Abstract
Background: Sarcopenia is closely associated with poor performance status and high mortality in cancer patients. The present study is to determine the correlation between sarcopenia and prognosis of hepatectomy for hepatolithiasis-associated intrahepatic cholangiocarcinoma (IHHCC). Methods: Sixty-seven eligible IHHCC patients who underwent hepatectomy, between January 2000 and August 2014 were retrospectively evaluated. Sarcopenia was determined from skeletal muscle index (SMI), assessed by skeletal muscle mass on axial computed tomography images. Factors contributing to overall survival (OS) and recurrence-free survival (RFS) were analyzed by univariate and multivariate analyses. Results: Sarcopenia occurred in 33 (49.3%) out of 67 patients. Median OS of the enrolled patients was 12 months. Sarcopenic patients had a shorter OS compared with non-sarcopenic patients (P < 0.001). On univariate analyses, sarcopenia was significantly associated with overall survival (OS) and recurrence-free survival (RFS; both P < 0.05). On multivariate analysis, sarcopenic patients suffered poor overall survival (P < 0.001) and recurrence-free survival (P = 0.011) compared with non-sarcopenic patients. Conclusions: Preoperative sarcopenia is an independent biomarker of poor prognosis in IHHCC patients following hepatectomy. The identification of sarcopenia may enhance a clinical consideration on decision making for IHHCC patients before surgery.
Keywords: Sarcopenia, hepatolithiasis-associated intrahepatic cholangiocarcinoma (IHHCC), hepatectomy, prognosis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common intrahepatic primary tumor after hepatocellular carcinoma, and the incidence of ICC has increased during the past years [1,2]. Hepatolithiasis is prevalent in Asian countries, including China, Japan, and Korea, but uncommon in Western countries [3,4]. However, as a consequence of increased disease incidence, mortality rates from ICC increased worldwide reaching rates of 1.3 per 100,000 in American Indian and Alaska Native groups and 1.4 per 100,000 in Asian populations [5]. It is well known that hepatolithiasis was associated with incidence of ICC. Seven percent of hepatolithiasis patients can develop intrahepatic cholangiocarcinoma [6]. The development of hepatolithiasis-associated intrahepatic cholangiocarcinoma (IHHCC) is linked to risk factors of enteric bacteria bile duct colonization and infections predisposed by hepatolithiasis and biliary enteric drainage [7]. For the reason of vague symptoms combined with hepatolithiasis and insidious in development, IHHCC often are diagnosed at an advanced stage, consequently, limiting the possibility of curative surgical resection [8,9]. Better-performing prognostic markers in IHHCC following hepatectomy are required to predict outcome well and improve survival.
Several cholangiocarcinoma related prognostic factors have been examined to predict morbidity and mortality after surgery. Carbohydrate antigen 19-9 (CA19-9) is a conventional serum biomarker used for cholangiocarcinoma diagnosis [10]. However, CA19-9 is strongly affected by other diseases, such as primary sclerosing cholangitis and bacterial cholangitis [11,12]. In addition, patients who are negative for Lewis antigen (7% of general population) have undetectable serum CA 19-9 concentrations [13]. Weight loss and body mass index (BMI), which are predictive factors in patients with cancer, remain unappreciated and neglected by clinicians [14]. Moreover, in the settings of increasing prevalence of overweight and obesity, the association of cancer with weight loss and BMI is not strong [15].
Sarcopenia, a syndrome characterized by decreased muscle mass and function, has been identified as a prognostic factor in patients with various malignancies such as pancreatic cancer [16], hepatocellular carcinoma [15,17], colorectal cancer [18,19] and penile cancer [20]. To our knowledge, however, no previous studies have focused on associations of sarcopenia with the prognosis of patients with IHHCC following hepatectomy. We hypothesized sarcopenia may be associated with poor prognosis in IHHCC patients following hepatectomy. The objective of this study was to assess the impact of sarcopenia on outcomes in patients following hepatectomy.
Patients and methods
Patients
Between January 2000 and August 2014, a total of 95 patients underwent open hepatic resection with curative intent for IHHCC in the Department of General Surgery, the First Affiliated Hospital, Wenzhou Medical College, Wenzhou, China. Sixty-seven patients with available postoperative data and axial CT images within 30 days before surgery were enrolled in the study cohort. The ethnic group of all patients was Chinese. Overall survival (OS) was defined as the time from the date of the operation to the date of death or to the date of the last follow-up. Recurrence free survival (RFS) was calculated from the date of surgery until first recurrence or death from any cause.
Clinical data
The data were captured via retrospective chart review of the original records of all patients. Clinical and pathological data were examined with respect to prognosis on the basis of the following variables: age, sex, BMI, serum albumin level, serum carbohydrate antigen 19-9 level, Child-Pugh classification, liver cirrhosis, American Society of Anesthesiologists (ASA) grade, tumor differentiation, tumor size, tumor number, tumor node metastasis), TNM staging (American Joint Committee on Cancer 7th ed. staging for intrahepatic cholangiocarcinoma), and postoperative complication. Curative resection was defined by the absence of tumor tissue macroscopically detectable after removal of the tumor. The postoperative complications included incisional infection, bile leakage, intraperitoneal abscess, pleural effusion, liver failure and encephalopathy. Postoperative complications were scored according to Clavien-Dindo [21]. The presence of complication during 30 days after surgery was defined as a complication with a Clavien grade ≥ 3 (those requiring surgical intervention).
Follow-up strategy and postoperative treatment
Patients were contacted by telephone and asked to report their physical condition. Postoperative IHHCC patients were recommended to receive adjuvant therapy according to the stage of IHHCC and performance status. They were then assessed every 3 months during the first postoperative year and then once every year thereafter. Particularly, CA199 test and abdominal US examination was performed. Abdominal computerized tomography scanning, magnetic resonance imaging, and positron emission tomography (PET), were selected as needed. Treatment for tumor recurrence included reoperation, systemic chemotherapy, and conservative treatment. Follow-up was defined from the time of surgery until the date of last contact or date of death.
Image analysis and definition of sarcopenia
Sarcopenia was assessed by the measurement of the cross-sectional areas (cm2) of total muscle area (TMA) in the L3 region using computed tomography images. Images taken within 30 days before surgery were included. Skeletal muscle was identified by Hounsfield unit thresholds of -30 to +150 [22]. TMA was measured at the third lumbar vertebra using the first slice in the inferior direction with both vertebral spines visible. After reaching a consensus with radiologists, two trained examiners (ZGT and BHL) in consensus, blinded for other variables and patient outcomes at a time of quantification, performed measurements of the areas of TMA by manual outlining on the CT images. Multiple muscles at the third lumbar vertebra were quantified (Figure 1). The measured TMA was normalized by the square of the height to acquire the skeletal muscle index (SMI). Cut-off values for skeletal muscle index (cm2/m2) according to overall survival were defined as 43.75 cm2/m2 for men and 41.10 cm2/m2 for women [17,23]. Sarcopenia was defined according to this cut-off values, and patients were assigned to sarcopenia group or non-sarcopenia group.
Figure 1.
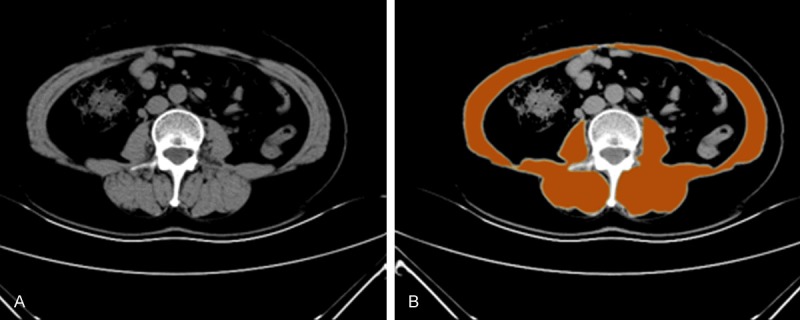
The measurement of the area of skeletal muscle. CT scan showing the area of skeletal muscle mass at the third lumbar vertebra (A), The area of skeletal muscle was highlighted orange (B).
Statistical analysis
Categorical variables with relevant outcome variables were assessed using the χ2 test or the Fisher exact test. For continuous data, Mann-Whitney’s test or independent-samples t test was used. Pearson correlation coefficients and linear regression were used to assess correlation between continuous variables. Univariate and multivariate Cox proportional hazard models tested the associations between variables and OS. OS was measured from the date of surgery to death from any cause or last follow-up. Survival curves were analyzed by the Kaplan–Meier method and compared with the log rank test. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 17 (SPSS, Chicago, IL, USA).
Results
Demographics and clinical characteristics
The clinical and pathological characteristics of 67 patients included in the study are detailed in Table 1. Median age was 61 years old (IQR 47-81), with a majority of women (female to male ratio = 2.1:1). The overall presence of sarcopenia was 49.3 % in our study population. Sarcopenic patients had significantly lower BMIs (21.2 vs. 23.3 kg/m2, P < 0.001) than non-sarcopenic patients. Regarding tumor characteristics, we found that sarcopenic patient was significantly correlated with poor tumor differentiation (P = 0.005), Lymphonodus metastasis (P = 0.018) and advanced TNM stage (P = 0.004). Other host-related factors including age, sex, serum albumin, Child-Pugh grade, tumor number, and tumor size, postoperative complications, were not related to the presence of sarcopenia. The VIF between age, BMI, and sarcopenia (VIF = 1.02 and 1.02) showed no evidence of multicollinearity.
Table 1.
Clinical and pathological characteristics of the 67 study patients
| Variables | Total (n = 67) | Scarcopenia (n = 33) | Nonsarcopenic (n = 34) | P |
|---|---|---|---|---|
| Sex (n), male/female | 22/45 | 9/24 | 13/21 | 0.339 |
| Median age [years (IQR)] | 61 (47-81) | 62 (47-81) | 59.5 (47-79) | < 0.001 |
| Median BMI [kg/m2 (IQR)] | 22.2 (16.7-28.1) | 21.2 (16.69-25.89) | 23.3 (19.3-28.1) | < 0.001 |
| Median SMI [cm2/m2 (IQR)] | 41.2 (26.7-57) | 37.0 (27.0-40.1) | 45.3 (41.2-57.1) | < 0.001 |
| Serum albumin mean (SD), g/L | 37.20 (4.87) | 36.70 (5.38) | 37.71 (4.34) | 0.398 |
| ASA score [No. (%)] | 0.959 | |||
| 1-2 | 57 (85.1) | 28 (84.8) | 29 (85.3) | |
| 3-4 | 10 (14.9) | 5 (15.2) | 5 (14.7) | |
| Serum CA-199 level (U/ml) > 37 [No. (%)] | 0.728 | |||
| No | 19 (28.4) | 10 (30.3) | 9 (26.5) | |
| Yes | 48 (71.6) | 23 (69.7) | 25 (73.5) | |
| Serum CEA level (ng/ml) > 5 [No. (%)] | 0.383 | |||
| No | 37 (55.2) | 20 (60.6) | 17 (50.0) | |
| Yes | 30 (44.8) | 13 (39.4) | 17 (50.0) | |
| Child-Pugh grade [No. (%)] | 0.945 | |||
| A | 51 (76.1) | 25 (75.8) | 26 (76.5) | |
| B | 16 (23.9 ) | 8 (24.2) | 8 (23.5) | |
| Liver cirrhosis [No. (%)] | 0.803 | |||
| No | 54 (80.6) | 27 (81.8) | 27 (79.4) | |
| Yes | 13 (19.4) | 6 (18.2) | 7 (20.6) | |
| Tumor size (cm) > 5 [No. (%)] | 0.39 | |||
| No | 44 (65.7) | 20 (60.6) | 24 (70.6) | |
| Yes | 23 (34.3) | 13 (39.4) | 10 (29.4) | |
| Tumor number [No. (%)] | 0.281 | |||
| Solitary | 51 (76.1) | 27 (81.8) | 24 (70.6) | |
| Multiple | 16 (23.9) | 6 (18.2) | 10 (29.4) | |
| Differentiation of IHHCC [No. (%)] | 0.005 | |||
| Poor/unknown | 27 (40.3) | 19 (57.6) | 8 (23.5) | |
| Well/moderate | 40 (59.7) | 14 (42.4) | 26 (76.5) | |
| TMN stage [No. (%)] | 0.004 | |||
| I-II | 44 (65.7) | 16 (48.5) | 28 (82.4) | |
| III-IV | 23 (34.3) | 17 (51.5) | 6 (17.6) | |
| Lymphonodus metastasis [No. (%)] | 0.018 | |||
| No | 56 (83.6) | 24 (72.7) | 32 (94.1) | |
| Yes | 11 (16.4) | 9 (27.3) | 2 (5.9) | |
| Clavien grade ≥ 3 [No. (%)] | 0.324 | |||
| No | 54 (80.6) | 25 (75.8) | 29 (85.3) | |
| Yes | 13 (19.4) | 8 (24.2) | 5 (14.7) |
Variables in bold are statistically significant (P < 0.05).
Among 67 study patients 53 cases died, 4 cases were lost to follow-up, and 10 cases survived. Sarcopenia patients had a significantly worse prognosis than non-sarcopenic patients in terms of both overall (P < 0.001) (Figure 2) and recurrence-free survival (P < 0.001) (Figure 3). The estimated median OS time was 21 months (95% CI, 16.86-25.14) in the non-sarcopenia group and 6 months (95% CI, 3.95-8.05) in the sarcopenia group. The recurrence rate for patients followed up during this study was 76.1% (51 patients), and the estimated median of disease-free survival in our series was 8 months. Sarcopenia patients had significantly shorter estimated median RFS than non-sarcopenic patients (4 months vs 12 months, respectively; P < 0.001).
Figure 2.
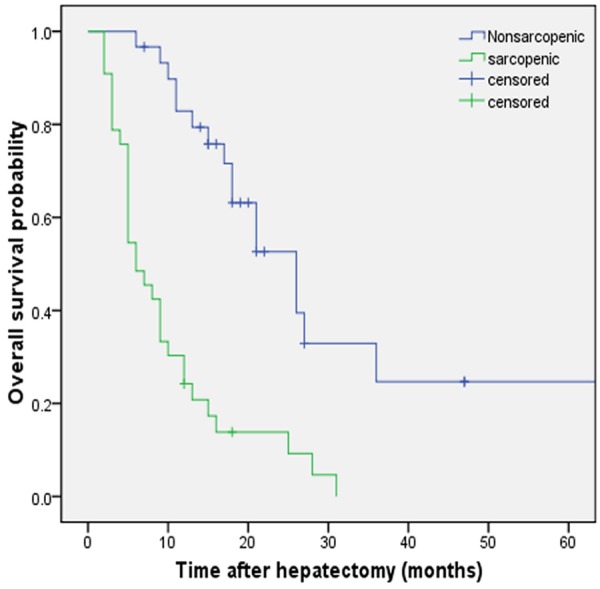
Overall survival of patients with IHHCC after hepatectomy. Kaplan-Meier curves show significant differences in overall survival (OS) probability after hepatectomy in sarcopenic and nonsarcopenic patients. P < 0.001 (log rank test).
Figure 3.
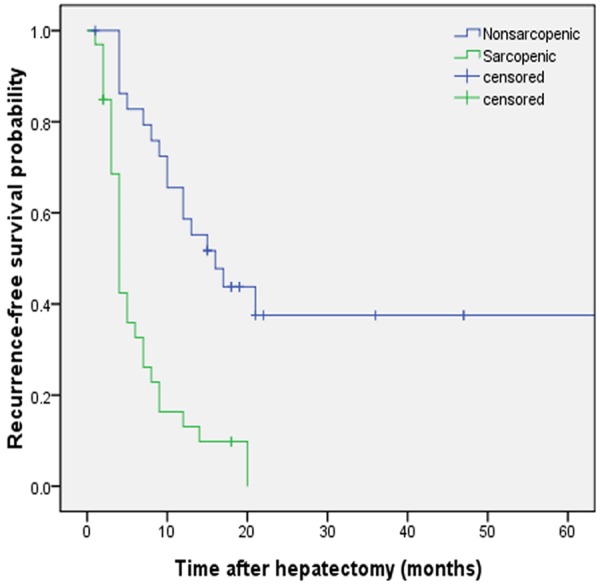
Recurrence-free survival of patients with IHHCC after hepatectomy. Kaplan-Meier curves show significant differences in recurrence-free survival (RFS) probability after hepatectomy in sarcopenic and nonsarcopenic patients. P < 0.001 (log rank test).
Table 2 shows variables associated with OS after liver resection for IHHCC in univariate and multivariate Cox proportional hazard models. On univariate analysis, the presence of sarcopenia, tumor size > 5 cm, TNM stage III+IV, lymph nodes metastasis, poor differentiation and lower serum albumin level were found to be associated with poor overall survival. Multivariable analysis identified three poor prognostic factors (sarcopenia, TNM stage III+IV and lower serum albumin level) that influenced overall survival. Table 3 provides univariate and multivariate Cox proportional hazards regression models for RFS. On univariate analysis, significant prognostic factors for RFS were the presence of sarcopenia, TNM stage III+IV, lymph nodes metastasis, poor differentiation and lower serum albumin level. Multivariable analysis identified three poor prognostic factors (sarcopenia, TNM stage III+IV and lower serum albumin level) that influenced RFS.
Table 2.
Univariate analysis and multivariate analysis of prognostic factors associated with OS
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age > 60 | 0.86 (0.60-1.49) | 0.591 | ||
| Sex female | 1.46 (0.80-2.67) | 0.217 | ||
| Body mass index > Median (22.2) | 0.8 (0.47-1.41) | 0.461 | ||
| Serum albumin ≤ 35 g/L | 2.85 (1.55-5.26) | 0.001 | 2.29 (1.18-4.47) | 0.015 |
| Serum CA-199 (U/ml) > 37 | 0.97 (0.52-1.80) | 0.912 | ||
| Serum CEA (ng/ml) > 5 | 1.45 (0.83-2.51) | 0.188 | ||
| ASA score 3-4 | 1.59 (0.70-3.61) | 0.265 | ||
| Child-Pugh B | 0.84 (0.42-1.68) | 0.623 | ||
| Liver cirrhosis | 1.28 (0.65-2.49) | 0.475 | ||
| Tumor size > 5 cm | 1.82 (1.04-3.20) | 0.035 | ||
| Multiple tumors | 1.06 (0.55-2.03) | 0.865 | ||
| Poor differentiation | 3.24 (1.81-5.83) | < 0.001 | ||
| Lymphonodus metastasis | 5.91 (2.78-12.59) | < 0.001 | ||
| TNM stage III+IV | 10.33 (4.73-22.60) | < 0.001 | 7.94 (3.54-17.81) | < 0.001 |
| Clavien grade ≥ 3 | 0.82 (0.39-1.75) | 0.609 | ||
| Sarcopenia | 3.40 (1.91-6.03) | < 0.001 | 3.01 (1.65-5.51) | < 0.001 |
CA19-9 carbohydrate antigen 19-9; CEA carcino-embryonic antigen; ASA score American Society of Anaesthesiologists score; TNM tumor-node-metastasis; HR hazard ratio; CI confidential interval; Variables in bold are statistically significant in multivariate analysis (P < 0.05).
Table 3.
Univariate analysis and multivariate analysis of prognostic factors associated with RFS
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age >60 | 0.75 (0.43-1.30) | 0.299 | ||
| Sex female | 1.81 (0.98-3.36) | 0.060 | 1.73 (0.92-3.25) | 0.087 |
| Body mass index > Median (22.2) | 1.06 (0.61-1.85) | 0.826 | ||
| Serum albumin ≤ 35 g/L | 2.84 (1.54-5.24) | 0.001 | 2.06 (1.05-4.03) | 0.035 |
| Serum CA-199 (U/ml) > 37 | 0.97 (0.52-1.81) | 0.922 | ||
| Serum CEA (ng/ml) > 5 | 1.59 (0.91-2.78) | 0.104 | ||
| ASA score 3-4 | 2.01 (0.96-4.18) | 0.064 | ||
| Child-Pugh B | 1.14 (0.60-2.14) | 0.694 | ||
| Liver cirrhosis | 1.32 (0.67-2.60) | 0.418 | ||
| Tumor size > 5 cm | 1.60 (0.90-2.86) | 0.111 | ||
| Multiple tumors | 1.45 (0.78-2.69) | 0.241 | ||
| Poor differentiation | 2.95 (1.67-5.21) | 0.001 | ||
| Lymphonodus metastasis | 5.25 (2.51-10.98) | < 0.001 | ||
| TNM stage III+IV | 19.72 (4.45-21.19) | < 0.001 | 7.22 (3.14-16.64) | < 0.001 |
| Clavien grade ≥ 3 | 1.03 (0.52-2.07) | 0.927 | ||
| Sarcopenia | 3.06 (1.72-5.45) | < 0.001 | 2.06 (1.20-4.02) | 0.011 |
CA19-9 carbohydrate antigen 19-9; CEA carcino-embryonic antigen; ASA score American Society of Anaesthesiologists score; TNM tumor-node-metastasis; HR hazard ratio; CI confidential interval; Variables in bold are statistically significant in multivariate analysis (P < 0.05).
Discussion
Hepatolithiasis-associated intrahepatic cholangiocarcinoma (IHHCC) has a poor outcome, and limited possibility of curative surgical resection is one of the most important reasons [24]. Given the rising incidence and the poor prognosis of IHHCC, better-performing prognostic factors are warranted. More recently, sarcopenia is growing as a novel prognostic factor for long-term or short-term outcomes in patients with malignancy. To investigate these findings in more detail, we assessed whether preoperative sarcopenia was correlated with IHHCC prognosis after hepatectomy. To our knowledge, this is the first retrospective analysis focused on associations of sarcopenia with the prognosis of patients with IHHCC following hepatectomy. This single-centre retrospective study shows that sarcopenia has a negative impact on both overall and recurrence-free survival in patients with IHHCC following partial hepatectomy.
Several studies have shown alternative cutoff points for sarcopenia based on lumbar SMI [23,25,26], however, no stratification according to BMI has been validated in the IHHCC population. Despite numerous definitions of sarcopenia, in the present study, sarcopenia was determined as the cut-off values from the study performed in patients undergoing hepatectomy of colorectal liver metastases [23]. According to the cut-off values, nearly half of IHHCC patients undergoing hepatectomy were sarcopenia. Sarcopenic patients had advanced age compared to non-sarcopenic patients, however, advanced age was not a significant predictor of poor OS on invariable analysis or multivariable analysis. No correlation was found between sarcopenia and sex, Child-Pugh classification, serum albumin levels or liver cirrhosis. Although previous studies show that sarcopenia is associated with lower serum albumin levels [15,17,27], we have not found a significant relation between sarcopenia and lower serum albumin levels. However, there was a slight trend of sarcopenia with lower serum albumin levels. We suspect that association between sarcopenia and lower serum albumin levels could have been further clarified in lager sample size.
Sarcopenia has been identified as a predictor of worse long-term outcomes in patients undergoing surgery with various malignancies. The study of Hiroshi Fukushima et al. reported that sarcopenia was predictive of worse overall survival in patients with advanced urothelial carcinoma [27]. In addition, Rebecca M. Dodson et al. reported the association between sarcopenia and worse long-term outcomes after intra-arterial therapy of hepatic malignancies, and 13.0% patients in the study population were intrahepatic cholangiocarcinoma [28]. Similarly, our findings showed that sarcopenia is a predictor of worse overall and recurrence-free survival in IHHCC patient undergoing hepatectomy. When compared to nonsarcopenic patients, sarcopenic patients have more poor differentiation IHHCC (P = 0.005), more lymphnodes metastasis (P = 0.018) and more advanced TNM stage tumor (P = 0.004). As previous studies described in hepatocellular carcinoma [15,17], the present study has also shown a close association between sarcopenia and tumor aggressiveness. Skeletal muscle depletion may result in increased metabolic condition of aggressive tumor biology. Thus, sarcopenia may predict poor prognostic in patients with malignancy.
Sarcopenia is associated with ageing, and it is more prevalent in older patients, particularly, combined with chronic disease and malignancy. Sarcopenia can reflect many clinical characteristic in patients with malignancy, such as low nutritional status and catabolism [15]. BMI reflectes nutrition and catabolism. In clinical setting, low BMI can be accquried conveniently and is related to sarcopenia. For ICC, radical surgery is the optimal therapy and offers a potential for cure [29]. However, late diagnosis and advanced tumor stage often limit the radical surgery and lead to poor prognosis. Recent studies have also shown a close association between sarcopenia and tumor aggressiveness [15,17]. Skeletal muscle depletion may result in increased metabolic condition of aggressive tumor biology. Moreover, the roles of adjuvant therapies for ICC remain poorly defined. Until now, poor long-term prognosis remains a great challenge for both the patient and surgeon. Thus, sarcopenia could be a valuable and earlier predictor for prognostic in patients with IHHCC. The impact of sarcopenia on postoperative complications has not been determined yet. In the present study, no correlation was found between sarcopenia and postoperative complications. In addition, a confounding bias occurs in the combination analysis of intrahepatic cholangiocarcinoma and hepatolithiasis, for hepatolithiasis has an impact on the incidences of postoperative infectious complication [30].
The underlying mechanism of sarcopenia is multifactorial but still poorly understood. The most evident metabolic explanation for muscle decline in elderly people is an imbalance between protein synthesis and breakdown rates. Other factors such as neurodegenerative disorder, anabolic hormone such as insulin, growth and sex hormones, cytokine, inflammatory response, nutritional intake and lifestyle could be associated with muscle decline [31]. The intervention and treatment of sarcopenia have shown the ability to reverse low skeletal muscle mass and function. The cellular processes underlying the mechanism of sarcopenia requires further study, in order to identify targets for treatment. For example, specific mitochondrial processes, including oxidative stress, quality control mechanisms and apoptotic signaling, were shown to make contribution on the development of sarcopenia [32]. The contribution of increased oxidative stress in elderly people has been recognized as one of the risk factors of sarcopenia. Khor et al. reported that vitamin E, a lipid soluble vitamin with potent antioxidant properties, can prevent muscle atrophy and promote muscle regeneration [33]. Moreover, the association of nutritional supplementation and physical exercise also has been show effective in the treatment of sarcopenia in old age [34,35]. Branched-chain amino acid (BCAA) supplementation has shown effective ability to improved survival of cirrhotic patients [36]. Identification of sarcopenia can be readily peformed before treatment for IHHCC patients through a secondary analysis of CT images, which is accurate and well-recognized in clinical practice. In addition, cross-sectional abdominal scanning by CT is routinely available for most IHHCC patients, generally used to assess tumor or hepatolithiasis characteristics and to look for abdominal metastases. In clinical setting, sarcopenia can be a objective and promising prognostic factor in IHHCC patients before surgery.
The present study had several limitations. First, for single-institution nature, the present study was limited by the small sample size and limited patient comorbidity data. Thus, it may be unreliable to demonstrate prognostic factors on outcomes with subgroup analysis in such a small cohort of patients. However, IHHCC is a rare disease, and a low rate of hepatectomy with curative intent is performed. Second, this was a retrospective study. Although sarcopenia is recommended to define by using the combination of skeletal muscle wasting and functional status [37]. Due to the retrospective nature, we were unable to acquire walking speed, grip strength or ECOG/Karnofsky score. Third, although skeletal muscle mass is connected with ethnicities, the cut-off value of SMI was defined according to a previous western study in the present study, which may have an impact on the result. Fourth, whether preoperative SMI reflects the precancer state or consequence of malignancy cannot be easily distinguished. Despite these limitations, the present study raises the probability that the presence of sarcopenia indicates overall survival and recurrence-free survival among IHHCC patients.
Conclusion
Sarcopenia, which is assessed on routinely preoperative CT scans, is a useful and objective biomarker for predicting the mortality and recurrence of after curative resection in IHHCC patients. The identification of sarcopenia may enhance a clinical consideration on decision making for IHHCC patients before surgery. Future studies are necessary to determine whether preventive strategies to maintain muscle mass can alter the present poor outcomes.
Acknowledgements
This study was supported by Zhejiang Provincial Top Key Discipline in Surgery and Wenzhou Key Laboratory Project in Surgery. The authors wish to thank Sulin Wang and Qiandong Zhu for technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Cancer Net. 2009;7:423–7. doi: 10.6004/jnccn.2009.0030. [DOI] [PubMed] [Google Scholar]
- 2.Luke C, Price T, Roder D. Epidemiology of cancer of the liver and intrahepatic bile ducts in an Australian population. Asian Pac J Cancer Prev. 2010;11:1479–85. [PubMed] [Google Scholar]
- 3.Shoda J, Tanaka N, Osuga T. Hepatolithiasis-epidemiology and pathogenesis update. Front Biosci. 2003;8:e398–409. doi: 10.2741/1091. [DOI] [PubMed] [Google Scholar]
- 4.Lesurtel M, Regimbeau JM, Farges O, Colombat M, Sauvanet A, Belghiti J. Intrahepatic cholangiocarcinoma and hepatolithiasis: an unusual association in Western countries. Eur J Gastroenterol Hepatol. 2002;14:1025–7. doi: 10.1097/00042737-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 5.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006;26:1047–53. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 6.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–79. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tocchi A, Mazzoni G, Liotta G, Lepre L, Cassini D, Miccini M. Late development of bile duct cancer in patients who had biliary-enteric drainage for benign disease: a follow-up study of more than 1,000 patients. Ann Surg. 2001;234:210–4. doi: 10.1097/00000658-200108000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, Firpi RJ, Morelli G, Clark V, Cabrera R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013;29:1259–67. doi: 10.3892/or.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang GW, Lin JH, Qian JP, Zhou J. Identification of prognostic factors and the impact of palliative resection on survival of patients with stage IV hepatolithiasis-associated intrahepatic cholangiocarcinoma. J Surg Oncol. 2014;109:494–9. doi: 10.1002/jso.23524. [DOI] [PubMed] [Google Scholar]
- 10.Scarlett CJ, Saxby AJ, Nielsen A, Bell C, Samra JS, Hugh T, Baxter RC, Smith RC. Proteomic profiling of cholangiocarcinoma: diagnostic potential of SELDI-TOF MS in malignant bile duct stricture. Hepatology. 2006;44:658–66. doi: 10.1002/hep.21294. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh PG, Navaneethan U, Shen B, Mc-Cullough AJ. Increased serum levels of carbohydrate antigen 19-9 and outcomes in primary sclerosing cholangitis patients without cholangiocarcinoma. Dig Dis Sci. 2013;58:850–7. doi: 10.1007/s10620-012-2401-3. [DOI] [PubMed] [Google Scholar]
- 12.Ker CG, Chen JS, Lee KT, Sheen PC, Wu CC. Assessment of serum and bile levels of CA19-9 and CA125 in cholangitis and bile duct carcinoma. J Gastroenterol Hepatol. 1991;6:505–8. doi: 10.1111/j.1440-1746.1991.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 13.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–54. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 14.Spiro A, Baldwin C, Patterson A, Thomas J, Andreyev HJ. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br J Cancer. 2006;95:431–4. doi: 10.1038/sj.bjc.6603280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2014;261:1173–83. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 16.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 17.Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y, Nishie A, Yamanaka T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100:1523–30. doi: 10.1002/bjs.9258. [DOI] [PubMed] [Google Scholar]
- 18.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–6. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida M, Watanabe M, Baba H. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22:2663–8. doi: 10.1245/s10434-014-4281-6. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Zargar-Shoshtari K, Caracciolo JT, Richard GJ, Poch MA, Pow-Sang J, Sexton WJ, Spiess PE. Sarcopenia as a predictor of complications in penile cancer patients undergoing inguinal lymph node dissection. World J Urol. 2015;33:1585–92. doi: 10.1007/s00345-014-1471-6. [DOI] [PubMed] [Google Scholar]
- 21.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 23.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–7. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 24.Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, Ducerf C, Rivoire M, Bachellier P, Chiche L, Nuzzo G, Regimbeau JM. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–29. doi: 10.1097/SLA.0b013e318236c21d. discussion 30. [DOI] [PubMed] [Google Scholar]
- 25.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 26.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31:1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima H, Yokoyama M, Nakanishi Y, Tobisu K, Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One. 2015;10:e0115895. doi: 10.1371/journal.pone.0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odson RM, Firoozmand A, Hyder O, Tacher V, Cosgrove DP, Bhagat N, Herman JM, Wolfgang CL, Geschwind JF, Kamel IR, Pawlik TM. Impact of sarcopenia on outcomes following intra-arterial therapy of hepatic malignancies. J Gastrointest Surg. 2013;17:2123–32. doi: 10.1007/s11605-013-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amamoto M, Ariizumi S. Surgical outcomes of intrahepatic cholangiocarcinoma. Surg Today. 2011;41:896–902. doi: 10.1007/s00595-011-4517-z. [DOI] [PubMed] [Google Scholar]
- 30.Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Kawai M, Hirono S, Tani M, Yamaue H. Risk factors for postoperative infectious complications after hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:67–73. doi: 10.1007/s00534-010-0313-1. [DOI] [PubMed] [Google Scholar]
- 31.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13:717–23. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 32.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khor SC, Abdul Karim N, Ngah WZ, Yusof YA, Makpol S. Vitamin E in sarcopenia: current evidences on its role in prevention and treatment. Oxid Med Cell Longev. 2014;2014:914853. doi: 10.1155/2014/914853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahar S, Kamaruddin NS, Badrasawi M, Sakian NI, Abd Manaf Z, Yassin Z, Joseph L. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin Interv Aging. 2013;8:1365–75. doi: 10.2147/CIA.S46826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc. 2013;14:10–7. doi: 10.1016/j.jamda.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193–9. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]


