Abstract
Background: Cervical cancer is one of the leading causes of cancer-related death in woman worldwide. In the present study, we investigated the role of microRNA 664 (miR-664) in regulating cancer migration and chemotherapy sensitivity in cervical cancer. Methods: Quantitative real-time PCR (qPCR) was used to assess the mRNA levels of miR-664 in both cervical cancer cell lines and cancer tissues from human patients. Lentiviral vector containing miR-664 mimics (lv-miR-664) was used to upregulate endogenous miR-664 in cervical cancer HeLa cells. The effects of miR-664 up-regulation on cervical cancer cell migration and cisplatin sensitivity were assessed by MTT and Cisplatin assays. Furthermore, the effect of miR-664 up-regulation on E-Cadherin expression was examined by western blot. E-Cadherin was then silenced by siRNA to examine its effect on miR-664 regulation on cervical cancer cell. Results: MiR-664 was downregulated in both cervical cancer cell lines and cancer tissues in patients. In HeLa cells, lentivirus mediated miR-664 up-regulation reduced cancer cell migration and increased chemosensitivity to cisplatin. Western blot showed E-Cadherin was upregulated upon miR-664 overexpression in HeLa cells. Genetic silencing of E-Cadherin by siRNA reversed the inhibitory effect of miR-664 up-regulation on cervical cancer cell migration. Conclusion: Our study demonstrated that miR-664 played an important role in regulating cervical cancer, possibly modulated by E-Cadherin.
Keywords: miR-664, cervical cancer, E-cadherin
Introduction
Cervical cancer is one of the most common malignant cancers in woman worldwide [1,2]. Every year, there are estimated more than half million of new cervical cancer cases, and more than quarter million cancer deaths among females [2]. Especially, more than 85% of those cancer incidences and mortalities occur in developing countries, such as China. In the past decades, tremendous advances have been made in understanding the molecular mechanism of carcinogenesis and cancer development of cervical cancer [3-5]. Yet, the complete scope of underlying mechanism of cervical cancer remains largely elusive. Therefore, identifying key molecular pathways and modulators in cervical cancer carcinogenesis and development is critical to develop novel therapeutic targets for the prevention and treatment of cervical cancer.
MicroRNAs (miRNAs) are a group of small-size, non-coding and single-stranded RNA that modulate gene expression by post-translational mRNA cleavage or degradation [6]. In recent decades, it has been shown that many miRNAs may modulate various stages of carcinogenesis, cancer growth and progression in cervical cancer through the modulation of genes [4,7-9].
Among many of the cancer regulating miRNAs, MicroRNA 664 (miR-664) has shown to be involved in many aspects of carcinogenesis, tumor growth, differentiation and apoptosis. In hepatocellular carcinoma, miR-664 was found to be upregulated in cancer cell lines and subsequent down-regulation treatment of miR-664 reduced cancer growth [10]. In papillary thyroid carcinoma and sporadic breast carcinoma, miR-664 was shown to be downregulated in cancer tissues but its functional role remains unknown [11,12]. As for the cervical cancer, no report, before our study, was available describing the expression and mechanisms of miR-664.
E-Cadherin is the member of transmembrane glycoprotein family that regulates calcium-dependent ion exchanges between adjacent cells [13]. In cervical cancer, previous studies had demonstrated that both the mRNA and protein levels of E-cadherin were downregulated in metastatic carcinomas [14-16]. It was also shown that E-Cadherin was directly targeted by microRNA miR-200 family in regulating epithelial-to-mesenchymal transition in many of the cancer forms [17,18]. However, it was not clear whether E-Cadherin was directly regulated by miRNAs in cervical cancer.
In the current study, we identified miR-664 as a tumor suppressor miRNA in cervical cancer. Our data showed that expression of miR-664 was markedly downregulated in cervical cancer cells and cervical cancer tissues. We also demonstrated that miR-664 up-regulation inhibited cervical cancer cell migration and increased cisplatin chemosensitivity. Furthermore, we showed that E-Cadherin was directly regulated by miR-664 in cervical cancer, and subsequent down-regulation of E-Cadherin reversed the inhibitory effect of miR-664 up-regulation on cancer cell growth.
Materials and methods
Cervical cancer cell lines
Five cervical cancer cell lines, SiHa, Hela, Caski, C-33A and Hela-229 were purchased from ATCC (American Type Culture Collection, Shanghai, China). A control cell line, normal human astrocyte (NHA) was also obtained from ATCC, Shanghai, China. All cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 10 mmol/L HEPES, 2 mmol/L L-glutamine, 50 μmol/L β-mercaptoethanol, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a tissue culture chamber with 95% O2 and 5% CO2.
Clinical tissues
The human cervical tissue samples were obtained from the patients in the department of Gynecology at Qingdao Municipal Hospital from September 2013 to July 2014. Normal cervical tissues were taken from areas of peripheral tissues adjacent to the tumors. Tissues were quickly removed, with partial of them in OCT compound and examined by a histologist to confirm the tumor pathology. Consent forms were obtained from the patients. All protocols of the present study were approved by the Human Research and Ethics Committees of at Qingdao Municipal Hospital in China.
Quantitative real-time reverse transcription-PCR (qRT-PCR)
Total RNA, either from cell lines or clinical tissues, was isolated using a TRIzol reagent kit (Invitrogen, USA) according to manufacturers’ protocol. Reverse transcription was performed by a TaqMan microRNA reverse transcription kit (Applied Biosystems, USA) according to the manufacturer’s protocol. The coding sequence for E-Cadherin primers were 5’-CTGCTGCAGGTCTCCTCTTG-3’ and 5’-TGTCGACCGGTGCAATCTTC-3’. Amplification of cDNA was conducted in 25 µl reaction tubes containing 0.2 µM dNTPs, 20 pmol of each primer, and 0.2U Tag polymerase in PCR buffer. The amplification conditions were 37 cycles of 15 s at 92°C and 1 min at 60°C. The internal controls for miR-664 and E-Cadherin were housekeeping gene U6 and GAPDH, respectively.
Lentiviral production and transfection
The oligonucleotides of hsa-miR-664-5p mimics and its non-specific control were purchased from Ribobio (RiboBio, Guangzhou, China). They were amplified and inserted in a pCDH-CMV-MCS-EF1-coGFP construct to make lentiviral expression vectors (System Biosciences, USA). Then, the lentiviral expression vectors and a pPACK packaging vector were co-transfected into HEK293T cells to produce lentivirus particles of miR-664-5p mimics (lv-miR-664) and its corresponding non-specific control (lv-NC). Cervical cancer cell line, HeLa cells, was then transfected with lv-miR-664 or lv-NC using a Lipofectamine 2000 reagent (Invitrogen, CA), according to manufacturer’s protocol
MTT migration assay
Cervical cancer cell invasion was performed using a quantitative cell migration assay kit (ECM500, Chemicon), according to the manufacturer’s protocol. Briefly, cancer cells were cultured in 96-well plates (10,000 well) for 24 h at 37°C. Lentivirus of lv-miR-664 or lv-NC was then added into culture for 3 days, followed by changing culture medium with 100 μL DMEM + 10% FBS. At the end of 3-day culture, a 20 μL MTT solution was added in the culture for 2 hours according to manufacturer’s protocol (Sigma Aldrich, USA). The absorbance of 560 nm was examined to decide the total number of migrated cells.
Cisplatin chemosensitivity assay
Cervical cancer HeLa cells were transfected with either lv-miR-664 or lv-NC lentivirus, and then plated in 6-well plates for 24 h. The viable cells were transferred at 1 × 104 per well to 24-well plates. Various concentrations of Cisplatin (1~200 μg/mL) were added and cells were further cultured for another 48 hours. Cell density was evaluated by measuring the fluorescence intensity of propidium iodide (PI) at 24 and 48 hours. The fluorescence intensities were assessed by a CytoFluor II multiwell plate reader (PerSeptive Biosystems), and normalized to the intensity while cells were treated with 1 μg/mL cisplatin. All experiments were conducted in triplicate.
Western blotting assay
For western blotting analysis, cells lysates was extracted from HeLa cells using a lysis buffer containing 50 mM Tris (pH 7.6), 150 mM NaCl, 1 mM EDTA, 10% glycerol, and 0.5% NP-40 and protease inhibitor cocktail (Invitrogen, USA). The obtained protein was dissolved in 10% SDS-PAGE gel and blotted on nitrocellulose membranes. The membranes were incubated with primary antibody against E-Cadherin (1:500, Cell Signaling, USA) according to manufacturer’s protocol, followed by horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, USA). The western blots were examined by an enhanced chemiluminesence system (Amersham Biosciences, USA) according to the manufacturer’s protocol.
Genetic silencing of E-Cadherin of siRNA
The E-Cadherin siRNA (ECad_siRNA) and non-specific control siRNA (NC_siRNA) were purchased from Stanta Cruz (Santa Cruz Biotechnology, USA). HeLa cells were plated in 6-well plates at density of 1 × 106 cells/well, and transfected with ECad_siRNA (100 nM) or NC_siRNA (100 nM) with Lipofectamine 2000 (Invitrogen, USA) according to manufacturer’s recommended protocol. HeLa cells were cultured for another 48 hours with the MTT migration essay.
Statistical analysis
All data were presented as the mean ± S.E.M. and evaluated with a two-tail, unpaired Student’s t test. Statistically significance was determined if P < 0.05. All experiments were repeated at least three times.
Results
MiR-664 is downregulated in cervical cancer cell lines and patient samples
As first step of the study, we investigated the expression patterns of miR-664 in both cervical cancer cell lines, as well as tissues from patient samples, with the method of qPCR. We found that, while compared with the mRNA level of miR-664 in control normal human astrocytes (NHA) cell line, the expression levels of miR-664 were significantly lower in all five cervical cancer cell lines we probed, including SiHa, HeLa, Caski, C-33A and HeLa-229 cells, than in non-carcinoma cells (Figure 1A, *: P < 0.05). We then examined the mRNA level of miR-664 in cervical cancer tissues (T) and adjacent non-cancer tissues (ANT) in 8 patients. We found that the expression levels of miR-664 were significantly lower in all cancer tissues, as compared to non-cancer tissues (Figure 1B, *, P < 0.05).
Figure 1.
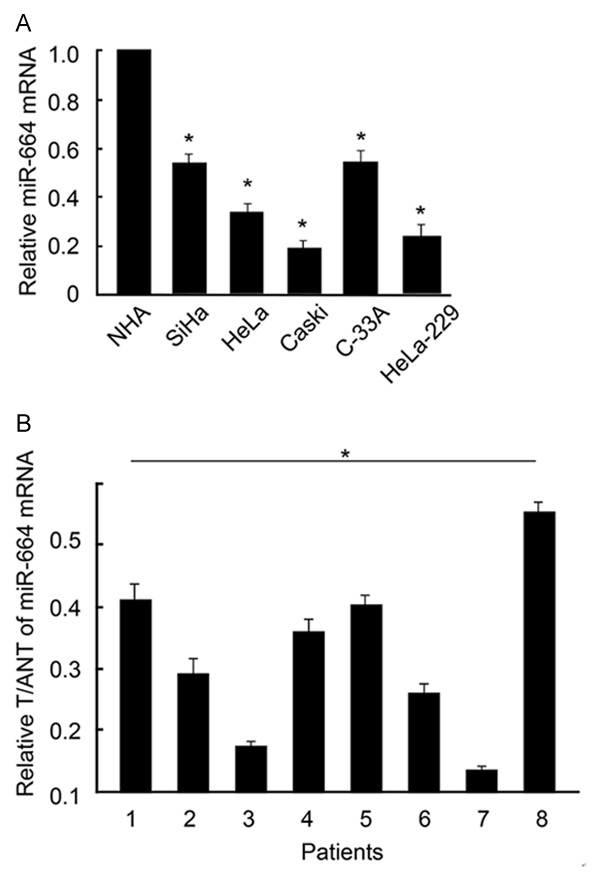
MiR-664 expression in cervical cancer cell lines and primary cancer tissues. A. In five cervical cancer cell lines and a control cell line, normal human astrocytes (NHA), qPCR was performed to compare the mRNA levels of miR-664. Relative values of mRNA expression were normalized to the level in NHA (*, P < 0.05). B. In eight patients with cervical cancer, miR-664 expression levels were compared between paired cancer tissues (T) and adjacent non-cancer tissues (ANT). Relative values of T/ANT were presented (*, P < 0.05).
Up-regulation of miR-664 inhibited cervical cancer cell migration
As we showed that miR-664 was significantly down-regulated in both cervical cancer cell lines and primary tissues of cervical cancer patients, we wondered whether miR-664 had any functional role in cervical cancer.
We firstly constructed lentivirus to over-express human miR-664 in cervical cancer. The efficiency of lentivirus to up-regulated endogenous miR-664 in cervical cancer cells was confirmed by qRT-PCR. In that assay, we demonstrated that the mRNA expression of miR-664 was significantly upregulated by lv-miR-664 in HeLa cells, as compared with non-specific control lentivirus (lv-NC) (Figure 2A, *: P < 0.05).
Figure 2.
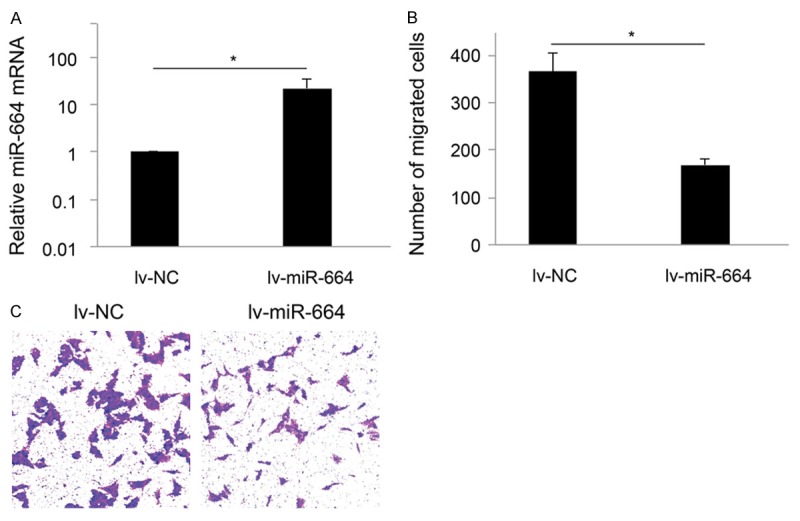
MiR-664 regulates cervical cancer cell migration. A. HeLa cells were transfected with lentiviral vectors expressing human miR-664 mimics (lv-miR-664) or non-specific control (lv-NC) for 48 hours. The endogenous mRNA levels of miR-664 after lentiviral transfections were assessed by qRT-PCR (*: P < 0.05). B. HeLa cells were transfected with lv-miR-664 or lv-NC for 72 hours, followed by measuring migration capability using a MTT trans-well. C. Comparing cervical cancer migration between the conditions of miR-664 up-regulation (lv-miR-664) and not (lv-NC), it showed that the number of migrated cells was significantly reduced with miR-664 up-regulation (*: P < 0.05).
We then assessed the effect of miR-664 up-regulation on migration capability of cervical cancer cells. HeLa cells were transfected with lv-miR-664 for 72 hours to upregulate endogenous mRNA level of miR-664. In parallel control experiment, HeLa cells were transfected with lv-NC. After lentiviral transfection, a MTT trans-well assay showed that up-regulation of miR-664 significantly reduced cervical cancer migration (Figure 2B, 2C, *: P < 0.05).
Up-regulation of miR-664 increased cervical cancer cell chemosensitivity to cisplatin
We then assessed whether miR-664 would have a functional role in regulating the chemotherapy sensitivity in cervical cancer. For that aim, we treated cervical cancer cell line HeLa cells with various concentrations of chemotherapy reagent 5 cisplatin (1~200 μg/mL), upon miR-664 up-regulation by lentivirus of lv-miR-664. In parallel control experiment, lv-NC was applied. We examined time-dependent effects at two time points, 24 hours and 48 hours after lentiviral transfection. After 24 hours of transfection, we found that up-regulation of miR-664 significantly increased HeLa cell chemosensitivity to cisplatin at concentrations between 5 and 100 μg/mL (Figure 3A, *: P < 0.05). After 48 hours, miR-664 up-regulation also significantly increased HeLa cell cisplatin chemosensitivity at concentrations between 5 and 50 μg/mL (Figure 3B, *: P < 0.05)
Figure 3.
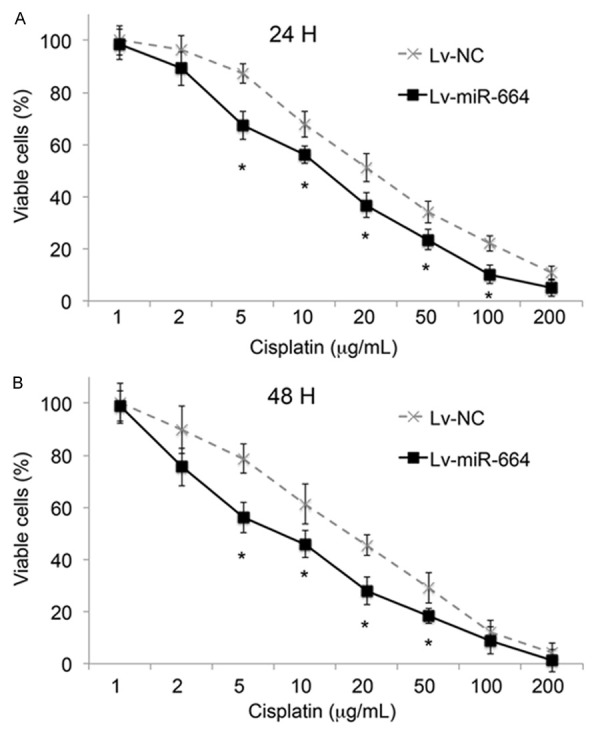
MiR-664 regulates cervical cancer cell chemosensitivity to cisplatin. Cervical cancer HeLa cells were transfected with lv-miR-664or lv-NC. Their chemosensitivity to cisplatin were assessed (A) 24 hours and (B) 48 hours after adding 1 to 100 μg/mL cisplatin in the culture. (*: P < 0.05, Student’s t-test).
Regulation of miR-664 on cervical cancer is modulated by E-Cadherin
Finally, we wondered what the molecular signaling pathways were involved in the regulation of miR-664 in cervical cancer. By western blotting analysis, we found that E-Cadherin was significantly upregulated while miR-664 was over-expressed in cervical cancer HeLa cells (Figure 4A). Then, we used siRNA (ECad_siRNA, 100 nM) to genetically silence E-Cadherin in HeLa cells and evaluated the cancer cell migration during the same time. In parallel control experiment, a non-specific control siRNA (NC_siRNA, 100 nM). Our result showed that E-Cadherin downregulation markedly reversed the inhibitory effect of miR-664 up-regulation on cancer cell migration capability in HeLa cells (Figure 4B, 4C, *: P < 0.05). Thus, it is very likely that the regulatory effect of miR-664 on cervical cancer is modulated through E-Cadherin.
Figure 4.
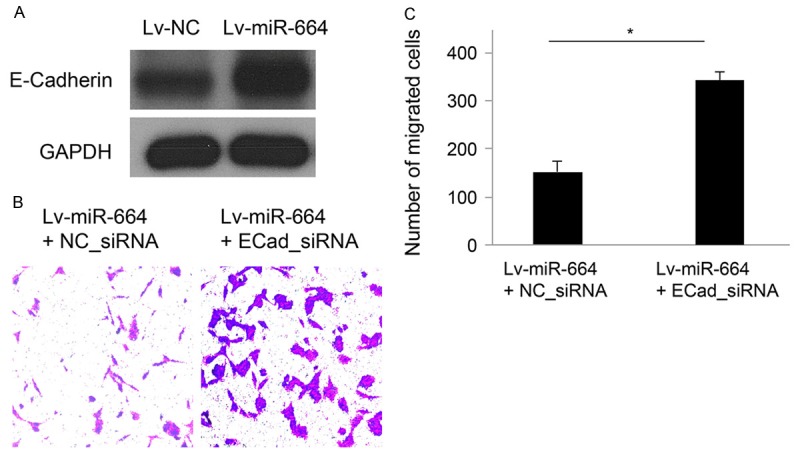
MiR-664 regulation in cervical cancer cell is modulated by E-Cadherin. A. HeLa cells were transfected with either lv-miR-664 or lv-NC for 48 hours. The endogenous protein levels of E-Cadherin were shown by western blotting analysis with internal control of GAPDH. B. HeLa cells were initially transfected with lv_miR-664 to upregulate endogenous miR-664 for 24 hours, followed by transfection of either E_Cadherin siRNA (ECad_siRNA, 100 nM) or non-specific control siRNA (NC_siRNA, 100 nM) for additional 24 hours. The migration of HeLa cells was assessed by MTT migration assay. C. Quantitative analysis showed that the number of migrated cells, initially reduced by miR-664 up-regulation, was reversely upregulated by E_Cadherin downregulation (*: P < 0.05).
Discussions
In the present study, we demonstrated, for the first time, that miR-664 had functional role in regulating cervical cancer. We firstly demonstrated that miR-664 expression was overall downregulated in both cervical cancer cell lines and primary cervical carcinoma tissues. The application of lentivirus to upregulate miR-664 in a functional MTT migration assay demonstrated that miR-664 overexpression significantly inhibited cancer cell migration. These data are consistent with previous reports showing that miR-664 was down-regulated in papillary thyroid carcinoma and sporadic breast carcinoma [11,12], but contrary to a previous report showing miR-664 was up-regulated in hepatocellular carcinoma [10]. Thus, our results suggest that, at least in cervical cancer, miR-664 may act as a tumor suppressor miRNA that is commonly down-regulated in cancer tissues and its up-regulation may inhibit cancer development.
Also in the present study, we showed that E-Cadherin is the direct target of miR-664 regulation in cervical cancer, by demonstrating that the protein expression of E-Cadherin was significantly upregulated by miR-664 overexpression. Furthermore, we showed that E-Cadherin was also directly involved in the regulation of miR-664 in cervical cancer, by demonstrating that genetic silencing of E-Cadherin reversed the inhibitory effect of miR-664 overexpression on cervical cancer cell migration. It has been shown in previous studies that E-Cadherin was normally downregulated, and overexpressing E-Cadherin reduced cancer cell development in cervical cancer [14-16]. Thus, our results showing E-Cadherin was interactively associated with miR-664 to regulate cancer cell growth and migration further supported the idea that E-Cadherin is a proliferative gene in cervical cancer.
In summary, our study revealed a new functional microRNA modulator, miR-664, in human cervical cancer. Our data suggests that miR-664 may act as a tumor suppressor to modulate cervical cancer cell growth and migration. Targeting miR-664 it may help to develop a new therapeutic method for patients with cervical cancer.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Seoud M, Tjalma WA, Ronsse V. Cervical adenocarcinoma: moving towards better prevention. Vaccine. 2011;29:9148–9158. doi: 10.1016/j.vaccine.2011.09.115. [DOI] [PubMed] [Google Scholar]
- 4.Pedroza-Torres A, Lopez-Urrutia E, Garcia-Castillo V, Jacobo-Herrera N, Herrera LA, Peralta-Zaragoza O, Lopez-Camarillo C, De Leon DC, Fernandez-Retana J, Cerna-Cortes JF, Perez-Plasencia C. MicroRNAs in cervical cancer: evidences for a miRNA profile deregulated by HPV and its impact on radio-resistance. Molecules. 2014;19:6263–6281. doi: 10.3390/molecules19056263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoff J, Yang B, Hung CF, Wu TC. Cervical Cancer: Development of Targeted Therapies Beyond Molecular Pathogenesis. Curr Obstet Gynecol Rep. 2014;3:18–32. doi: 10.1007/s13669-013-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–1761. doi: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou C, Li G, Zhou J, Han N, Liu Z, Yin J. miR-107 Activates ATR/Chk1 Pathway and Suppress Cervical Cancer Invasion by Targeting MCL1. PLoS One. 2014;9:e111860. doi: 10.1371/journal.pone.0111860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granados Lopez AJ, Lopez JA. Multistep model of cervical cancer: participation of miRNAs and coding genes. Int J Mol Sci. 2014;15:15700–15733. doi: 10.3390/ijms150915700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres A, Torres K, Maciejewski R, Harvey WH. MicroRNAs and their role in gynecological tumors. Med Res Rev. 2011;31:895–923. doi: 10.1002/med.20205. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Cho ME, Li TW, Peng H, Ko KS, Mato JM, Lu SC. MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J Clin Invest. 2013;123:285–298. doi: 10.1172/JCI63861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zhang H, Zhang P, Li J, Shan Z, Teng W. Upregulation of miR-2861 and miR-451 expression in papillary thyroid carcinoma with lymph node metastasis. Med Oncol. 2013;30:577. doi: 10.1007/s12032-013-0577-9. [DOI] [PubMed] [Google Scholar]
- 12.Bueno RC, Canevari RA, Villacis RA, Domingues MA, Caldeira JR, Rocha RM, Drigo SA, Rogatto SR. ATM down-regulation is associated with poor prognosis in sporadic breast carcinomas. Ann Oncol. 2014;25:69–75. doi: 10.1093/annonc/mdt421. [DOI] [PubMed] [Google Scholar]
- 13.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denk C, Hulsken J, Schwarz E. Reduced gene expression of E-cadherin and associated catenins in human cervical carcinoma cell lines. Cancer Lett. 1997;120:185–193. doi: 10.1016/s0304-3835(97)00308-x. [DOI] [PubMed] [Google Scholar]
- 15.de Boer CJ, van Dorst E, van Krieken H, Jansen-van Rhijn CM, Warnaar SO, Fleuren GJ, Litvinov SV. Changing roles of cadherins and catenins during progression of squamous intraepithelial lesions in the uterine cervix. Am J Pathol. 1999;155:505–515. doi: 10.1016/S0002-9440(10)65146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T. Expression of E-cadherin and alpha- and beta-catenin mRNAs in uterine cervical cancers. Tumour Biol. 1997;18:206–212. doi: 10.1159/000218033. [DOI] [PubMed] [Google Scholar]
- 17.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]


