Abstract
This study aims to investigate the effects and mechanisms of simvastatin on podocyte injuries in diabetic rats. Streptozotocin was used to induce diabetes in a rat model. Three groups were tested: normal control (NC) group, diabetes mellitus control (DM) group, and simvastatin (SVT) group. The serum creatinine, cholesterol, and urinary albumin excretion rate (UAER) were measured 4 to 8 weeks after administering either saline or the drug. Renal pathological changes were observed, and immunohistochemistry was performed to determine the expression of nephrin, podocin, and vascular endothelial growth factor (VEGF). Real-time PCR was performed to detect the mRNA expression levels of nephrin, podocin, and VEGF. Serum creatinine levels and the UAER were higher in the DM group than in the NC group (P < 0.01). The protein and mRNA expression levels of nephrin and podocin were lower in the DM group than in the NC group (P < 0.01); whereas, the expression of VEGF protein and mRNA was higher in the DM group than in the NC group (P < 0.01). Simvastatin (SVT) could reduce serum creatinine levels and the UAER, maintain the expression of nephrin and podocin, reduce the expression of VEGF, and improve the pathological changes of podocytes, which were much more pronounced at 8 weeks (P < 0.01). Simvastatin could maintain the distribution of nephrin and podocin in podocytes, inhibit VEGF expression, and thus improve podocyte injuries and protect kidney functions in diabetic rats.
Keywords: Diabetic nephropathy, podocyte, vascular endothelial growth factor, simvastatin
Introduction
Diabetes mellitus is a an increasingly common and serious disease that threatens global human health. Every year, the morbidity rate of diabetes mellitus increases [1]. Diabetic nephropathy (DN) is among the most serious microvascular complications in diabetes, which can result in end-stage renal disease (ESRD) and eventually death [2]. The main clinical manifestations of DN include proteinuria, reduced glomerular filtration rate, and eventually ESRD [3]. Major pathological features of DN are the proliferation of renal glomerular mesangial cells and matrix, tubular atrophy, interstitial fibrosis, and glomerular sclerosis. The pathogenesis of DN is complex, and has not been fully elucidated to date. In recent years, numerous studies have shown that an impairment of podocytes, which are a major cell type of the glomerulus, plays an important role in the pathogenesis of DN [4]. Podocytes, which are positioned at the inner lining of the glomerular capsule, together with the basement membrane and endothelial cells constitute the glomerular filtration barrier. Podocyte hypertrophy, degeneration, shedding and loss result in a disruption of the glomerular filtration barrier, which leads to proteinuria and deterioration in DN. The podocyte proteins, nephrin and podocin, are major constituents of the slit diaphragm required to maintain the selective permeability of the glomerular filtration barrier [5,6]. Proteinuria, glomerulosclerosis, and renal function deterioration can be caused by the downregulation of nephrin and podocin, which result in structural and functional damage to the podocyte slit diaphragm [7].
Research concerning changes in the expression of podocyte proteins has become more popular in recent years. Vascular endothelial growth factor (VEGF), which is mainly secreted by podocytes, might be overexpressed in DN and thus might be involved in the pathophysiology of proteinuria and DN. Recently, crosstalk between VEGF and nephrin signaling has been suggested; however, further studies are re-quired to confirm bona fide crosstalk. Statins are 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, which inhibit the synthesis of mevalonate, thereby reducing intracellular cholesterol levels and lowering systemic lipid levels. Statins have also been shown to affect cellular processes not directly related to lipid metabolism including: inhibition of cell proliferation and inflammation, and regulation of the cell cycle and the expression of microRNAs [8]; thus, statins could potentially be used in other clinical applications. In DN, statins inhibited mesangial cell proliferation, reduced extracellular matrix accumulation, improved endothelial function, regulated renal blood flow, protected podocytes, and alleviated renal tubulointerstitial injury [9,10]. Blanco et al. found that in obese Zucker diabetic rats (OZR) models, atorvastatin delayed podocyte injury (PI) and preserved kidney function through lipid-lowering and non-lipid-lowering effects [11]. Shibata et al. confirmed that fluvastatin protected against PI by inhibiting foot process apoptosis and podocyte (F-actin) cytoskeletal protein disassembly [12]. Although the protective effects of statins in DN are evident, the specific mechanisms remain to be elucidated.
Therefore, the objectives of our study were to determine whether simvastatin could protect against glomerular PI and DN in diabetic rats and to further explore the mechanisms of simvastatin’s effects, which would establish a theoretical basis for its potential clinical applications.
Materials and methods
Animal care and preparation of the diabetic model
A total of 60 male specific pathogen free (SPF) Sprague Dawley (SD) rats, 8 weeks old and weighing 180-200 g, were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and bred at the Experimental Animal Center of Capital Medical University (Beijing, China). The rats were kept in a well-ventilated room, which was maintained at room temperature (22-25°C) and at a relative humidity of 50-70%, in a 12:12 h light-dark cycle. The rats were given ad libitum access to standard pellet feed and water for 7 days. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Capital Medical University.
Forty out of the 60 rats were randomly selected, fasted and injected once intraperitoneally with streptozotocin (STZ dosage: 60 mg·kg-1 body weight; dissolved in 0.1 M citrate buffer, pH 4.4) (Sigma, USA). After 72 h, a sample of tail vein blood was collected, and the blood sugar level measured; rats with a blood sugar level ≥16.7 mM were deemed positive for diabetes. The remaining 20 rats were injected with an equal volume of citrate buffer, which represented the negative control. Among the 40 rats injected with STZ, one was deemed negative for diabetes based on the blood sugar level. In this case, we may have failed to properly inject STZ into the abdominal cavity. Among the 39 diabetic rats, 3 died of dehydration induced by high sugar levels or diabetic ketoacidosis.
Experimental design and groups
The 36 diabetic rats were randomly divided into two groups: the diabetes mellitus control group (DM group, n=18) or the simvastatin group (SVT group, n=18). The 20 non-diabetic rats constituted the normal control group (NC group). Rats from the NC and DM group were orally administered normal saline, and those in the SVT group were administered simvastatin (10 mg·kg-1·day-1, brand name: Zocor; Lot number: 011124) (Hangzhou MSD Pharmaceutical Co. Ltd., China). The day that rats were orally administrated saline or SVT was considered day 0 of the study. During the study, rats had ad libitum access to water and feed, and the bedding materials were kept dry. Two rats died of suffocation during oral administration of saline or SVT; as a result, 18 rats remained in each group (DM and SVT).
Sample collection
At 4 and 8 weeks after oral administration of either saline or SVT, rats were placed into metabolic cages and urine was collected over a 24-h period. Rats were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (40 mg·kg-1 body weight), and the heart blood was collected and centrifuged to obtain the serum. Kidneys were dissected, and a sample of the tissue was fixed in 10% neutral formalin. Another sample of the renal cortex (1 mm3) was fixed in 2.5% glutaraldehyde (0.1 M phosphate buffer, pH 7.4) at 4°C for 4 h. The remainder of the renal cortex was stored in liquid nitrogen.
Metabolite, protein, and lipid detection
The serum blood glucose (BG), serum creatinine, triglyceride (TG), total cholesterol (TC) and low density lipoprotein-C (LDL-C) levels were detected using an automatic biochemical analyzer, AU5400 (Olympus, Japan). Urine albumin level was detected with an automatic protein analysis system (Dade Behring BN Prospec, USA) that uses the chemiluminescence method. The urinary albumin excretion rate (UAER) was calculated as UAER = Urinary albumin level/1,440 min.
Histopathological observation
For light microscopy, formalin-fixed renal tissues were paraffin-embedded and cut into 4-μm-thick slices. Slices were dewaxed and rehydrated, stained with periodic-acid Schiff (PAS) stain and observed with an Olympus U-25ND25 microscope (Olympus, Japan).
For transmission electron microscopy (TEM), glutaraldehyde-fixed renal cortex tissues were rinsed with 0.18 mM sucrose phosphate buffer, and then fixed in 1% osmium tetroxide at 4°C for 2 h. Stained tissues were dehydrated in a graded acetone series, embedded by impregnating with Epon 812 resin in acetone, and sectioned. Semi-thin sections were stained with 1% toluidine blue and positioned under an optical microscope. Ultrathin sections were placed on copper grids, and stained with uranyl acetate at room temperature for 15-30 min. Grids were rinsed with distilled water and dried with filter paper before and after staining with lead citrate at room temperature for 10-20 min. Sections were imaged with a JEM-1230 transmission electron microscope (Electronics Co. Ltd., Japan). The lengths of the glomerular basal membranes (m) and the numbers of foot processes (n) were measured, and the foot process width (FPW) was calculated using the formula FPW = π/4·(Σm/Σn) [13].
Immunohistochemistry
The Elivision two-step method was used. Four micron paraffin sections were dewaxed, rehydrated, and digested with trypsin for antigen retrieval. Endogenous peroxidases were blocked with 3% hydrogen peroxide and rinsed with PBS buffer (0.01 M, pH 7.4). Rabbit anti-rat nephrin (1:75 dilution) (Boster, Wuhan, China), rabbit anti-rat podocin (1:100 dilution) (Boster, Wuhan, China) and rabbit anti-rat vascular endothelial growth factor (VEGF) (1:50 dilution) (Santa Cruz, USA) were added dropwise. The anti-nephrin and anti-podocin antibodies were incubated at 37°C for 30 min; the anti-VEGF antibody was incubated at 4°C overnight. The secondary antibody (IgG) was then added dropwise and incubated at 37°C for 30 min. DAB staining was performed under a microscope. Staining was followed by dehydration with a graded alcohol series, xylene hyalinization, and neutral gum mounting. PBS was used in place of the primary antibody and was considered the negative control.
In semi-quantitative immunohistochemical analysis, brown granules inside glomeruli were defined as positive expression. The Image Pro Plus 6.0 professional image analysis software was used for the semi-quantitative analysis of the immunohistochemical results. In each slice, 10 non-overlapping fields-of-view (× 400) were selected, and the average optical density and the area of immunopositive signals of nephrin, podocin and VEGF inside the glomerulus were measured. We calculated a positive signal index (RI), which expresses the relative immunopositive content, using the following formula: RI = average optical density of positive signal × positive signal area/glomerular area × 100%. The average RI values for each group were compared.
Real-time PCR
Total RNA was extracted using a TRIzol one-step method and reverse transcribed into cDNA using reverse transcriptase at 37°C for 50 min (hold at 70°C for 15 min). The resulting cDNA from the NC group was then used as a template, and the primers, which were designed for conventional PCR amplification, were used to construct plasmids for each gene. The Promega plasmid extraction kit was used to isolate plasmids. The copy numbers of plasmids were calculated, and the plasmids were serially diluted 10-fold to prepare standards for quantitative PCR amplification. The dilution series of plasmids ranged between 103 to 1010 copies/μL. For instance, the following 4 concentrations might be used for a standard curve: 105, 106, 107 and 108 copies/μL. The ABI Prism 7000 real-time PCR instrument (ABI, USA) was used for real-time quantitative PCR detection, and the associated software was used to design the two-step real-time PCR reactions. The results of sample measurement were calculated and converted to copy numbers according to the standard curve.
The sequences of PCR primers used to construct plasmids are shown in Table 1, and the PCR primers and probe sequences used for quantitative PCR are shown in Table 2.
Table 1.
PCR primer sequences for nephrin, podocin and VEGF plasmid construction
| Gene | Primers | Product |
|---|---|---|
| Nephrin-F | 5’-TGCTGAAGGCGAGGACAT-3’ | 275 bp |
| Nephrin-R | 5’-TGTAGGAAACGGGTGTTGTGAAG-3’ | |
| Podocin-F | 5’-CCAAAGCCATCCAGTTCC-3’ | 300 bp |
| Podocin-R | 5’-TCTCAGCCGCCATCCTCA-3’ | |
| VEGF-F | 5’-GTCACTATGCAGATCATGCGGA-3’ | 253 bp |
| VEGF-R | 5’-TTACACGTCTGCGGATCTTGG-3’ |
Table 2.
Real-time PCR primers and probe sequences of nephrin, podocin and VEGF
| Gene | Primers | Product | Probes |
|---|---|---|---|
| Nephrin-F | 5’-GAAGTACGAATGGACCCCTATGA-3’ | 63 bp | 5’-FAM-CTTCGCTGGCCTGAGGTCCA-TAMRA-3’ |
| Nephrin-R | 5’-TCCCCTCGGATCCTCACA-3’ | ||
| Podocin-F | 5’-CAGCTGGGCTTCAGCACTCT-3’ | 62 bp | 5’-FAM-TGGCTGTGGAAGCTGAGGCAC-TAMRA-3’ |
| Podocin-R | 5’-CCGCACTTTGGCCTGTCTT-3’ | ||
| VEGF-F | 5’-CGCAAGAAATCCCGGTTTAA-3’ | 69 bp | 5’-FAM-TCCTGGAGCGTTCACTGTGAGC-TAMRA-3’ |
| VEGF-R | 5’-CAAATGCTTTCTCCGCTCTGA-3’ |
Statistical analysis
Data were expressed as x̅ ± s.d. Comparisons between groups were performed using the t-test. Correlations among variables were determined with multivariate regression analysis. P < 0.05 was considered a significant difference. The SPSS16.0 software was used for statistical analysis.
Results
Effect of SVT on serum creatinine and lipid levels in diabetic rats
At 4 and 8 weeks after administering saline, serum creatinine levels were elevated in the DM group compared to in the NC group. At 4 weeks after administering either saline or SVT, serum creatinine levels were reduced in the SVT group compared to in the DM group, although the difference was not statistically significant. However, at 8 weeks, serum creatinine levels were significantly reduced in the SVT group compared to in the DM group (P < 0.01). At 4 and 8 weeks after administering saline, the UAER was higher in the DM group than in the NC group (P < 0.01). At 4 weeks after administering either saline or SVT, the UAER was not significantly between the SVT group and the DM group; whereas, at 8 weeks, the UAER was significantly reduced in the SVT group compared to the DM group (P < 0.01). At 4 and 8 weeks after administering saline or SVT, there was no significant difference in blood lipids between the SVT group and the DM group (Table 3).
Table 3.
Changes of BG, Scr, UAER, TG, TC and LDL-C among the experimental groups (x̅ ± s)
| Group | N | BG (mmol/l) | Scr (umol/l) | UAER (μg/min) | TG (mmol/l) | TC (mmol/l) | LDL-C (mmol/l) | |
|---|---|---|---|---|---|---|---|---|
| Week 4 | NC | 9 | 3.41±1.76 | 54.92±6.27 | 0.81±0.28 | 1.54±0.26 | 0.41±0.12 | 0.72±0.13 |
| DM | 9 | 29.99±3.30** | 65.58±8.66* | 9.65±1.23** | 1.82±0.17 | 0.64±0.15** | 0.88±0.13 | |
| SVT | 9 | 31.75±7.61** | 58.63±4.88 | 10.81±9.47 | 1.90±0.23 | 0.91±0.56 | 0.57±0.35 | |
| Week 8 | NC | 9 | 1.23±0.99 | 58.80±4.20 | 0.93±0.11 | 1.57±0.22 | 0.34±0.05 | 0.58±0.08 |
| DM | 9 | 25.41±3.10** | 75.43±4.41** | 11.13±1.05** | 1.89±0.11** | 0.70±0.12** | 0.96±0.11** | |
| SVT | 9 | 26.66±5.24** | 59.95±2.96ΔΔ | 4.41±1.54ΔΔ | 1.83±0.16 | 0.70±0.13 | 0.94±0.10 |
Compared with NC group in the same period, P < 0.05;
Compared with NC group in the same period, P < 0.01;
compared with DM group in the same period, P < 0.01.
Effect of SVT on histopathological changes in diabetic rats
Light microscopy imaging of PAS stained tissues revealed that pathological changes of the renal tissue were not obvious at 4 weeks. However, at 8 weeks, mild mesangial proliferation was evident in tissues from the DM group. In addition, although the proliferative width was less than the diameter of capillaries showing segmental distribution, tubular epithelial cells fell off and exhibited vacuolar degeneration. These pathological features were milder in the SVT group than in the DM group (Figure 1).
Figure 1.
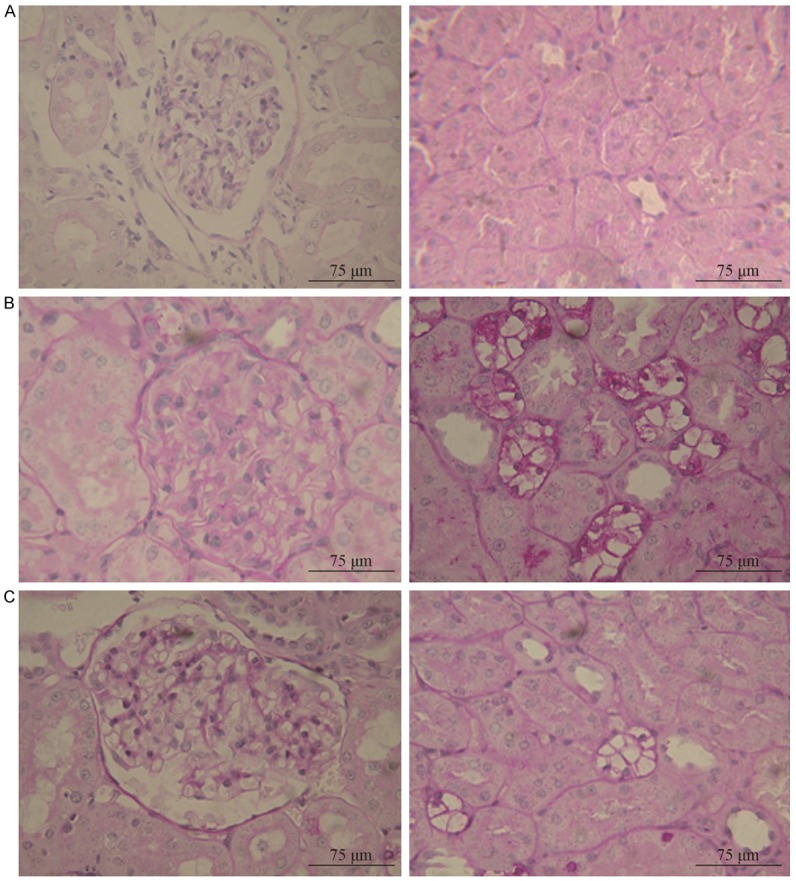
Renal pathological changes under light microscope in the post-modeling week 8 (PAS staining, × 400). Note: A for normal control (NC) group; B for diabetes mellitus control (DM) group; C for simvastatin (SVT) group.
Pathological changes to the slit diaphragm were also assessed using transmission electron microscopy. Glomerular structures in the NC group were normal: foot processes were normal and evenly distributed, and structures of the slit membrane were clear. Four weeks after administering saline or SVT, pathological changes to glomeruli were not obvious in the DM group or the SVT group. However, at 8 weeks, pathological features in tissues of the DM group were evident and included: mild diffusive widening of the glomerular mesangium and matrix, slight swelling of foot processes, fusion of small segments of foot processes, and the partial reduction and disappearance of slit membranes. The FPW was increased in the DM group compared to in the NC group (P < 0.01). Treatment with SVT partially prevented the pathological changes, including the fusion of foot processes and an increase in FPWs (P < 0.05), induced by high blood sugar levels (Figure 2).
Figure 2.
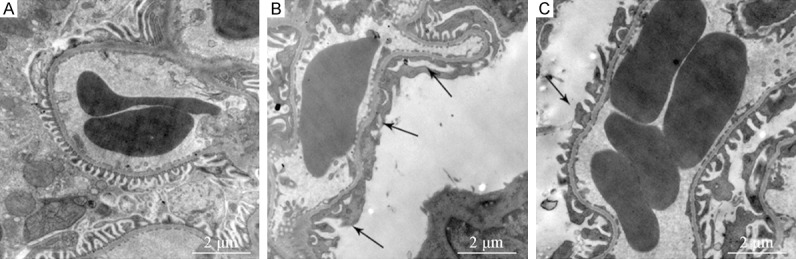
Changes of podocyte foot processes among the experimental group under TEM in the post-modeling week 8 (TEM, × 15000). Note: A for normal control (NC) group; B for diabetes mellitus control (DM) group; C for simvastatin (SVT) group.
Effect of SVT on nephrin, podocin, and VEGF protein expression in diabetic rats
Nephrin and podocin were expressed on the glomerular slit membrane and were evenly and linearly distributed along the glomerular capillary loop (positive expression was brown). The expression of nephrin and podocin was reduced and unevenly distributed in the DM group compared to the NC group at 4 weeks; these changes were more significant at 8 weeks. After 4 and 8 weeks of SVT treatment, the expression of nephrin and podocin (P < 0.01 and P < 0.05, respectively) and their even, linear distribution were partially restored in the SVT group compared to in the DM group (Table 4; Figure 3).
Table 4.
Immunohistochemical semiquantitative analysis of nephrin, podocin and VEGF in renal tissues among groups (RI value) (%, x̅ ± s)
| Group | N | Nephrin | Podocin | VEGF | |
|---|---|---|---|---|---|
| Week 4 | NC | 9 | 8.11±1.21 | 7.59±1.73 | 0.31±0.15 |
| DM | 9 | 4.08±0.52** | 4.41±1.04** | 1.86±0.72* | |
| SVT | 9 | 5.64±0.85ΔΔ | 5.62±0.20Δ | 0.89±0.27Δ | |
| Week 8 | NC | 9 | 8.45±1.50 | 7.49±1.43 | 0.11±0.05 |
| DM | 9 | 1.85±0.41** | 1.84±0.43** | 2.28±0.97** | |
| SVT | 9 | 3.80±0.40ΔΔ | 2.73±0.65Δ | 1.25±0.54Δ |
Compared with NC group in the same period, P < 0.05;
compared with NC group in the same period P < 0.01;
compared with DM group in the same period P < 0.05;
compared with DM group in the same period P < 0.01.
Figure 3.
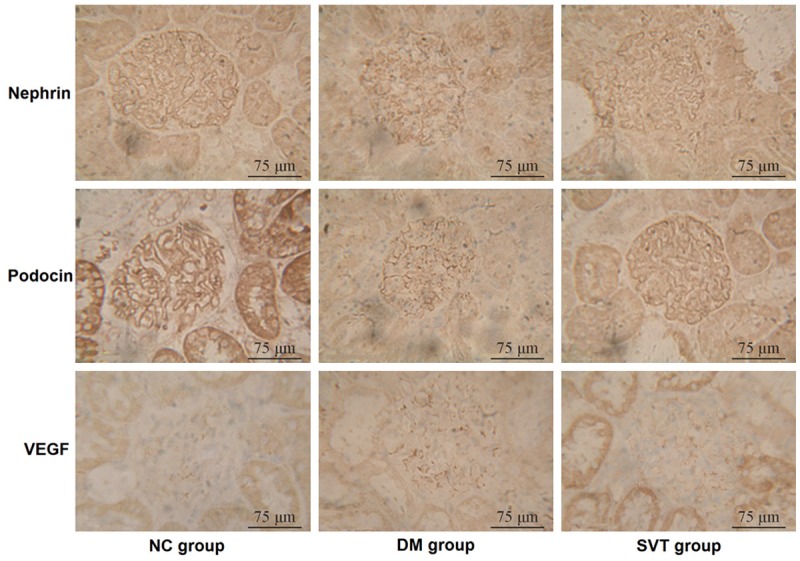
Expression of glomerular nephrin, podocin and VEGF in the post-modeling week 8 among groups (immunohistochemistry, × 400).
Four and eight weeks after administering saline or SVT, the expression of VEGF was higher in the DM group than in the NC group (P < 0.05 and P < 0.01, respectively), whereas it was lower in the SVT group than in the DM group (P < 0.05) (Table 4; Figure 3).
Effect of SVT on nephrin, podocin and VEGF mRNA expression in diabetic rats
Four and eight weeks after administering saline or SVT, the mRNA expression of nephrin and podocin was reduced in the DM group compared to in the NC group (P < 0.01, Table 5) and increased in the SVT group compared to in the DM group (P < 0.01, Table 5). At these same timepoints, the mRNA expression of VEGF was higher in the DM group than in the NC group (P < 0.01, Table 5) and lower in the SVT group compared to in the DM group (P < 0.05, P < 0.01, Table 5).
Table 5.
mRNA expression of nephrin, podocin and VEGF among groups (lg copies/μl, x̅ ± s)
| Group | N | Nephrin | Podocin | VEGF | |
|---|---|---|---|---|---|
| Week 4 | NC | 9 | 7.96±0.25 | 8.08±0.15 | 1.76±0.21 |
| DM | 9 | 4.90±0.22** | 6.42±0.09** | 3.96±0.19** | |
| SVT | 9 | 6.33±0.36ΔΔ | 7.05±0.15ΔΔ | 2.47±0.16Δ | |
| Week 8 | NC | 9 | 7.93±0.47 | 8.14±0.10 | 1.99±0.09 |
| DM | 9 | 5.31±0.22** | 6.25±0.20** | 4.87±0.17** | |
| SVT | 9 | 7.18±0.27ΔΔ | 7.47±0.14ΔΔ | 3.31±0.14ΔΔ |
Compared with NC group in the same period P < 0.01;
compared with DM group in the same period, P < 0.05;
compared with DM group in the same period, P < 0.01.
Correlation analysis
The UAER was negatively correlated with nephrin and podocin mRNA expression (r = -0.685, r = -0.721, P < 0.01), and positively correlated with VEGF mRNA expression (r = 0.633, P < 0.01). Expression of nephrin and podocin mRNA was negatively correlated with VEGF mRNA (r = -0.929, r = -0.931, P < 0.01).
Discussion
Podocytes, which are one of the main cell types that constitute the glomerular filtration barrier, are injured in diabetes, resulting in proteinuria and DN [14]. Foot processes, which interdigitate to form bridging structures known as slit diaphragms, are characteristic features of podocytes. Multiple proteins, including nephrin, podocin, CD2AP, podocalyxin, TRPC6, etc., maintain the integrity of the podocyte slit diaphragm [15]. Nephrin is the earliest discovered protein that is specifically located in the podocyte slit membrane area. Podocin connects nephrin and CD2AP via its C-terminus to form a “zipper-type” filtration barrier and to maintain the normal structures and functions of the glomerular filtration membrane. Previous studies have shown that the expression of nephrin and podocin was lower in diabetic rats than that in normal controls [16]. In diabetic patients, reduced expression of nephrin and podocin occurred earlier than ultrastructural changes of the podocyte and the occurrence of proteinuria [17]. Therefore, nephrin can be used to potentially indicate early glomerular podocyte injuries [18]. In our studies, TEM showed that foot processes were mildly swollen and had fused in small segmental areas, and that partial slit membranes were reduced or missing 8 weeks after inducing a diabetic state. The expression of nephrin and podocin mRNAs and proteins were reduced in diabetic rats compared to in normal rats, and positively correlated with the urinary albumin excretion rate. These results are consistent with those previously reported in the above-mentioned studies: proteinuria and DN are correlated with the reduced expression the podocyte membrane-associated proteins, nephrin and podocin, and changes in expression of nephrin and podocin might change the permeability of the glomerular filtration membrane by affecting the integrity of the foot processes.
VEGF is mainly secreted by podocytes, and can bind to VEGF receptors on glomerular endothelial cells and mesangial cells. Thus, VEGF and VEGF signaling are involved in the pathophysiology of DN [19]. When blood glucose levels are high, VEGF might be overexpressed, which would promote the proliferation of endothelial cells, increase glomerular capillary permeability, result in high glomerular filtration, promote oxidative stress, enhance transforming growth factor-beta (TGF-β) levels [20], and eventually result in proteinuria and glomerulosclerosis. Previous studies have shown that early in DN, injured podocytes release large amounts of VEGF, and that VEGF mRNA expression in the circulation and in kidney tissues was increased [21,22]. Serum VEGF levels were positively correlated with the urinary albumin excretion rate [23]. Even when albuminuria was not evident, VEGF was detected in urine, suggesting that VEGF may be used as a sensitive biomarker for the early diagnosis of DN [24]. In recent years, crosstalk between VEGF-A, a member of the VEGF family, and nephrin signaling was suggested [25,26]. Previous studies have also found that Notch signaling might influence VEGF and nephrin signaling. Activation of Notch-1 signaling in diabetic patients or in diabetic rats result in the increased production of VEGF by podocytes, downregulation of nephrin, and increased apoptosis. Conversely, inhibiting Notch-1 signaling inhibited VEGF signaling and improved proteinuria in diabetic patients and rats [27]. In our study, we observed that the expression of VEGF mRNA and protein increased in diabetic rats compared to that in normal rats, and was negatively correlated with the expression of nephrin and podocin.
Statin’s ability to lower serum cholesterol, especially LDL-C, has been widely accepted as its mechanism-of-action in clinical applications and has been suggested to account for its protective effects in kidney diseases, which include reducing proteinuria, improving the glomerular filtration rate and renal function. In recent years, other pleiotropic effects of statins are being recognized: alleviate inflammation, retard proliferation, prevent oxidative stress, improve endothelial functions [28] and reduce podocyte injuries, etc. [29]. Recent studies have also shown that statins could reduce urinary albumin excretion and serum cystatin C levels in DN patients independently of changes to blood pressure and blood lipids [30,31]; however, the exact mechanisms were not elucidated. In our study, we observed that 8 weeks after administering simvastatin, it reduced the serum creatinine levels and urinary albumin excretion in diabetic rats, and partially prevented pathological changes, such as glomerular mesangial proliferation and renal tubule vacuolar degeneration, in diabetic rats. Importantly, we did not observe any effects on serum lipids in rats treated with simvastatin, which demonstrated that the protective effects of simvastatin on renal functions and structures may not rely on its lipid-lowering effects. Simvastatin could also alleviate the pathological changes of podocytes in diabetic rats. With SVT treatment, we observed improvement of the fusion of foot processes, partial restoration of the normal structures of the slit membrane, upregulation of protein and mRNA levels of podocyte-associated proteins (nephrin and podocin), maintenance of the even and linear distribution of nephrin and podocin in the slit membrane, and reduction of VEGF expression in renal tissues of diabetic rats, which was negatively correlated with the expression of nephrin and podocin in podocytes.
In summary, this study confirmed that podocyte injuries were closely related to proteinuria and DN. Simvastatin could prevent podocyte injury, improve kidney lesions, maintain the proper expression and distribution of podocyte-associated proteins (nephrin and podocin), and inhibit VEGF expression. Our findings might provide a theoretical basis for statins’ clinical applications in DN, although the exact molecular mechanisms still require further elucidation.
Disclosure of conflict of interest
None.
References
- 1.Centers for Disease Control and Prevention. Diabetes Report Card 2012: national and state profile of diabetes and its complications. Available from: http://www.cdc.gov/diabetes/pubs/pdf/DiabetesReportCard.pdf.
- 2.Packham DK, Alves TP, Dwyer JP, Atkins R, de Zeeuw D, Cooper M, Shahinfar S, Lewis JB, Lambers Heerspink HJ. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis. 2012;59:75–83. doi: 10.1053/j.ajkd.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Rocco MV, Berns JS. KDOQI in the era of global guidelines. Am J Kidney Dis. 2009;54:781–787. doi: 10.1053/j.ajkd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Xing Y, Ye S, Chen Y, Hu W, Chen Y. Hydrochloride pioglitazone protects diabetic rats against podocyte injuries through preserving glomerular podocalyxin expression. Arq Bras Endocrinol Metabol. 2014;58:630–639. doi: 10.1590/0004-2730000003141. [DOI] [PubMed] [Google Scholar]
- 5.Welsh GI, Saleem MA. Nephrin-signature molecule of the glomerular podocyte? J Pathol. 2010;220:328–337. doi: 10.1002/path.2661. [DOI] [PubMed] [Google Scholar]
- 6.Piscione TD, Licht C. Genetics of proteinuria: an overview of gene mutations associated with nonsyndromic proteinuric glomerulopathies. Adv Chronic Kidney Dis. 2011;18:273–289. doi: 10.1053/j.ackd.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Lai FM, Lai KB, Chow KM, Li KT, Szeto CC. Messenger RNA expression of podocyte-associated molecules in the urinary sediment of patients with diabetic nephropathy. Nephron Clin Pract. 2007;106:c169–c179. doi: 10.1159/000104428. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava SP, Shi S, Koya D, Kanasaki K. Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair. 2014;7:12. doi: 10.1186/1755-1536-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care. 2005;28:2728–2732. doi: 10.2337/diacare.28.11.2728. [DOI] [PubMed] [Google Scholar]
- 11.Blanco S, Vaquero M, Gómez-Guerrero C, López D, Egido J, Romero R. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18:557–565. doi: 10.1016/j.amjhyper.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Shibata S, Nagase M, Fujita T. Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol. 2006;17:754–764. doi: 10.1681/ASN.2005050571. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg JG, van den Bergh Weerman MA, Assmann KJ, Weening JJ, Florquin S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 2004;66:1901–1906. doi: 10.1111/j.1523-1755.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Y, Zhang X, Cai X, Wang K, Chen Y, Deng Y. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS One. 2014;9:e85690. doi: 10.1371/journal.pone.0085690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu HJ, Tzeng TF, Liou SS, Da Lin S, Wu MC, Liu IM. Polysaccharides from Liriopes Radix ameliorate streptozotocin-induced type I diabetic nephropathy via regulating NF-κB and p38 MAPK signaling pathways. BMC Complement Altern Med. 2014;14:156. doi: 10.1186/1472-6882-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jim B, Ghanta M, Qipo A, Fan Y, Chuang PY, Cohen HW, Abadi M, Thomas DB, He JC. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One. 2012;7:e36041. doi: 10.1371/journal.pone.0036041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patrakka J, Tryggvason K. Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med. 2007;13:396–403. doi: 10.1016/j.molmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol. 2012;32:385–393. doi: 10.1016/j.semnephrol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovind P, Tarnow L, Oestergaard PB, Parving HH. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl. 2000;75:S56–S61. [PubMed] [Google Scholar]
- 22.Hohenstein B, Hausknecht B, Boehmer K, Riess R, Brekken RA, Hugo CP. Local VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in man. Kidney Int. 2006;69:1654–1661. doi: 10.1038/sj.ki.5000294. [DOI] [PubMed] [Google Scholar]
- 23.Baelde HJ, Eikmans M, Lappin DW, Doran PP, Hohenadel D, Brinkkoetter PT, van der Woude FJ, Waldherr R, Rabelink TJ, de Heer E, Bruijn JA. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71:637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 24.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF-a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 26.Lin CL, Wang FS, Hsu YC, Chen CN, Tseng MJ, Saleem MA, Chang PJ, Wang JY. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 2010;59:1915–1925. doi: 10.2337/db09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A. Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem. 2011;286:39933–39944. doi: 10.1074/jbc.M111.241620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gensini GF, Gori AM, Dilaghi B, Rostagno C, Gaw A, Blanco-Colio LM, de Teresa E, Egido J, Farsang C, Leiter LA, Martineau P, Nozza A, Langer A. Effect of atorvastatin on circulating hsCRP concentrations: a sub-study of the achieve cholesterol targets fast with atorvastatin stratified titration (ACTFAST) study. Int J Cardiol. 2010;142:257–264. doi: 10.1016/j.ijcard.2008.12.213. [DOI] [PubMed] [Google Scholar]
- 29.Takemoto M, Ishikawa T, Onishi S, Okabe E, Ishibashi R, He P, Kobayashi K, Fujimoto M, Kawamura H, Yokote K. Atorvastatin ameliorates podocyte injuries in patients with type 2 diabetes complicated with dyslipidemia. Diabetes Res Clin Pract. 2013;100:e26–e29. doi: 10.1016/j.diabres.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Kimura S, Inoguchi T, Yokomizo H, Maeda Y, Sonoda N, Takayanagi R. Randomized comparison of pitavastatin and pravastatin treatment on the reduction of urinary albumin in patients with type 2 diabetic nephropathy. Diabetes Obes Metab. 2012;14:666–669. doi: 10.1111/j.1463-1326.2012.01566.x. [DOI] [PubMed] [Google Scholar]
- 31.Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb. 2011;18:1018–1028. doi: 10.5551/jat.9084. [DOI] [PubMed] [Google Scholar]


