamin D3 (Qingdao Double Whale Pharmaceutical Co., Ltd.) from gestational week 20 through 32, while those in the control group received a placebo (containing the same excipients, but no vitamin D3) (Qingdao Double Whale Pharmaceutical Co., Ltd.), for a total of 12 consecutive weeks. Placebo pearls were similar in color, shape, size, and package to the vitamin D3 ones and contained edible paraffin. Subjects were requested not to alter their regular physical activity or normal dietary intakes throughout the study and not to take any supplements other than the one provided to them by the investigators. 4 mL of peripheral blood were collected at baseline and 12 weeks after the intervention from the antecubital vein at gestational week 32 and anticoagulated with heparin; detection took place within 24 hours in room temperature.
Preeclampsia indicators
C-reactive protein (CRP) and urine microalbumin were measured with a BN ProSpec specific protein analyzer and matched reagents (Siemens, Berlin, Germany).
Peripheral blood 25-hydroxy vitamin D detection
Fasting venous blood samples were collected and centrifuged at 500 × g for five minutes to collect plasma and immediately stored at -80°C. Electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Germany) was used to detect serum 25-(OH) vitamin D concentration according to the manufacturer’s protocol; the intra-batch and inter-batch coefficients of variation were less than 5% and 10%.
Detection of TLR4 on peripheral blood monocytes
10 μL FITC mouse anti-human-CD14 monoclonal antibody, 10 μL phycoerythrin (PE) mouse anti-human TLR4, and isotype reference materials (BD Biosciences, Franklin Lakes, NJ) were added to 100 μL of peripheral blood and incubated in the dark at room temperature for 30 minutes. Erythrocyte lysis solution (BD Biosciences, Franklin Lakes, NJ) was used to destroy red blood cells, followed by a saline wash, centrifugation at 500 × g for six minutes, and re-suspension in 400 μL PBS. A FACSCanto machine (BD Biosciences) was used for detection and CD14 positive monocytes were used for gating; read mean fluorescence intensity (MFI) or the percentage of TLR4 positive of monocytes.
Preparation of primary monocytes and their activation of TLR4
The blood samples collected after 12 weeks intervention in sterile heparinized tubes were diluted with an equal volume of phosphate-buffered saline (PBS), pH 7.4 and centrifuged over Ficoll gradients (Amersham Pharmacia) to isolate peripheral blood mononuclear cells (PBMCs). The resulting PBMCs were further passed over Percoll gradients (Amersham Pharmacia) at a concentration of 5 × 107 cells/mL to deplete lymphocytes. The resulting primary monocytes were relatively pure (80-90%) as determined by CD14+ staining and flow cytometry. Finally, the purified monocytes were were then re-suspended with complete RPMI-1640 culture medium (2 mM L-glutamine and penicillin-streptomycin) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco, Life Technologies) to a concentration of 1 × 105 cells/mL and divided into two wells. One well received 50 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO). Cells were cultured in a 5% CO2 incubator at 37°C for 18 hours, and then supernatant was collected and stored at -80°C.
Cytokine detection
Two mL of peripheral blood were centrifuged at 500 × g for five minutes and serum were stored immediately in a refrigerator at -80°C. Serum cytokines, tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-8, and IL-10 were measured using detection reagents (Bio-Rad Laboratories, Inc, Hercules, CA) and a Luminex 2000 (Luminex Corporation, Austin, TX) according to the manufacturer’s protocol. Each sample was measured twice and concentrations were averaged.
Statistical analysis
Stata7.0 statistical software (StataCorp, College Station, TX) was used to conduct a data normality test. Normally distributed data were expressed as mean ± standard deviation, and comparisons between the two groups were done using a Student’s t-test. Skewed data were expressed as median M (25-75 percentile), and comparison between the two groups was done with Mann-Whitney U test as a two-sided test. The Linear correlation coefficient was calculated using the Spearman correlation coefficient. A P < 0.05 was considered statistically significant.
Results
Vitamin D3 supplementation affects pregnancy outcome
The cesarean section rate, urinary albumin, CRP, incidence of preeclampsia [10], systolic blood pressure, and diastolic blood pressure in the group that received vitamin D supplementation were significantly lower compared to the placebo group. The vitamin D supplementation group also gave birth to significantly heavier babies (Table 1).
Table 1.
The effect of vitamin D supplementation on pregnancy outcome
| Placebo group (n=30) | Vitamin D group (n=30) | P-Value | |
|---|---|---|---|
| Birth parameters | 26.5 ± 5.2 | 27.0 ± 5.3 | > 0.05 |
| Cesarean section (%) | 10 (33.3) | 4 (13.3 ) | < 0.05 |
| Gestational age (weeks) | 39.1 ± 1.3 | 39.4 ± 1.3 | > 0.05 |
| Preterm delivery (%) | 1 (3.3) | 0 (0) | > 0.05 |
| Newborn weight (g) | 3 141.0 ± 495.9 | 3 313.6 ± 341.1 | < 0.05 |
| Urinary albumin (mg/L) | 422.5 ± 70.9 | 115.1 ± 4.9 | < 0.05 |
| CRP (mg/L) | 5.78 ± 1.12 | 3.26 ± 0.82 | < 0.05 |
| Monocytes (1 × 109/L) | 0.39 ± 0.15 | 0.55 ± 0.21 | < 0.05 |
| 1-min Apgar score | 8.9 ± 0.2 | 8.9 ± 0.3 | > 0.05 |
| 5-min Apgar score | 9.9 ± 0.2 | 9.9 ± 0.3 | > 0.05 |
| Preeclampsia rate (%) | 13 (43.3%) | 6 (20.0%) | < 0.05 |
| Low birth weight (%) | 2 (6.7) | 0 (0) | > 0.05 |
| Systolic blood pressure | 130.4 ± 8.9 | 112.0 ± 10.0 | < 0.05 |
| Diastolic blood pressure | 92.6 ± 4.4 | 81.5 ± 8.4 | < 0.05 |
Vitamin D supplementation decreases TLR4 expression on monocytes
After vitamin D3 supplementation for 12 weeks, peripheral blood cells were analyzed with flow cytometry equipped with forward scattered (FS) and side scattered (SS) light to allow for the differentiation of monocytes (Figure 1A). Positive frequency and mean fluorescence intensity (MFI) of CD14+ monocytes TLR4 were also determined (Figure 1D, 1E). Both the positive frequency and MFI of monocytes TLR4 in the Vitamin D group were significantly lower than in the placebo group (P < 0.05).
Figure 1.
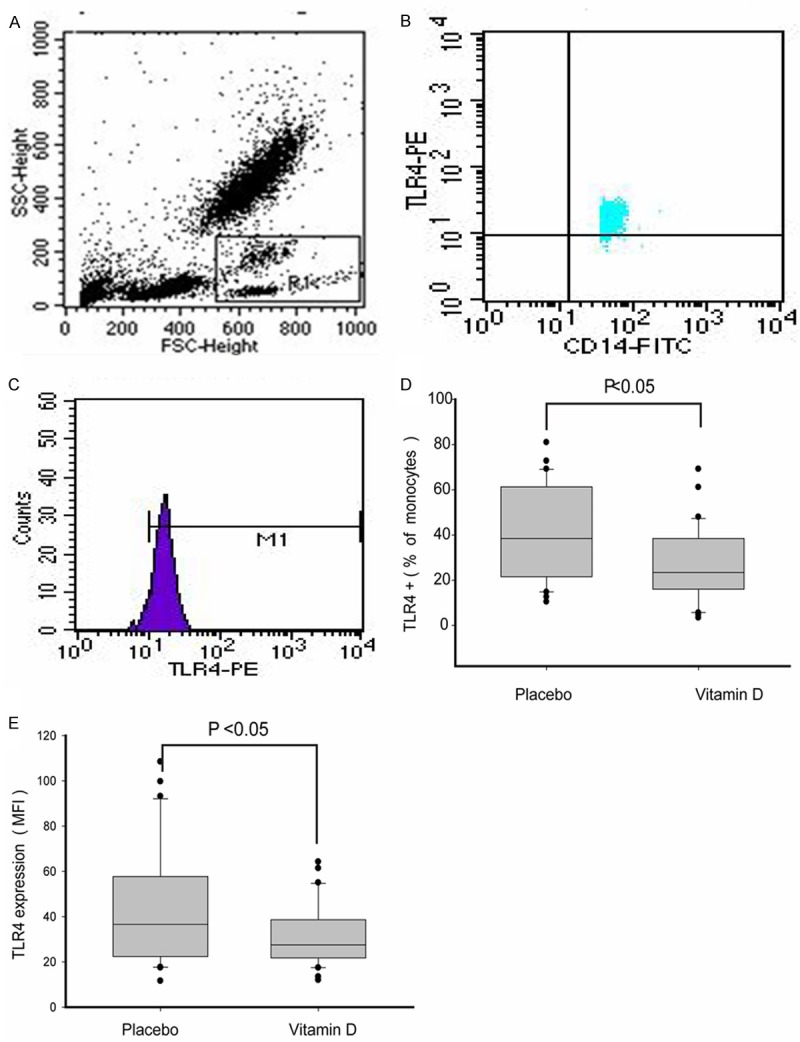
Analysis of monocytes TLR4 by flow cytometry. A. Representative flow cytometry plot showing forward and side scatter characteristics with gating used to identify monocytes (R1); B. Percentage of TLR4 positive monocytes. C. Mean fluorescence intensity (MFI) of monocytes TLR4; D. Comparison of the percentage of monocyte TLR4+ between Vitamin D and placebo groups. E. Comparison of the MFI monocyte TLR4+ between Vitamin D and placebo groups. Note: Statistical significance was determined by Mann-Whitney U test.
25-hydroxy vitamin D concentration in peripheral blood
After 12 weeks of vitamin D supplementation, the concentration of serum 25-hydroxy vitamin D was significantly higher compared to the serum levels before supplementation. As expected, the vitamin D group had significantly higher 25-hydroxy vitamin D levels after supplementation compared to the placebo group (Figure 2A). The concentration of 25-hydroxy vitamin D in peripheral serum of placebo-treated patients was negatively correlated with monocytes TLR4 levels, with a correlation coefficient of -0.532 (P=0.0025) (Figure 2B).
Figure 2.
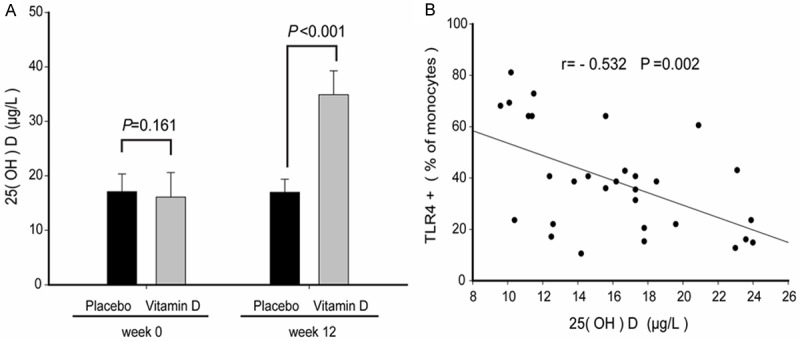
The concentration of serum 25-hydroxy vitamin D in pregnant women. A. The concentration of 25-hydroxy vitamin D in peripheral blood. B. The correlation of 25-hydroxy vitamin D and monocyte TLR4+.
LPS stimulation alters monocytes cytokine secretion
After stimulation with 50 ng/mL LPS, monocytes from patients supplemented with vitamin D had significantly lower secretion of TNF-α, IL-6, and IL-8 compared to the placebo-treated group, while the concentration of IL-10 was significantly higher (Figure 3).
Figure 3.
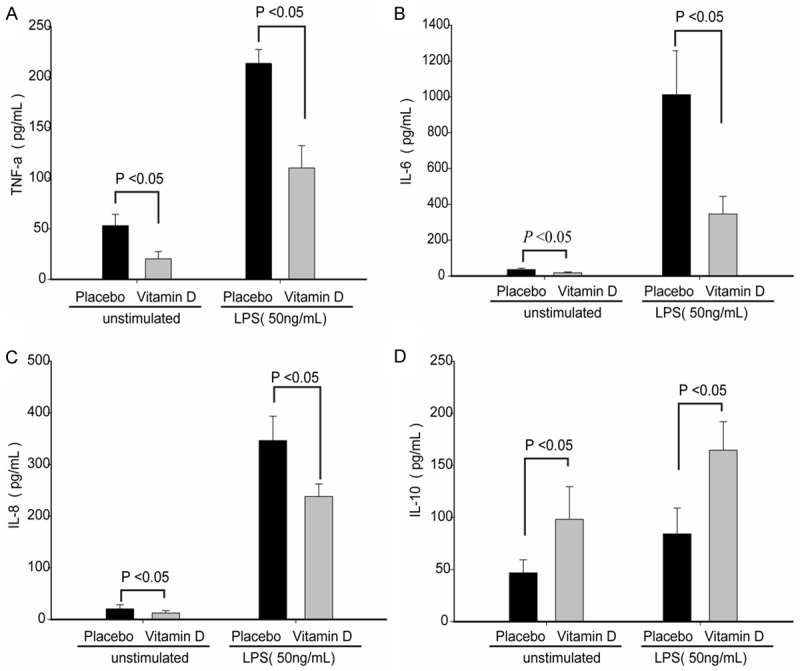
LPS stimulation alters cytokine secretion of monocytes from patients supplemented with vitamin D.
Comparing concentrations of cytokines in serum
Concentrations of serum TNF-α, IL-8, and IL-6 were significantly lower after vitamin D supplementation compared to the placebo group, while serum IL-10 was significantly higher (P < 0.05) (Figure 4).
Figure 4.
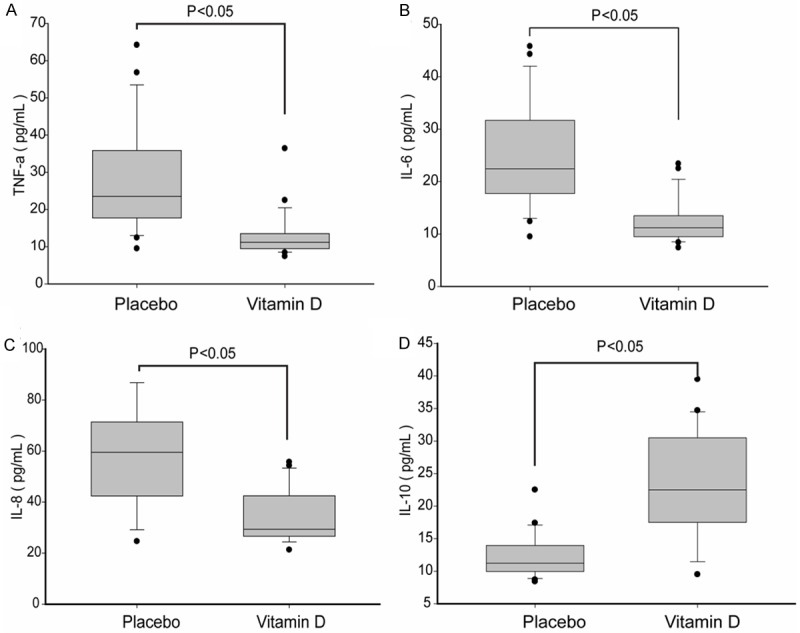
Comparison of the concentrations of cytokines in serum between vitamin D supplementation and placebo group.
Discussion
Vitamin D is a fat-soluble steroid hormone that exists in several forms within the human body, mainly as vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). The chemically active form of 25-hydroxy vitamin D [25-(OH)D] circulates in the bloodstream and regulates calcium and phosphorus metabolism to maintain bone health, and regulates immune function, glucose metabolism, cell proliferation and differentiation, and angiogenesis. Vitamin D deficiency has been linked to a three-fold increase in preeclampsia risk [11,12], and during weeks 24-26 of pregnancy, women with serum levels of 2-5(OH)D < 50 nmol/L have been found to have significantly increased risk of preeclampsia [13].
In this study, pregnant women at risk of developing preeclampsia received 2,000 IU vitamin D3 over the course of twelve weeks. This dose is higher than the recommended daily dose of 400 IU for pregnant women, but much lower than the dose advised by the Institute of Medicine (IOM) [14] and this is no problem in this short study of 12 weeks. In a meta-analysis study it has been shown that pregnant women who received vitamin D supplements in early pregnancy had lower odds of pre-eclampsia [15]. Vitamin D supplementation in healthy nulliparous women did affect pregnancy outcome with regard to pre-eclampsia [16]. But there is little known about the underlying mechanism.
Toll-like receptor is a biological pattern recognition receptor that initiates the immune response by recognizing and binding pathogen-related molecular patterns (PAMP). It is an important transmembrane receptor and signal transduction receptor in the innate immune response [17]. TLR4 can bind to LPS, teichoic acid, respiratory syncytial virus F protein, endogenous ligands such as heat shock protein, high mobility group protein-l, hyaluronic acid, fibrinogen, and fibronectin, among other molecules, on the surfaces of Gram-negative bacteria [18]. Many studies have suggested a link between the TLR4 signaling pathway and preeclampsia, but it is not known which of these ligands is involved. Analysis of the TLR4 signaling pathway in placenta revealed that TLR4, MyD88, and NF-κB are highly expressed in placental tissues from preeclampsia patients, resulting in localized and systematic inflammatory reaction and oxidative damage [19]. Monocytes from preeclampsia patients express significantly increased levels of TLR4, have abnormal reactivity to LPS ligand activation, and produce pro-inflammatory cytokines at high concentrations [20].
We found that vitamin D3 supplementation in patients at risk of developing preeclampsia led to significantly lower detection of TLR4 on peripheral blood monocytes. This is consistent with previous in vitro findings showing that 1, 25-dihydroxy vitamin D could block TLR2 and TLR4 activation by LPS in human monocytes THP-1 [21]. However, other studies suggest that vitamin D only affects TLR2 in monocytes, and has no effect on TLR4 [22]. This may be due to differences in the cell types used.
After TLR4 activation by LPS, monocytes produce a surplus of pro-inflammatory cytokines. LPS first binds to lipopolysaccharide binding protein (LBP) in the plasma, which then promotes binding to CD14 and MD-2 on the monocytes membrane [23]. The resulting complex is recognized by TLR4 and activates intracellular signaling pathways, including the tyrosine kinase pathway, protease C pathway, and NF-κB pathways [24]. The activation of a variety of transcription factors serves to regulate the expression of cytokines at the transcriptional level, synthesizing effector cells and secreting large amounts of inflammatory mediators or chemokines [25].
In our study, monocytes were activated with LPS and the levels of secreted cytokines were measured. Monocytes from pregnant women who received vitamin D3 supplementation secreted a significantly lower amount of the proinflammatory cytokines TNF-α, IL-6, and IL-8 compared to cells derived from women given the placebo, while the inflammatory cytokine IL-10 was significantly higher. Consistent with this finding, women treated with vitamin D3 had significantly lower peripheral serum TNF-α, IL-8, and IL-6, with an increase in IL-10. This is consistent with previous findings in which 1,25-(OH)D was used to treat adipose tissue and IL-6 and IL-8 were decreased [26]. Furthermore, monocytes TLR4 was negatively correlated with the concentration of 25-hydroxy vitamin D in peripheral serum in the placebo group.
Previous studies have suggested that preeclampsia patients have high concentrations of TLR4 ligands that produce an inflammatory reaction through the activation of TLR4 in the peripheral circulation. Fibrinogen concentrations in preeclampsia patients have been shown to be significantly higher than in normal pregnant women and to activate mononuclear cell TLR4 [27]. Similarly, high concentrations of heat shock protein 70, which can be detected in early-onset preeclampsia patients, are able to activate TLR4 signal pathways and are correlated with concentrations of TNF-α, IL-1β, IL-12, and soluble TNF receptor-I [28,29].
Here, we show that patients at risk of developing preeclampsia can significantly lower their risk by taking a daily dose of 2,000 IU vitamin D3 during gestational weeks 20-32. Vitamin D supplementation served to decrease the expression of TLR4 in peripheral blood monocytes and significantly reduce the pro-inflammatory cytokines TNF-α, IL-8, and IL-6 secreted by monocytes. It can be presumed that vitamin D3 can inhibit the TLR4 signaling in the peripheral blood monocytes, thereby down-regulating inflammatory pathways and reducing endothelial cell damage. Future clinical studies will focus on establishing appropriate vitamin D supplement doses for pregnant women of different populations, regions, and races.
Acknowledgements
This work was supported by a grant-in-aid from innovative team fund of autoimmune disease diagnosis and treatment, Yancheng, China.
Disclosure of conflict of interest
None.
Abbreviations
- TLR4
toll-like receptor 4
- FCM
flow cytometry
- MFI
mean fluorescence intensity
- LPS
lipopolysaccharide
- PBS
Phosphate-buffered saline
- IL
interleukin
- TNF
tumor necrosis factor
- CRP
C-reactive protein
References
- 1.Bian Z, Shixia C, Duan T. First-Trimester Maternal Serum Levels of sFLT1, PGF and ADMA Predict Preeclampsia. PLoS One. 2015;10:e0124684. doi: 10.1371/journal.pone.0124684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andraweera PH, Dekker GA, Jayasekara RW, Dissanayake VH, Roberts CT. Polymorphisms in the inflammatory pathway genes and the risk of preeclampsia in Sinhalese women. J Matern Fetal Neonatal Med. 2015;22:1–5. doi: 10.3109/14767058.2015.1034102. [DOI] [PubMed] [Google Scholar]
- 3.Biswas SK, Chittezhath M, Shalora IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53:11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 4.Frazão JB, Errante PR, Condino-Neto A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch Immunol Ther Exp (Warsz) 2013;61:427–443. doi: 10.1007/s00005-013-0243-0. [DOI] [PubMed] [Google Scholar]
- 5.Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–317. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 7.Díaz L, Noyola-Martínez N, Barrera D, Hernández G, Avila E, Halhali A, Larrea F. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009;81:17–24. doi: 10.1016/j.jri.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomized placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 10.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 11.Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, Wu Y, Luo ZC, Fraser WD. Longitudinal vitamin D status in pregnancy and the risk of preeclampsia. BJOG. 2012;119:832–839. doi: 10.1111/j.1471-0528.2012.03307.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxy vitamin D levels in early onset severe preeclampsia. Am J Obstet Gynecol. 2010;203:366, e1–e6. doi: 10.1016/j.ajog.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker AM, Haeri S, Camargo CA Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–109. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth DE. Vitamin D supplementation during pregnancy: safety considerations in the design and interpretation of clinical trials. J Perinatol. 2011;31:449–459. doi: 10.1038/jp.2010.203. [DOI] [PubMed] [Google Scholar]
- 15.Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, Magnus P, Meltzer HM. Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology. 2009;20:720–726. doi: 10.1097/EDE.0b013e3181a70f08. [DOI] [PubMed] [Google Scholar]
- 16.Hypponen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, Bánhidy F, Lawlor D, Czeizel AE. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63:331–340. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 17.Wardill HR, Van Sebille YZ, Mander KA, Gibson RJ, Logan RM, Bowen JM, Sonis ST. Toll-like receptor 4 signaling: a common biological mechanism of regimen-related toxicities: an emerging hypothesis for neuropathy and gastrointestinal toxicity. Cancer Treat Rev. 2015;41:122–128. doi: 10.1016/j.ctrv.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Morris MC, Gilliam EA, Li L. Innate immune programing by endotoxin and its pathological consequences. Front Immunol. 2015;5:680–686. doi: 10.3389/fimmu.2014.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernardi FC, Felisberto F, Vuolo F, Petronilho F, Souza DR, Luciano TF, de Souza CT, Ritter C, Dal-Pizzol F. Oxidative damage, inflammation, and Toll-like receptor 4 pathway are increased in preeclamptic patients: a case-control study. Oxid Med Cell Longev. 2012;2012:636419–636429. doi: 10.1155/2012/636419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-ofi E, Coffelt SB, Anumba DO. Monocyte subpopulations from pre-eclamptic patients are abnormally skewed and exhibit exaggerated responses to toll-like receptor ligands. PLoS One. 2012;7:e42217. doi: 10.1371/journal.pone.0042217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma R, Jung JH, Kim JY. 1, 25-Dihydroxyvitamin D3 up-regulates TLR10 while down-regulating TLR2, 4, and 5 in human monocyte THP-1. J Steroid Biochem Mol Biol. 2014;141:1–6. doi: 10.1016/j.jsbmb.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Ojaimi S, Skinner NA, Strauss BJ, Sundararajan V, Woolley I, Visvanathan K. Vitamin D deficiency impacts on expression of toll-like receptor-2 and cytokine profile: a pilot study. J Transl Med. 2013;11:176. doi: 10.1186/1479-5876-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66–75. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deguchi A, Tomita T, Omori T, Komatsu A, Ohto U, Takahashi S, Tanimura N, Akashi-Takamura S, Miyake K, Maru Y. Serum amyloid A3 binds MD-2 to activate p38 and NF-kappaB pathways in a MyD88-dependentmanner. J Immunol. 2013;191:1856–1864. doi: 10.4049/jimmunol.1201996. [DOI] [PubMed] [Google Scholar]
- 25.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 26.Wamberg L, Cullberg KB, Rejnmark L, Richelsen B, Pedersen SB. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: results from an intro study and a randomized controlled trial. Horm Metab Res. 2013;45:456–462. doi: 10.1055/s-0032-1331746. [DOI] [PubMed] [Google Scholar]
- 27.Al-ofi E, Coffelt SB, Anumba DO. Fibrinogen, an endogenous ligand of Toll-like receptor 4, activates monocytes in pre-eclamptic patients. J Reprod Immunol. 2014;103:23–28. doi: 10.1016/j.jri.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Peraçoli JC, Bannwart-Castro CF, Romao M, Weel IC, Ribeiro VR, Borges VT, Rudge MV, Witkin SS, Peracoli MT. High levels of heat shock protein 70 are associated with pro-infl ammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol. 2013;100:129–134. doi: 10.1016/j.jri.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Molvarec A, Szarka A, Walentin S, Beko G, Karádi I, Prohászka Z, Rigó J Jr. Serum heat shock protein 70 levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in women with preeclampsia. Clin Chim Acta. 2011;412:1957–1962. doi: 10.1016/j.cca.2011.06.042. [DOI] [PubMed] [Google Scholar]


