Abstract
To evaluate the effectiveness of tissue engineered skin in the management of diabetic foot ulcer. We searched PubMed, EMBASE and ISI Web of Science database in order to obtain the randomized controlled trial with interventions of tissue engineered skin. A meta-analysis was used to compare the effectiveness between tissue engineered skin and conventional treatment in the patients with diabetic foot ulcer. This meta-analysis was performed by STATA 11 software. The risk factor was analyzed by random effect model pooled odds ratio (OR) and 95% confidence interval (CI). Moreover, the funnel plot was used to assess the published bias of articles. Eight studies were included, and a total of 1060 cases were involved for this meta-analysis. The OR of tissue engineered skin for diabetic foot ulcers was 1.76 (95% CI: 1.35-2.30). A subgroup analysis was conducted for different types of tissue engineering skin, combined OR was 1.91 (95% CI: 1.12-3.27) for Derma graft, 2.05 (95% CI: 1.20-3.50) for Graft skin and 1.57 (95% CI: 0.91-2.70) for Hyalo graft 3D. Applying tissue engineered skin is more effective in the improvement of wound closure in patients with diabetic foot ulcers, compared with conventional treatment.
Keywords: Tissue engineered skin, diabetic foot ulcers, meta-analysis
Introduction
Diabetic foot, a chronic complication of type 2 diabetic mellitus (T2DM), is the result of the interaction among neuropathy, vascular disease and infection. It is the leading cause of diabetes amputation. In China, the incidence of diabetic foot is 8.57% [1]. Diabetic foot ulcers, the most common independent predictors of amputation, are the serious consequences of poor infective tolerance and extensive peripheral vascular disease caused by diabetes. Amputation not only seriously affects the quality of patients’ life, but also increases the risk of the contralateral amputation. Recently, diabetic foot ulcers are becoming a major and growing public health problem in the world.
Currently, the treatment for diabetic foot is the lack of specification guidelines. A growing number of clinical studies have reported a variety of technology for the treatment of diabetic foot ulcers. Lipo-prostaglandin E1 [2] is one of commonly used drugs to improve limb blood circulation. It can selectively combined with pathological changes, and improve the treatment efficiency. Autologous platelet-rich gel [3] is a gel-like substance which can obviously promote ulcer tissue repair and regeneration. This gel-like substance provides a large number of growth factors and cytokines thus stimulates angiogenesis, fibroblast proliferation and collagen synthesis. Tissue engineered skin is regarded as a kind of novel biological treatment materials. In recent years, it is more and more widely used in the world, such as Graftskin [4], Dermagraft [5] and Hyalograft 3D [6]. However, the discussion about the treatment effects of tissue engineered skin is still controversial. Here, we performed a meta-analysis to compare the effectiveness between tissue engineered skin and conventional treatment in the patients with diabetic foot ulcer.
Methods
Study selection
We collected literatures by searching PubMed, EMBASE database and Cochrane Library from July, 1995 to December, 2014. Studies were chose by using the following keywords or text words: “tissue engineering skin”, “human skin equivalent”, “human-tissue graft”, “Graftskin”, “Dermagraft”, “Hyalograft 3D” “diabetic foot ulcer” and “diabetic foot wound”. For each paper, additional studies were selected from its references, citations and from the PubMed option “Related Articles”. The criteria were used to select published studies: (1) Discussed the patients with diabetic foot ulcer; (2) Investigated the tissue engineering skin. The criteria were used to exclude published studies: (1) letters and reviews, (2) lack of data information, (3) non-English language literature, (4) Overlapping data sets. Xu checked the titles, abstracts, full texts and reference lists of the identified studies carefully.
Quality assessment
This research was systematically evaluated according to the guidelines of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guideline [7,8] to ensure the quality of this meta-analysis.
Data extraction
According to the selection criteria, the data was extracted from each identified paper. First author’s name, year of publication, total number of cases, ulcer area, history of ulcer, intervention measure, Comparison intervention, follow-up time, etc. were collected in a form (Table 1). Additional data were reviewed as the following: 95% confidence intervals (CI) and p value.
Table 1.
Summary of studies
| First author | Year | Patients (No.) | Ulcer area (cm2) | History of ulcer | Intervention measure | Comparison intervention | Follow-up time | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| TG | CG | |||||||
| Gentzkow | 1996 | 37 | 13 | >1 | ≥6 months | Dermagraft | CT | 14 months |
| Richard | 1997 | 109 | 126 | >1 | 45 weeks | Dermagraft | CT | 32 weeks |
| Veves | 2001 | 112 | 96 | 1-16 | ≥2 weeks | Graftskin | CT | 3 months |
| Jason | 2002 | 24 | 22 | 1-20 | ≥2 weeks | Dermagraft | CT | NR |
| Sams | 2002 | 9 | 8 | 1-16 | ≥2 weeks | Graftskin | CT | 3 months |
| Caravaggi | 2003 | 43 | 36 | >2 | ≥1 months | Hyalograft | CT | 11 weeks |
| Marston | 2003 | 130 | 115 | 1-20 | ≥2 weeks | Dermagraft | CT | 4 weeks |
| Luigi | 2011 | 90 | 90 | >1 | ≥2 weeks | Hyalograft | CT | 20 weeks |
TG: Treatment group; CG: Control group; CT: Conventional treatment; NR: Not reported; Dermagraft: Human fibroblast-derived dermis; Graftskin: Living human skin equivalents; Hyalograft: HYAFF 11-based autologous dermal and epidermal grafts.
Statistical analysis
In this study, the random effect model or fixed effect model was used for meta-analysis, according to the heterogeneity between studies. Heterogeneity was tested by the Q test (P<0.10 was considered indicative of statistically significant heterogeneity) and the I2 statistic (values of 25%, 50% and 75% were considered to represent low, medium and high heterogeneity, respectively). The fixed effect model was used when there was no significant heterogeneity (I2<50%); otherwise the random effect model was used. p values were calculated by I2 tests. p values <0.05 were regarded as statistically significant for all included studies. Calculation of dichotomous variables was carried out using the OR with the 95% confidence interval (CI) as the summary statistic. The Mantel-Haenszel method was used to combine ORs. Begg’s test was used to evaluate the publication bias. Analyses were performed using STATA statistical software (Version 11.0).
Results
1060 cases of diabetic foot ulcer were collected in this meta-analysis
This meta-analysis was performed on the basis of the remaining eight studies [9-13] (Figure 1). The main information of these studies was shown in Table 1. Inclusion criteria: 1. Study types: randomized controlled trial 2. Study object: diabetics in accordance with WHO diagnostic standard; course ≥2 weeks; Full-thickness ulcer wound (after debridement) 3. Intervening measure: Patients treated with tissue engineering skin, including the wound debridement preparation before transplantation and the same treatment measures against conservative treatment group; the control treatment is wet dressing conservative treatment released by the diabetes association. Exclusion criteria: 1. Local infection of the wound. 2. With other diseases, such as nephritis, nervous system disease, etc. 3. Using the system steroids and immunosuppressant.
Figure 1.
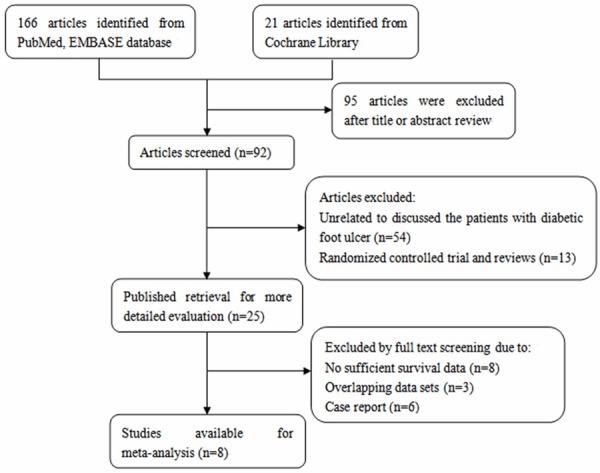
Flow chart of study selection in this meta-analysis.
Tissue engineered skin improved wound closure in diabetic foot ulcers
The 801 patients among six studies were from USA, 259 patients among other two studies were from Italy. The risk factor was analyzed by random effect model pooled OR and 95% CI. The combined analysis of the ten studies showed that the OR of tissue engineered skin for diabetic foot ulcers was 1.76 (95% CI: 1.35-2.30) (Figure 2). Furthermore, a subgroup analysis was conducted for different types of tissue engineering skin, combined OR was 1.91 (95% CI: 1.12-3.27) for Dermagraft, 2.05 (95% CI: 1.20-3.50) for Graftskin and 1.57 (95% CI: 0.91-2.70) for Hyalograft 3D (Figure 3). Taken together, all the data showed that tissue engineered skin, especially Graftskin, could improve wound closure in diabetic foot ulcers.
Figure 2.
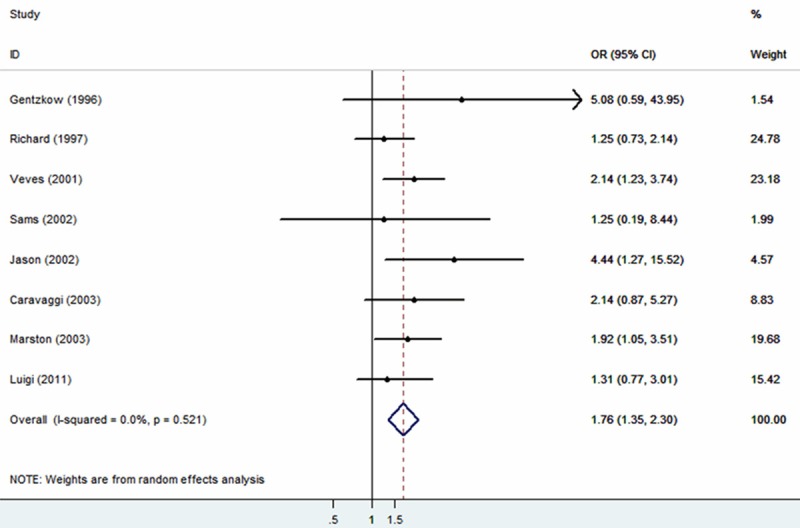
Meta-analysis (Forest plot) of the evaluable studies assessing the association between tissues engineered skin and conventional treatment.
Figure 3.
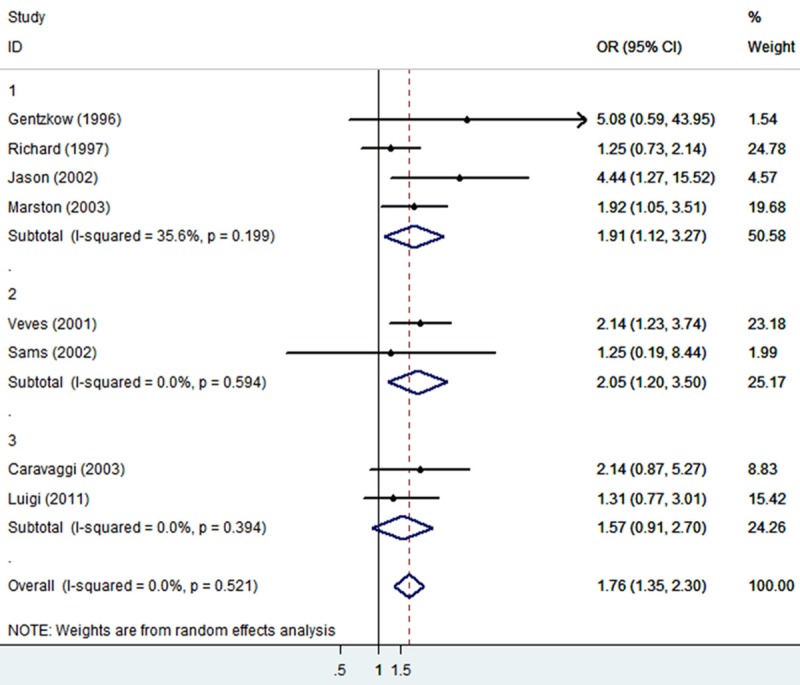
Subgroup analysis for Graftskin, Dermagraft and Hyalograft.
Funnel plot analysis did not show any evidence of publication bias (Begg’s test z = 1.11, P = 0.234, continuity corrected) (Figure 4).
Figure 4.
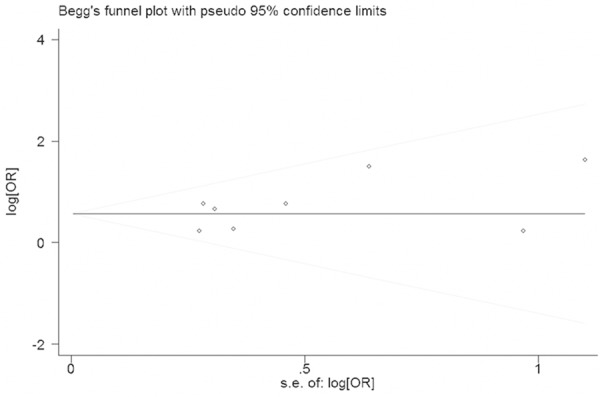
Begg’s funnel plot with 95% confidence intervals for publication bias testing.
Discussion
The WHO defines diabetic foot as diabetes mellitus patients with lower limb distal nerve abnormalities and different levels of peripheral vascular lesions of foot infection, ulcer and/or deep tissue destruction. Foreign studies reported that the accumulative prevalence of diabetic foot in diabetes mellitus patients’ life can be as high as 15%. Each year about more than one million patients with diabetes need amputation in the world. A large amputation occurs about every 30 seconds, prevention and treatment of foot ulcers can obviously reduce the cutting rate of limb [14]. Diabetic foot ulcers healing with diabetes basic diseases exist complicated pathophysiologic connection, blood vessels, immune function and biochemical indexes of neuropathy anomalies would affect tissue repair. Diabetic foot ulcers treatment need comprehensive measures, including infection control, weight control, relief shoes or device, surgical debridement and timely replacement of dressings to keep the local moist environment of the wound. However, although through such comprehensive treatment, DFU patients still have poor healing, scar, and the function, appearance and psychology problems.
At present, the tissue engineered skin types include Graftskin, Dermagraft and Graftjacket. Although the mechanism is not clear, some studies reported that tissue engineered skin can effectively treat chronic disunion wound, fill trauma matrix, product growth factors and cytokines [15-17], which are needed in the process of natural wound healing. It not only has the epidermis (composed) human keratinocytes and dermal layer (by fiber cells in adults into), and also contains human skin cells. Sabolinski reported that tissue engineered skin belongs to regenerative tissue [18,19], due to the functions of tissue engineered skin in morphology, biological function, releasing factor, and so on. However, compared with human skin, tissue engineered skin has no blood vessels, hair follicles, sweat glands, Langerhans’ cells, melanin cells, macrophages, and lymphatic is fine Cell, etc.
In our study, we first discussed the effectiveness between tissue engineered skin and conventional treatment in the patients with diabetic foot ulcer. A total of 1060 cases among eight studies were included. The OR of tissue engineered skin for diabetic foot ulcers was 1.76 (95% CI: 1.35-2.30). A subgroup analysis was conducted for different types of tissue engineering skin, combined OR was 1.91 (95% CI: 1.12-3.27) for Dermagraft, 2.05 (95% CI: 1.20-3.50) for Graftskin and 1.57 (95% CI: 0.91-2.70) for Hyalograft 3D. It is more effective for applying tissue engineered skin in the improvement of wound closure in patients with diabetic foot ulcers.
This meta-analysis is not somewhat perfect due to heterogeneity, biases and other limitations; however, we paid attention to the effectiveness of tissue engineered skin in the patients with diabetic foot ulcer. Quantitative synthesis of the studies was demonstrated that applying tissue engineered skin in the improvement of wound closure is good for the patients with diabetic foot ulcers. Larger-scale and more standard investigations are required to contribute to the role of tissue engineered skin in patients with diabetic foot ulcers and clinical application.
Conclusion
Taking together, applying tissue engineered skin is more effective in the improvement of wound closure in patients with diabetic foot ulcers compared with conventional treatment. It would drive us to pay more attention to the tissue engineered skin in diabetic foot ulcers therapy.
Acknowledgements
We thank our mentor and other teachers for critical reading of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Fard AS, Esmaelzadeh M. Assessment and treatment of diabetic foot ulcer. Int J Clin Pract. 2007;61:1931–8. doi: 10.1111/j.1742-1241.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- 2.Miyata T, Yamada N, Miyachi Y. Efficacy by ulcer type and safety of lipo-PGE1 for Japanese patients with diabetic foot ulcers. J Atheroscler Thromb. 2010;17:805–16. doi: 10.5551/jat.3608. [DOI] [PubMed] [Google Scholar]
- 3.Akingboye AA, Giddins S. Application of autologous derived-platelet rich plasma gel in the treatment of chronic wound ulcer: diabetic foot ulcer. J Extra Corpor Technol. 2010;42:20–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Eaglstein WH, Iriondo M. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839–43. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 5.Advanced Tissue Sciences and Smith & Nephew present Dermagraft data. Ostomy Wound Manage. 1997;43:77–8. 80. [PubMed] [Google Scholar]
- 6.Martin BR. Sangalang, Outcomes of allogenic acellular matrix therapy in treatment of diabetic foot wounds: an initial experience. Int Wound J. 2005;2:161–5. doi: 10.1111/j.1742-4801.2005.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu X. Prognostic role of microRNA-21 in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2011;41:1245–53. doi: 10.1111/j.1365-2362.2011.02535.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu W. Effects of common polymorphisms rs11614913 in miR-196a2 and rs2910164 in miR-146a on cancer susceptibility: A meta-analysis. PLoS One. 2011;6:e20471. doi: 10.1371/journal.pone.0020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentzkow GD, Iwasaki SD, Hershon KS, Mengel M, Prendergast JJ, Ricotta JJ, Steed DP, Lipkin S. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350–4. doi: 10.2337/diacare.19.4.350. [DOI] [PubMed] [Google Scholar]
- 10.Sams HH, Chen J, King LE. Graftskin treatment of difficult to heal diabetic foot ulcers: one center’s experience. Dermatol Surg. 2002;28:698–703. doi: 10.1046/j.1524-4725.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanft JR, Surprenant MS. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast-derived dermis. J Foot Ankle Surg. 2002;41:291–9. doi: 10.1016/s1067-2516(02)80047-3. [DOI] [PubMed] [Google Scholar]
- 12.Caravaggi C. HYAFF 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: a prospective, multicenter, controlled, randomized clinical trial. Diabetes Care. 2003;26:2853–9. doi: 10.2337/diacare.26.10.2853. [DOI] [PubMed] [Google Scholar]
- 13.Uccioli L. Two-step autologous grafting using HYAFF scaffolds in treating difficult diabetic foot ulcers: results of a multicenter, randomized controlled clinical trial with long-term follow-up. Int J Low Extrem Wounds. 2011;10:80–5. doi: 10.1177/1534734611409371. [DOI] [PubMed] [Google Scholar]
- 14.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM American Diabetes Association. Preventive foot care in diabetes. Diabetes Care. 2004;(Suppl 1):S63–4. doi: 10.2337/diacare.27.2007.s63. [DOI] [PubMed] [Google Scholar]
- 15.Allenet B, Paree F, Lebrun T, Carr L, Posnett J, Martini J, Yvon C. Cost-effectiveness modeling of Dermagraft for the treatment of diabetic foot ulcers in the french context. Diabetes Metab. 2000;26:125–32. [PubMed] [Google Scholar]
- 16.Saap LJ, Donohue K, Falanga V. Clinical classification of bioengineered skin use and its correlation with healing of diabetic and venous ulcers. Dermatol Surg. 2004;30:1095–100. doi: 10.1111/j.1524-4725.2004.30334.x. [DOI] [PubMed] [Google Scholar]
- 17.Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif Organs. 1997;21:1203–10. doi: 10.1111/j.1525-1594.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 18.Sabolinski ML, Alvarez O, Auletta M, Mulder G, Parenteau NL. Cultured skin as a ‘smart material’ for healing wounds: Experience in venous ulcers. Biomaterials. 1996;17:311–20. doi: 10.1016/0142-9612(96)85569-4. [DOI] [PubMed] [Google Scholar]
- 19.Hu S, Kirsner RS, Falanga V, Phillips T, Eaglstein WH. Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006;14:427–33. doi: 10.1111/j.1743-6109.2006.00148.x. [DOI] [PubMed] [Google Scholar]


