Abstract
Background: Preeclampsia is associated with inadequate invasion of trophocytes and spiral artery remodeling. As a β-D-glucuronidase enzyme, Heparanase is related to tumor angiogenesis, development and invasion. Trophocytes have similar characteristics to tumor cells, and heparanase could therefore play an important role in the pathogenesis of preeclampsia. Methods: The expression of heparanase in severe preeclampsia and normal placentas was detected via real-time PCR, immunohistochemistry and western blotting. The effects of heparanase on trophocytes migration and invasion were investigated by culturing the HTR-8/Svneo cell line with recombinant human heparanase protein in vitro. Results: The levels of inactive 65-kDa heterologous heparanase dimers were obviously increased, and the content of the 50-kDa active polypeptide was decreased in severe preeclampsia. Furthermore, exogenous heparanase protein could reduce the migration and invasion of HTR-8/Svneo cells. Conclusion: Our results suggested that heparanase might be an important factor in the pathogenesis of severe preeclampsia.
Keywords: Preeclampsia, heparanase, trophocyte, invasion
Introduction
Preeclampsia, particularly severe preeclampsia, as a leading cause of maternal and fetal morbidity and mortality, is one of the most serious complications of pregnancy [1]. Although the complete etiology and pathogenesis of preeclampsia remain unknown, it is widely accepted that preeclampsia is associated with inadequate invasion of trophocytes and spiral artery remodeling [2].
Heparanase (HPA), a β-D-glucuronidase enzyme, is a unique enzyme in humans that degrades heparin sulfate proteoglycan (HSPG) [3]. HSPG, which is an important component of the extracellular matrix (ECM) and is widely distributed on the cell surface, plays an important role in the growth and development of normal and pathological tissues [4]. The activation of heparanase is closely correlated with certain pathophysiological changes, including inflammatory reactions [5], angiogenesis [6], and tumor development, invasion and metastasis [7].
Trophocytes show similar characteristics to tumor cells, such as invasion and angiogenesis, and heparanase plays an important role in the processes of embryo implantation and placentation [8]. Therefore, we hypothesized that heparanase might contribute to the pathogenesis of severe preeclampsia. In present study, we investigated HPA mRNA and protein expression in the placentas of pregnant women with severe preeclampsia and the effects of HPA on the migration and invasion ability of the extravillous trophocytes (EVT) cell line HTR-8/Svneo in vitro.
Materials and methods
Materials
The study was approved by the Institutional Ethics Committee of Sichuan University West China Second Hospital and written informed consent was obtained from each patient prior to enrollment.
Preeclampsia was defined as described by the guidelines of the Obstetrics and Gynecology branch of the Chinese Medical Association. Briefly, women suffering from gestational hypertension with proteinuria were enrolled in the preeclampsia group [9]. The diagnostic criteria were as follows: blood pressure of 140 mmHg systolic or higher, or 90 mmHg diastolic or higher; and a 24-hour urinary protein level greater than or equal to 300 mg, or proteinuria greater than or equal to 1+ by dipstick testing, after the 20th week of pregnancy in a previously normotensive and non-proteinuria woman. Preeclampsia was considered severe if one or more of the following criteria were met: (1) blood pressure of 160 mmHg systolic or higher, or 110 mmHg diastolic or higher on two occasions at least 6 hours apart while the patient was under bed rest; (2) proteinuria or 5 g of protein or higher in a 24-hour urine specimen, or 3+ or greater in two random urine samples collected at least 4 hours apart; (3) oliguria of less than 500 mL in 24 hours; (4) cerebral or visual disturbances; (5) pulmonary edema or cyanosis; (6) epigastric or right upper-quadrant pain; (7) impaired liver function; (8) thrombocytopenia; and (9) fetal growth restriction. The exclusion criteria included multi-gestational pregnancy, diabetes, heart diseases, chronic hypertension, intrahepatic cholestasis of pregnancy, fetal malformation, chronic nephritis, renal hypertension, and nephritic syndrome.
Placentas were obtained from 50 puerperas who underwent caesarean section between October 2010 and October 2011 at Sichuan University West China Second Hospital 20 from women with normal full-term pregnancies and 30 from patients with severe preeclampsia. Immediately after caesarean delivery, the central zone of the placental tissue was collected [10] and then stored in liquid nitrogen for future RNA and protein extraction.
Methods
RNA extraction, reverse transcription, and real-time RT-PCR
Total RNA from stored placental tissues was extracted using the TRIzol reagent (Takara, Japan). First-strand cDNA was generated via reverse transcription. After a sufficient amount of cDNA was obtained, we performed PCR amplification using a real-time PCR cycler (Eppendorf, Germany). The sequences of the primers used in these assays were as follows: GAPDH forward: 5’-CAGAGCAAGAGAGGCATC-3’; GAPDH reverse, 5’-GAAGATGGTGATGGGATTTC-3’; HPA forward, 5’-CCTGA AGGCTGGTGGAGAAG-3’; and HPA reverse, 5’-GGTAGCAGTCCGTCCAT TCA-3’. GAPDH was used as an internal control. The amplification system included 5 μL of 2.5× RealMasterMix (2.5 μL), dd H2O (1.8 μL), cDNA (0.5 μL), the forward primer (0.1 μL) and the reverse primer (0.1 μL). The PCR cycling conditions for β-actin were as follows: Stage 1, 50°C for 2.00 min (1 cycle); Stage 2, 95°C for 10.00 min (1 cycle); Stage 3, 95°C for 15 s followed by 60°C for 15 s (40 cycles); and Stage 4, 95°C for 15 s first, then 60°C for 15 s and 60°C for 20 min, followed by 95°C for 15 s (1 cycle). The reaction conditions for HPA were as follows: Stage 1, 50°C for 2.00 min (1 cycle); Stage 2, 95°C for 10.00 min (1 cycle); Stage 3, 95°C for 15 s, followed by 60°C for 15 s and then 72°C for 20 s (45 cycles); and Stage 4, 95°C for 15 s first, then 60°C for 15 s and 60°C for 20 min, followed by 95°C for 15 s (1 cycle). The results were analyzed as described by Thomas D Schmittgen and Kenneth J Livak [11].
Immunohistochemistry assay
We used immunohistochemistry to detect the expression of HPA in 50 placental tissues. Formalin-fixed and paraffin-embedded tissues were cut into serial sections with a thickness of 4 μm. The sections were stained with hematoxylin and eosin (HE) for histological examination. The sections were gradually deparaffinized and rehydrated with xylene and ethanol, and endogenous peroxidase activity was blocked with a 3% hydrogen peroxide solution at room temperature for 10 minutes in the dark. The sections were then subjected to microwave antigen retrieval in citrate buffer (10 mM, pH 6.0) for 15 min and subsequently cooled at room temperature and washed with distilled water for 1 min. Phosphate-buffered saline (10 mM, pH 7.2-7.6) was used to wash the sections three times for 5 min each time. Then, the sections were separately incubated with a rabbit anti-human HPA polyclonal antibody (Ab85543, Abcam, UK) at 4°C overnight. Following incubation in a horseradish peroxidase (HRP)-conjugated polymeride (GK500505A, Genetic Technology, China) at 37°C for 40 min, the sections were stained with DAB solution (3,3’-diaminobenzidinetetrahydrochloride) (Genetic Technology, China) under microscopic observation and then counterstained with hematoxylin, dehydrated and mounted. Negative controls were processed with rabbit polyclonal IgG (Ab27478, Abcam, UK). The slides were evaluated with a light microscope (Olympus, Japan).
Western blot analysis
Total protein extracted from the placental tissues was analyzed via 10% SDS-polyacrylamide gel electrophoresis and then transferred to a 0.22 μm polyvinylidene fluoride (PVDF) membrane (Millipore, Germany) using a semi-dry western blot transfer system (Bio-Rad, USA) for 90 min at 200 mA. The membrane was subsequently washed with TBST (TBS containing 0.05% Tween-20) for 1 min, blocked in TBST with 5% non-fat dry milk and incubated with a primary rabbit anti-human HPA polyclonal antibody (Ab85543, Abcam, UK) for 1.5 h at room temperature (RT). Thereafter, the membrane was washed three times in TBST (5 min each) and incubated with a secondary HRP-conjugated goat anti-rabbit antibody (ZDR-5306, ZSGB-Bio, China) for 90 min at RT. After washing four times with TBST, the membrane was stained using Immobilon Western kit (Millipore, Germany). The blots were then detected via chemiluminescence autoradiography. The resultant protein bands were quantified using QualityONE software (Bio-Rad, USA). GAPDH (Ab37168, Abcam, UK) was used as an internal control.
Cell culture and treatment
An immortalized first-trimester EVT cell line, HTR-8/Svneo, which was derived from a short-lived primary EVT cell line, was used in the present study. This cell line has been employed in many studies to simulate the behavior of trophocytes. The HTR-8/SVneo cell line in this study was provided by Dr. Yali Hu of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, China, and incubated in RPMI1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin and 100 µg/mL streptomycin (Beyotime Institute of Biotechnology) under standard culture conditions (37°C in 5% humidified CO2 incubator). Cultured HTR-8/SVneo cells in the log phase of growth were used in this study.
Scratch migration assay
The effects of HPA on the migration capacity of HTR-8/SVneo cells were assessed in a 6-well culture plate. The density of HTR-8/SVneo cells was adjusted to 1×105 cells/mL, and 2 mL of HTR-8/SVneo cells was added to every well. The cells were incubated for 48 h (37°C in a 5% humidified CO2 incubator). Once 70-80% confluence was observed, a 1 mm linear “scratch” was made in the adherent cell monolayer with a 200 μL pipette tip. Then, different concentrations of recombinant human heparanase protein (R&D, USA) were added to the culture medium. The linear distance between the cells on either side of the scratch was measured at 10 locations in each well at 0, 6, 12 and 24 hours after scratching. Each experiment was conducted in triplicate.
Cell invasion assay
The HTR-8/SVneo cells (1×105 cells/mL) were added to the upper well of a Transwell chamber (Corning, USA) with a polyethylene terephthalate membrane (PET membrane) with a 12 µm pore diameter. The PET membrane was pre-treated with Matrigel at a concentration of 300 μL/mL. The HTR-8/SVneo cells were incubated with HPA in 24-well plates, and after 24 h of incubation, the remaining cells were removed from the upper surface of the membrane using a sterile cotton swab, while the cells that had migrated to and invaded the lower surface were fixed with 70% methyl alcohol and stained with trypan blue. Finally, the number of migrated cells was examined using a digital microscope. The cell numbers were counted in five random fields for each chamber, and the average value was calculated. Each experiment was conducted in triplicate.
Statistical analysis
All of the data were expressed as mean ± SD (standard deviation). All of the statistical analyses were performed using the SPSS statistical software package (SPSS Inc., Chicago, IL, USA). Furthermore, a P-value less than 0.05 was considered statistically significant.
Results
Clinical characteristics
Clinical data were obtained from the puerpera who participated in the study (Table 1). The placental tissues were classified into two groups: the severe preeclampsia (SPE, n=30) and normal pregnancy groups (N, n=20). The results of one-way ANOVA showed that the women with severe preeclampsia had a higher pre-BMI than the controls (P<0.05), while the gestational age at birth, fetal weight and fetal length were significantly lower than the controls (P<0.05).
Table 1.
Clinical characteristics of women with and without severe preeclampsia
| Characteristics | Control (n=20) | SPE (n=30) |
|---|---|---|
| Maternal age (years) | 29.97±4.32 | 30.24±5.75 |
| Pre-BMI (kg/m2) | 20.25±4.37 | 21.97±3.40* |
| SBP (mmHg) | 111.26±7.76 | 150.76±12.91* |
| DBP (mmHg) | 70.62±7.49 | 93.16±12.38* |
| Delivery weeks (w) | 38.00±1.81 | 33.91±4.10* |
Data presented as mean ± standard deviation;
P<0.05.
HPA mRNA expression in placental tissues
The mean ± SD 2-ΔCT of the severe preeclampsia group was 0.028±0.011, while that of the normal group was 0.016±0.002. The result of Student’s t-test showed that there was no difference (P=0.3175) between the two groups in this study.
Localization of HPA in placental tissues
HPA expression was observed in all of the placental tissues. Positive staining with HPA was mainly localized in the cytoplasm of trophocytes in the normal group but was found in both the cytoplasm and nucleus in the severe preeclampsia group (Figure 1).
Figure 1.
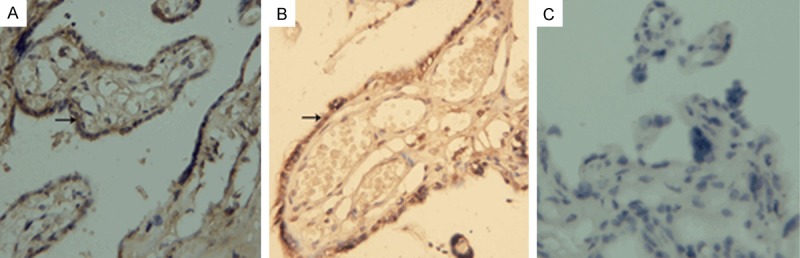
HPA staining in placental tissues. A: Placenta of normal control (original magnification ×400). B: Placenta of severe preeclampsia (original magnification ×400). C: Negative control (original magnification ×400).
Protein expression of HPA in placental tissues
Western blot analysis showed two bands, of approximately 65 kDa and 50 kDa. The expression of the 65-kDa HPA protein in the severe preeclampsia group was significantly higher than in the normal group (0.61±0.04 vs. 0.41±0.04, P<0.05), whereas that of 50-kDa protein was lower than in the normal group (0.09±0.03 vs. 0.30±0.03, P<0.05) (Figure 2).
Figure 2.
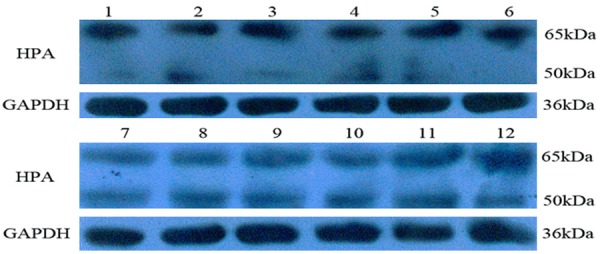
HPA expression in placenta tissues. (1-6) Placenta of severe preeclampsia. (7-12) Placenta of normal control.
Recombinant human HPA protein inhibits cell migration
The effect of recombinant human HPA protein on HTR-8/SVneo cell migration was assessed in vitro using the scratch assay. As shown in Figure 3, HTR-8/SVneo cell migration was delayed in the groups treated with recombinant human HPA protein compared with the negative controls at 12 and 24 hours.
Figure 3.
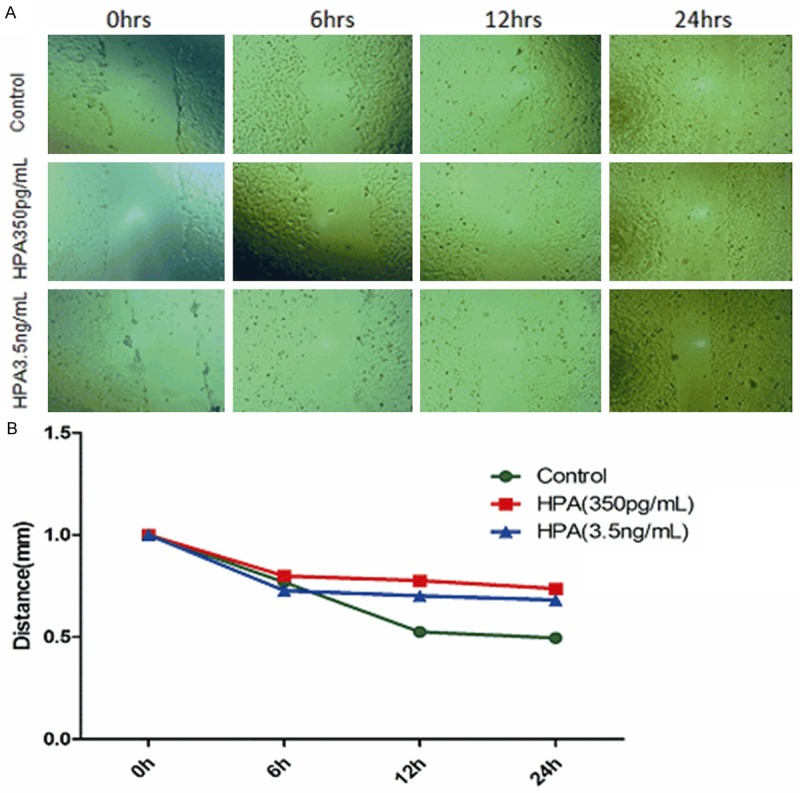
In vitro assessment of HPA treatment of HTR-8/SVneo cells migration (A) HTR-8/SVneo cells showing re-growth following in vitro scratch test (original magnification ×400). (B) Distance between edges of linear scratch following treatment with 350 pg/mL or 3.5 ng/mL.
Recombinant human HPA protein inhibits cell invasion
To observe the effect of HPA on HTR-8/SVneo cell invasion, a Matrigel invasion assay was performed. The results showed that the number of transmembrane cells in the negative control group was 167.0±14.2, whereas it was 30.4±4.4 in the recombinant human HPA protein-treated group. This difference was significant (P<0.05) (Figure 4).
Figure 4.
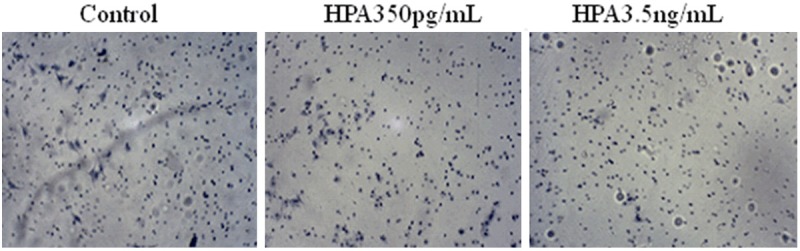
In vitro assessment of HPA treatment of HTR-8/SVneo cells invasion.
Discussion
The abnormal location of HPA
HPA exists in lysosomes under normal physiological conditions in vivo. In the present study, HPA was abnormally located in the nuclei of trophocytes in placentas with severe preeclampsia. There are many transcription factors necessary to maintain normal trophocytes function in the nucleus, such as AP-1 and nuclear factor κB [12]. These factors are prevented from degradation when bound to heparin sulfate (HS). It appeared that the overexpression of HPA in the nucleus degraded HS, and hence, the activities of HS-bound transcription factors were decreased. Therefore, the normal biological behavior of trophocytes was destroyed, possibly leading to severe preeclampsia [13].
The 65-kDa heterologous dimmers and the 50-kDa polypeptide
HPA is initially synthesized as inactive 65-kDaheterologous dimers. After HPA undergoes proteolysis, an 8-kDa active peptide, a 50-kDa active polypeptide and a 6-kDa connecting peptide are produced [14]. When the connecting peptide is completely removed, the 50-kDa and 8-kDa active polypeptides are released. The 50-kDa polypeptides, with high biological activity, play the most important roles in the physiological functions of HPA [14,15].
The expression of heterologous HPA dimmers and the 50-kDa active peptide
Our results showed that the expression of heterologous HPA dimers was markedly increased in placentas with severe preeclampsia, whereas that of the 50-kDa active peptide was significantly reduced compared with the placentas from normal pregnancies. In the severe preeclampsia group, HPA mainly existed in the form of heterologous dimers and was not fully activated. Due to a lack of the 50-kDa active peptide, the full physiological function of HPA could not be exerted. ECM and basement membrane structures could not be effectively degraded by HPA, and trophocytes therefore could not effectively invade the spiral arterioles, decidua and muscular tissue of the uterus. This outcome ultimately constituted an obstacle to vascular remodeling, and thus, preeclampsia occurred. Heterologous HPA dimers also have biological functions. They can cause the release of some cytokines with a high affinity for the HS side chains of HSPG, such as soluble fms-like tyrosine kinase 1 (sFlt-1) and Fibronectin (FN) [16]. Searle reported that HPA could modulate sFlt-1 release, and excessive expression of HPA could therefore significantly increase sFlt-1 in the blood circulation of pregnant rats [17]. Other researchers have confirmed that abnormal sFlt-1 is associated with the onset of preeclampsia [18].
HPA decrease in trophocytes invasion in vitro
To further examine whether HPA overexpression decreases the migration and invasion abilities of trophocytes, different concentrations of recombinant human HPA protein were used when scratch migration and cell invasion assays were performed.
The HTR-8/SVneo cell line is an immortalized trophocytes cell line that was established by introducing the gene encoding the simian virus 40 large T antigens into first trimester human trophocytes. The HTR-8/SVneo cell line has been reported to exhibit a number of characteristics similar to those of parental trophocytes cells. Therefore, this cell line has been a useful tool for investigations of placental function and tumor progression. In the present study, the HTR-8/SVneo cell line was used as a model. Our results showed that exogenous HPA inhibited the migration and invasion ability of HTR-8/SVneo cells when the minimum exogenous HPA concentration reached 350 pg/mL. A higher concentration did not further affect the migration and invasion ability of HTR-8/SVneo cells.
The present study found that HPA overexpression and abnormal nuclear localization might be important factors in the occurrence of severe preeclampsia. This result was different from that of Ronit H.K. et al. [19]. We believe that the different ethnicities and primary antibodies involved might have been the causes. Our group has begun to construct an animal model. We plan to inject an HPA overexpression plasmid into the placentas of pregnant rats and then observe the outcomes of pregnancy. Such an experiment would better identify the correlation between HPA and preeclampsia.
Acknowledgements
This study was financially supported by the grants from the Sichuan Provincial Science & Technology Project (No. 2014JY0081), the Office of Science & Technology of Chengdu (No. 2014-HM01-00043-SF) and the Research Fund for the Doctoral Program of Higher Education of China (No. 20120181120046).
Disclosure of conflict of interest
None.
References
- 1.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87:134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava PK. Endo-beta-D-glucuronidase (heparanase) activity of heat-shock protein/tumour rejection antigen gp96. Biochem J. 1994;301:919. doi: 10.1042/bj3010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlodavsky I, Goldshmidt O, Zcharia E, Atzmon R, Rangini-Guatta Z, Elkin M, Peretz T, Friedmann Y. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol. 2002;12:121–9. doi: 10.1006/scbi.2001.0420. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–77. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- 6.Naomoto Y, Gunduz M, Takaoka M, Okawa T, Gunduz E, Nobuhisa T, Kobayashi M, Shirakawa Y, Yamatsuji T, Sonoda R, Matsuoka J, Tanaka N. Heparanase promotes angiogenesis through Cox-2 and HIF1alpha. Med Hypotheses. 2007;68:162–5. doi: 10.1016/j.mehy.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Li L, Wang Y, Zhang J, Wei G, Sun Y, Shen F. Downregulating the expression of heparanase inhibits the invasion, angiogenesis and metastasis of human hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;358:124–9. doi: 10.1016/j.bbrc.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza SS, Daikoku T, Farach-Carson MC, Carson DD. Heparanase expression and function during early pregnancy in mice. Biol Reprod. 2007;77:433–41. doi: 10.1095/biolreprod.107.061317. [DOI] [PubMed] [Google Scholar]
- 9.Association of Obstetricians and Gynecologists of Chinese Medicine. The guidebooks of diagnosis and treat for hypertension in pregnancy. Chinese Journal of Obstetrics and Gynecology. 2012;47:476–80. [Google Scholar]
- 10.Gui S, Ni S, Jia J, Gong Y, Gao L, Zhang L, Zhou R. Inconformity of CXCL3 plasma level and placenta expression in preeclampsia and its effect on trophoblast viability and invasion. PLoS One. 2014;9:e114408. doi: 10.1371/journal.pone.0114408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 12.Busch SJ, Martin GA, Barnhart RL, Mano M, Cardin AD, Jackson RL. Trans-repressor activity of nuclear glycosaminoglycans on Fos and Jun/AP-1 oncoprotein-mediated transcription. J Cell Biol. 1992;116:31–42. doi: 10.1083/jcb.116.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart MD, Sanderson RD. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol. 2014;35:56–9. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenzie E, Young K, Hircock M, Bennett J, Bhaman M, Felix R, Turner P, Stamps A, McMillan D, Saville G, Ng S, Mason S, Snell D, Schofield D, Gong H, Townsend R, Gallagher J, Page M, Parekh R, Stubberfield C. Biochemical characterization of the active heterodimer form of human heparanase (Hpa1) protein expressed in insect cells. Biochem J. 2003;373:423–35. doi: 10.1042/BJ20030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–91. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 16.Pikas DS, Li JP, Vlodavsky I, Lindahl U. Substrate specificity of heparanases from human hepatoma and platelets. J Biol Chem. 1998;273:18770–7. doi: 10.1074/jbc.273.30.18770. [DOI] [PubMed] [Google Scholar]
- 17.Searle J, Mockel M, Gwosc S, Datwyler SA, Qadri F, Albert GI, Holert F, Isbruch A, Klug L, Muller DN, Dechend R, Muller R, Vollert JO, Slagman A, Mueller C, Herse F. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro-brief report. Arterioscler Thromb Vasc Biol. 2011;31:2972–4. doi: 10.1161/ATVBAHA.111.237784. [DOI] [PubMed] [Google Scholar]
- 18.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haimov-Kochman R, Friedmann Y, Prus D, Goldman-Wohl DS, Greenfield C, Anteby EY, Aviv A, Vlodavsky I, Yagel S. Localization of heparanase in normal and pathological human placenta. Mol Hum Reprod. 2002;8:566–73. doi: 10.1093/molehr/8.6.566. [DOI] [PubMed] [Google Scholar]


