Abstract
To prospectively observe imaging features and local control of advanced lung tumors after radiofrequency ablation (RFA), and to propose a follow-up protocol post-ablation. 58 stage IV malignant lung tumor patients were enrolled in our study. One hundred of lung lesions were performed 77 sessions of RFA. Enhanced computed tomographic (CT) images of pre-ablation, 1-month, and 3-month post-ablation and thereafter every 3 months were obtained. Positron emission tomographic/CT (PET/CT) was performed pre-ablation, 3-month post-ablation and thereafter every 6 months. The CT size, shape, enhancement, and PET/CT metabolic activity of the ablated zone were analyzed to assess local lesion control. There was significant difference in lesion size between pre-ablation and 1-month post-ablation (P=0.000), 1 and 3-month post-ablation (P=0.000), 3 and 6-month post-ablation (P=0.006). Metabolic activity of the ablated zone after 3 months decreased markedly as compared with pre-ablation (p=0.001). Local control rate was 88%, and forms of definite recurrence or residual included increased size, nodular enhancement, and central enhancement. Time to local progression (TTLP), progression-free survival (PFS), and overall survival (OS) were 15.4±7.5, 9.6±5.8 and 18.0±7.0 months respectively. No death related to operation occurred, and the main complication rate was 29%, of which 9% needed clinical management. RFA is a safe and effective approach for local control of lung tumors even if in advanced patients. To obtain definite CT evaluation, lesion size at 1-month post-ablation as the baseline is appropriate, with efficacy assessment 6-month post-ablation. PET/CT is a useful tool to predict recurrence or residual at least 3 months post-ablation.
Keywords: Radiofrequency ablation, advanced lung tumors, imaging features, local control, follow-up protocol
Introduction
For patients with advanced lung tumors, the therapeutic options are severely limited, because of not candidates for surgery, poor lung function, or comorbidities [1,2]. However, radiofrequency ablation (RFA) is gaining increasing attraction as an effective therapy for patients with primary and secondary malignancies of the lung [3,4], some preliminary results suggest a survival benefit and appreciable local control [5,6]. The patient can undergo repeat treatment if residual or recurrence is detected early, and thus reliable post-RFA imaging follow-up is critical.
Up to now, no standard follow-up imaging protocol has been established or accepted uniformly, and rarely have long time survival analyses been reported. We designed to observe the imaging features and local control of the advanced lung tumors after RFA, and to propose a follow-up post-ablation protocol.
Materials and methods
Patient selection and study design
The study was approved by the ethics committee of our hospital. The risks and benefits were explained to each patient and informed consent was obtained. Inclusion criteria for this study were as follows: patients with a definite pathologic diagnosis of lung malignant tumor; Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 to 2, and an life expectancy ≥3 months; the number of per hemi-thorax tumors ≤3, and any lesion size ≤5 cm. Patients were excluded if any of the following conditions was existent: patients that declined to participate; uncorrected coagulopathy (INR>1.5, PLT<50×109); existence of critical organ failure.
The enhanced CT images of pre-ablation, 1-month, 3-month after ablation and thereafter every 3 months were obtained. The PET/CT was performed pre-ablation, 3 months after ablation and thereafter every 6 months (Figure 1). The CT size, shape, enhancement, and PET/CT metabolic activity of the ablated zone were applied to describe the imaging evolution, and to assess local control of the lesion. Post-ablation at least a 12-month follow-up was given. The TTLP, PFS and OS of the patient were duly recorded.
Figure 1.
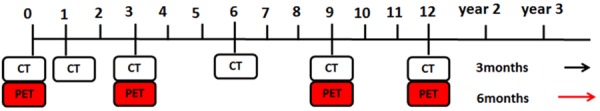
The follow-up imaging protocol post-ablation. Initial PET/CT was required for staging and observing metabolic activity of lesions. CT was performed pre-ablation, 1-month, 3-month after ablation and thereafter every 3 months. PET/CT was taken pre-ablation, 3-month after ablation and thereafter every 6 months.
Study population
Between February 2012 and December 2013, 58 stage IV malignant lung tumor patients were enrolled in our study. The enrolled population consisted of 33 males and 25 females. The average patient age was 55 years (range, 24-81 years). Of the patients, 14 presented with primary lung tumors and 44 presented with metastatic lung tumors. Patient characteristics were summarized in Table 1.
Table 1.
Patient and tumor characteristics
| N | |
|---|---|
| Patient | |
| Age (y) | 54±12.3 (24-81) |
| Sex (M/F) | 33/25 |
| Tumor type (primary/metastasis) | 14/44 |
| TNM (I/II/III/IV) | (0/0/0/58) |
| Lesion | |
| Size (mm) | 19.4±9.5 (6.3-45.8) |
| Location (upper/middle/lower lobe) | 35/16/49 |
Lesions and percutaneous RFA technique
A total of 100 lung lesions were performed 77 sessions of RFA. The average lesion size was 19.4 mm (range, 6.3-45.8 mm). The lesion distribution was as follow: 35 in the upper lung, 16 in the middle lung and 49 in the lower lung. Tumor characteristics were summarized in Table 1.
RFA was performed in patients who were under conscious sedation with intramuscular diazepam (2 ml:10 mg, China) and fentanyl patch (4.2 mg, China) with CT guidance by interventional radiologists, who had more than five years of ablation experience. Local anesthesia was injected at the entry site, and the ablation applicators were placed into position. Applicator type, number, percutaneous entry route, and duration of ablation were based on tumor size, location, tolerance of the patient, and planned treatment goals.
Generally, at our institution, for lesions that were located adjacent to vital organs (such as mediastinum, aorta, main pulmonary artery, main bronchus and esophagus), the internally cooled single needle puncture (17 gauge, Olympus) was preferred, with 2-4 cm active tip. The other lesions can be treated by using a cluster radiofrequency electrode (17 gauge, MedSphere), with mode of temperature control or impedance control for choice. In principle, when ground glass opacity (GGO) around the lesion reached 5-10 mm (Figure 2), the ablation was finished. Immediately after ablation, a chest CT scan was performed to evaluate operation complications, including pneumothorax and hemorrhage. Then patients were transferred to the ward for observation, and a chest radiography was obtained next morning. If in stable condition, patients can be arranged discharge.
Figure 2.
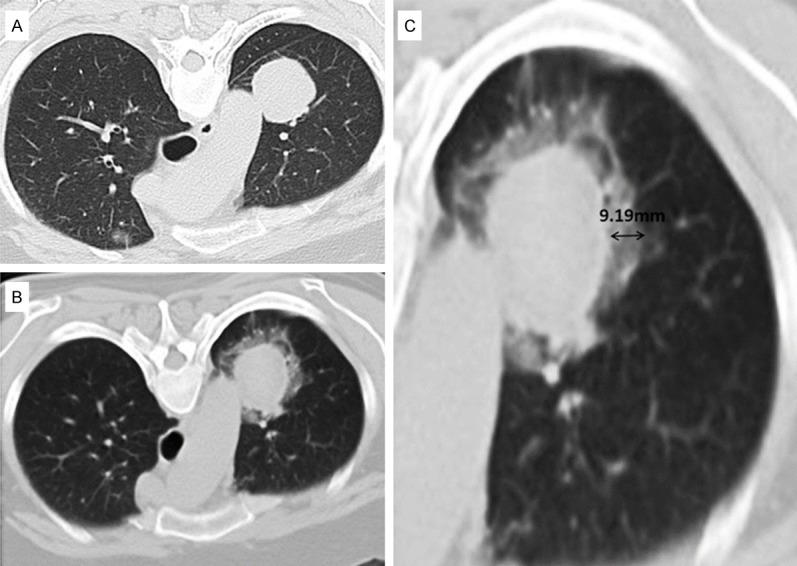
The perilesional GGO with CT scan immediately post-ablation. A: Lesion pre-ablation. B: GGO surrounding the lesion immediately post-ablation. C: When GGO reached 5-10 mm (double-headed arrow), ablation was finished.
Follow-up images and clinical evaluation
In the follow-up of present study, CT scan was performed with a 64-row helical multidetector CT scanner (Siemens, Germany) at 1-month, 3-month after ablation and thereafter every 3 months. In contrast-enhanced CT scan, 100 mL of iohexol was administered at a flow rate of 2-3 mL/sec, and images were acquired 30 seconds after injection. PET/CT was performed 3-month after ablation and thereafter every 6 months. The inject dose of 18F-fluorodeoxyglucose (18F-FDG) was 0.15-0.21 mCi/Kg, and scan began 45-60 minutes later.
Clinical evaluation was based on Revised RECIST guideline (version 1.1) [7]. Local control was defined when target lesion was complete response (CR) or partial response (PR) or stable disease (SD). PFS was defined from the first day after ablation to progression of target lesions and/or progression of non-target lesions and/or appearance of new lesions. OS was defined from the first day after ablation to patient death or the last follow-up.
Statistical analysis
Data were analyzed with SPSS v.19.0 statistical software package. Statistical analysis of lesion size and metabolic activity of the ablated zone used paired Student’s t-test, p<0.05 was considered to indicate a statistically significant difference. Kaplan-Meier analysis was used to analyze the PFS and OS of the patients.
Results
Imaging features
The post-RFA imaging features include the CT size, shape, enhancement, and metabolic activity at PET/CT image. In this study, the post-RFA period was divided into an early phase (≤1-mon after RFA), an intermediate phase (>1-month to 3-month after RFA), and a late phase (>3-month after RFA).
Lesion size, shape and enhancement of CT
The lesion size pre-ablation and at 1, 3, 6, 12-month post-ablation was 19.4±9.5, 28.3±10.2, 20.6±8.1, 18.6±8.8, and 16.4±10.6 mm respectively. There was significant difference in terms of lesion size between pre-ablation and 1-month post-ablation (P=0.000), 1 and 3-month post-ablation (P=0.000), 3 and 6-month post-ablation (P=0.006); while significant difference wasn’t found when comparing 6 and 12-month post-ablation (P=0.300). Typically, the ablated zone got larger in early phase, then retracted, disappeared or stable in size at last.
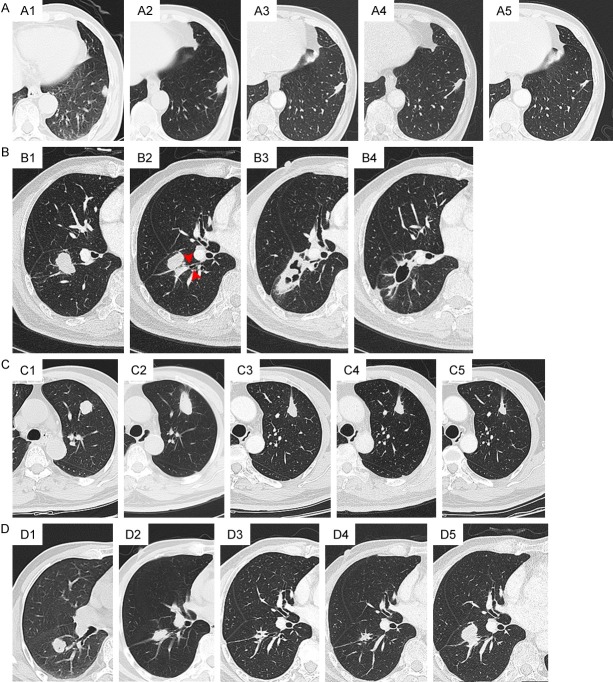
The size of the ablated zone immediately post-ablation should be larger than the original tumor because it includes both the tumor and the perilesional lung tissue, which shows as GGO in most cases. Categories of prognosis of the ablated zone were as follows (Figure 3): (A) disappearance or scan left, (B) cavity formation, (C) stable lesion, and (D) local recurrence or residual. In early and intermediate phase, the ablated zone continued to demonstrate a marked reduction in contrast material uptake as compared with pre-ablation but the peripheral, whose benign enhancement may continue to be seen, might persist for as long as 6 months after RFA. After 6 months, enhancement continued to decrease and not exceed the enhancement at 3 months. The appearance of continuation central or nodular enhancement >10 mm and/or >15 HU compared to plain scan suggested progression of recurrence or residual.
Figure 3.
Categories of CT images evolution of the ablated zone. (A) Disappearance or scan left. (A1) lesion pre-ablation. (A2) 1-month post-ablation. (A3) 8-monthpost-ablation. (A4) 1-yearpost-ablation. (A5) 2-year post-ablation, a scan left at last. (B) Cavity formation. (B1) Lesion pre-ablation, which connected with a bronchus (red arrow in B2). (B3) 1-month post-ablation. (B4) 3-month post-ablation, a thin-wall cavity formed. (C) Stable lesion. (C1) Lesion pre-ablation. (C2) 1-month post-ablation. (C3) 8-month post-ablation. (C4) 1-year post-ablation. (C5) 2-year post-ablation, no obvious alteration of size and shape of the lesion occurred. (D) Local recurrence or residual. (D1) Lesion pre-ablation. (D2) 2-month post-ablation. (D3) 8-month post-ablation. (D4) 1-year post-ablation, no recurrence was detected, (D5) but till 20-month post-ablation, nodular recurrence occurred.
Metabolic activity of PET/CT
Metabolic activity of the ablated zone decreased markedly after 3 months as compared with that pre-ablation (p=0.001). A few lesions still remained uniformly surrounding high uptake of FDG 3-month post-ablation, but not obviously 9 months later, which pointed to inflammatory response. Increased metabolic activity, new uptake of FDG, and irregular or nodular high uptake of FDG (maximum standard uptake value (SUVmax) ≥3) of the ablated zone after 3 months were all findings concerning recurrence or residual.
Clinical evaluation
Of the 58 patients, the number of deaths was 11, and 9 patients were lost of follow-up. In this study, the local control rate was 88%, and 52% of the target lesion was not found definite recurrent or residual lesions during follow-up. In definite recurrent or residual lesions, 63% showed increased of more than 20% as compared with minimum size, 27% showed nodular enhancement measuring more than 10 mm, and 10% showed central enhancement greater than 15 HU. TTLP, PFS, and OS were 15.4±7.5, 9.6±5.8, and 18.0±7.0 months respectively (Figure 4). The mean hospital stays was 4±2.1 days (range, 2-15 days). No death related to operation occurred, the main complication rate was 29%, which included pneumothorax, pulmonary hemorrhage, hydropneumothorax, severe subcutaneous emphysema, and inflammatory cavity. Of all the complications, the rate that needed clinical management was only 9%.
Figure 4.
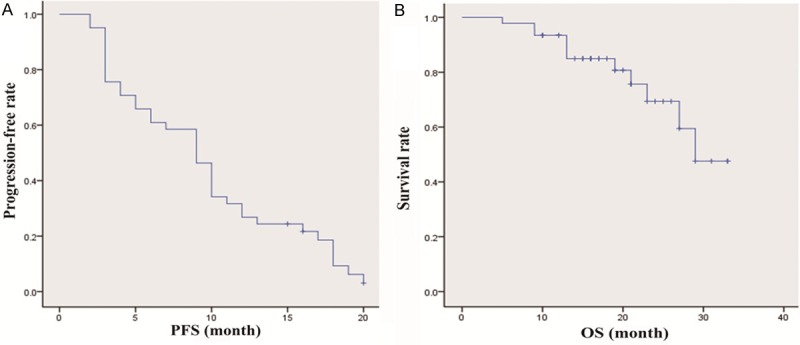
Curves of PFS and OS. A: The progression-free survival (PFS) was 9.6±5.8 months. B: The overall survival (OS) was 18.0±7.0 months.
Discussion
Patients with advanced lung tumors, for not candidates for surgery, poor lung function, or comorbidities, the therapies are limited and challenging [2]. RFA as a minimally invasive therapy is gaining increasing attention, and a growing need has arisen to establish not only the optimal follow-up imaging protocol but also imaging reference for assessing treatment response. Moreover, the early identification of tumor residual or recurrence could make possible expeditious retreatment or alternative therapy [3].
Chest radiographs are routinely obtained after the procedure to evaluate the procedure-related complications, including pneumothorax and pleural effusion. CT is most commonly used for post-RFA surveillance because of its high resolution, availability, low cost and short scan time [1,8,9]. Although magnetic resonance imaging (MRI) is not usually for patients’ follow-up, due in part to its poor lung visualization, but it has also been reported to be a useful modality for the evaluation of ablated tumors [10]. Okuma et al reported the apparent diffusion coefficient (ADC) on diffusion-weighted imaging (DWI) could predict the early treatment response to RFA of lung tumors [11]. Multiple studies showed PET/CT findings not only predicted regrowth of tumors earlier than CT, but also allowed surveillance of extrathoracic tumor progression [12-14]. However, there has been controversy with regard the use of PET and PET/CT to assess the treatment response with RFA. This was mainly due to the false-positive of ablation zone especially in the first three months post-ablation [5,13,15]. In addition, there were some incidences of false-negative outcomes in the surveillance of new extrathoracic lesions. Moreover, the radiation exposure and expensive cost should be taken into account.
In this study, the follow-up of the patient was taken by CT and PET/CT. We found the ablated zone at 1-month post-ablation larger than the original size, then retracted and persistently regressed, which mostly continued to 6-month post-ablation, and then disappeared or was stable at last. Hence, we propose that it is more appropriate to take lesion size at 1-month as the baseline, and to assess efficacy 6-month post-ablation. As for the recurrence or residual after ablation, the forms including increased in size of more than 20% as compared with minimum size, nodular enhancement measuring more than 10 mm, and central enhancement greater than 15 HU, which were consistent with literatures [3,5,7]. Metabolic activity of the ablation zone after 3 months decreased markedly as compared with pre-ablation, although a few lesions remained uniformly surrounding high uptake of FDG. In order to derive a definitive evaluation, too early follow-up of PET/CT is not recommended [5], and many investigators consider that it had better at least 3 months after ablation to take PET/CT [13,15].
The local control rate in our study was 88%, which was consistent with previously published reports [4,16]. Also, 48% of the target lesions were found recurrence or residual during follow-up, which ranged from 7% to 55% according to literatures [17-20]. No death related to operation occurred, and the main complication rate was 29%, of which 9% needed clinical management, including pneumothorax, pulmonary hemorrhage, hydropneumothorax, severe subcutaneous emphysema, and inflammatory cavity. The complications of rib fractures and implantation metastasis were not observed in this study and were rarely reported in the literature [8], but should be paid attention to.
Patients had a rapid recovery and a high life quality after ablation, some could even be discharged the second day post-ablation. Besides, some preliminary results have also suggested a survival benefit [18-20], although still need further studies to confirm.
Conclusion
RFA is a safe and effective approach for local control of lung tumors even if in advanced patients. To obtain a definite CT evaluation, it is more suitable to take lesion size 1-month post-ablation as the baseline, and to assess efficacy 6-month after ablation. PET/CT is a useful tool to predict recurrence or residual at least 3 months after ablation.
Acknowledgements
This work was supported by grants from the Science Technology Commission of Shanghai Municipality (No. 12DZ1940605 and No. 14140902202).
Disclosure of conflict of interest
None.
References
- 1.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–655. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 2.Sesti J, Donington JS. Managing lung cancer in high-risk patients: what to consider. Expert Rev Respir Med. 2014;8:443–452. doi: 10.1586/17476348.2014.918508. [DOI] [PubMed] [Google Scholar]
- 3.Chheang S, Abtin F, Guteirrez A, Genshaft S, Suh R. Imaging Features following Thermal Ablation of Lung Malignancies. Semin Intervent Radiol. 2013;30:157–168. doi: 10.1055/s-0033-1342957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, Helmberger T, Gillams AR, Frilling A, Ambrogi M, Bartolozzi C, Mussi A. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 5.Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics. 2012;32:947–969. doi: 10.1148/rg.324105181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba Y, Watanabe M, Kawanaka K, Iwagami S, Ishimoto T, Iwatsuki M, Yoshida N, Yamashita Y, Baba H. Radiofrequency ablation for pulmonary metastases from esophageal squamous cell carcinoma. Dis Esophagus. 2014;27:36–41. doi: 10.1111/dote.12034. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Alexander ES, Hankins CA, Machan JT, Healey TT, Dupuy DE. Rib fractures after percutaneous radiofrequency and microwave ablation of lung tumors: incidence and relevance. Radiology. 2013;266:971–978. doi: 10.1148/radiol.12120933. [DOI] [PubMed] [Google Scholar]
- 9.Palussiere J, Marcet B, Descat E, Deschamps F, Rao P, Ravaud A, Brouste V, de Baere T. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol. 2011;34:989–997. doi: 10.1007/s00270-010-0048-z. [DOI] [PubMed] [Google Scholar]
- 10.Levine RA, Vogel JA. Cardiovascular and metabolic effects of adenosine 3’,5’-monophosphate in vivo. Nature. 1965;207:987–988. doi: 10.1038/207987a0. [DOI] [PubMed] [Google Scholar]
- 11.Okuma T, Matsuoka T, Yamamoto A, Hamamoto S, Nakamura K, Inoue Y. Assessment of early treatment response after CT-guided radiofrequency ablation of unresectable lung tumours by diffusion-weighted MRI: a pilot study. Br J Radiol. 2009;82:989–994. doi: 10.1259/bjr/13217618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuma T, Okamura T, Matsuoka T, Yamamoto A, Oyama Y, Toyoshima M, Koyama K, Inoue K, Nakamura K, Inoue Y. Fluorine-18-fluorodeoxyglucose positron emission tomography for assessment of patients with unresectable recurrent or metastatic lung cancers after CT-guided radiofrequency ablation: preliminary results. Ann Nucl Med. 2006;20:115–121. doi: 10.1007/BF02985623. [DOI] [PubMed] [Google Scholar]
- 13.Higaki F, Okumura Y, Sato S, Hiraki T, Gobara H, Mimura H, Akaki S, Tsuda T, Kanazawa S. Preliminary retrospective investigation of FDG-PET/CT timing in follow-up of ablated lung tumor. Ann Nucl Med. 2008;22:157–163. doi: 10.1007/s12149-007-0113-0. [DOI] [PubMed] [Google Scholar]
- 14.Harada S, Sato S, Suzuki E, Okumura Y, Hiraki T, Gobara H, Mimura H, Kanazawa S, Kaji M, Fujiwara T. The usefulness of pre-radiofrequency ablation SUV(max) in 18F-FDG PET/CT to predict the risk of a local recurrence of malignant lung tumors after lung radiofrequency ablation. Acta Med Okayama. 2011;65:395–402. doi: 10.18926/AMO/47265. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi M, Honjo H, Shigihara T, Shishido F, Suzuki H, Gotoh M. A phase II study of radiofrequency ablation therapy for thoracic malignancies with evaluation by FDG-PET. J Cancer Res Clin Oncol. 2014;140:1957–1963. doi: 10.1007/s00432-014-1743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargellini I, Bozzi E, Cioni R, Parentini B, Bartolozzi C. Radiofrequency ablation of lung tumours. Insights Imaging. 2011;2:567–576. doi: 10.1007/s13244-011-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochiai S, Yamakado K, Kodama H, Nomoto Y, Ii N, Takaki H, Sakuma H. Comparison of therapeutic results from radiofrequency ablation and stereotactic body radiotherapy in solitary lung tumors measuring 5 cm or smaller. Int J Clin Oncol. 2015;20:499–507. doi: 10.1007/s10147-014-0741-z. [DOI] [PubMed] [Google Scholar]
- 18.Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, Mayo-Smith WW. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 19.Chua TC, Sarkar A, Saxena A, Glenn D, Zhao J, Morris DL. Long-term outcome of image-guided percutaneous radiofrequency ablation of lung metastases: an open-labeled prospective trial of 148 patients. Ann Oncol. 2010;21:2017–2022. doi: 10.1093/annonc/mdq098. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Han Y, Zhao J, Wang X, Cheng Q, Li X, Xu H, Gao K. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg. 2011;39:348–351. doi: 10.1016/j.ejcts.2010.06.004. [DOI] [PubMed] [Google Scholar]