Abstract
Objective: To investigate the antifibrotic effect of Pyk2-related non-kinase (PRNK) and explore the possibility of using adenovirus carrying PRNK gene for targeted inhibition of Pyk2 to treat myocardial fibrosis. Method: Adenovirus carrying PRNK gene was constructed and the angiotention II (Ang II)-induced rat cardiac fibroblasts (CFs) were transfected with the adenovirus. The expressions of PRNK and phosphorylated Pyk2 proteins in CFs were detected. After the preparation of rat model of abdominal aortic stenosis, the rats were infected by the adenovirus expressing PRNK gene. Four groups were set up: sham operation group, PRNK group, drug intervention group and operation group. Myocardial collagen volume fraction (CVF) and perivascular collagen area (PVCA) were measured through Van Gieson (VG) staining, and the content of blue-stained collagen was analyzed by Masson’s trichrome staining. TUNEL method was used to detect myocardial cell apoptosis, and secretions of type I and IV collagen in myocardial tissues were detected by ELISA; expressions of PRNK and phosphorylated Pyk2 proteins were detected by Western Blot. Results: Adenoviral vector carrying PRNK gene was successfully constructed; rat CFs were effectively transfected by the adenovirus that expressed PRNK gene stably. The adenoviruses were injected into rats with myocardial interstitial fibrosis via the tail vein. CVF, PVCA and grayscale of blue-stained collagen in the treatment groups were significantly lower than those in the control group, while the apoptosis rate of CFs in the former was significantly higher than that in the latter. In the transfection group, PRNK protein was upregulated in CFs, and the phosphorylated Pyk2 protein was downregulated. PPARγ agonist rosiglitazone (RSG) was injected as a comparison. The secretions of type I and IV collagen in myocardial tissues and serum did not show significant differences, and they were all much lower than those of the control. Conclusion: Adenoviral vector provides an effective means for the transfer of genes in researches on the mechanism and prevention and control of myocardial fibrosis. Targeted inhibition of Pyk2 using PRNK is a new pathway to achieve an antifibrotic action. Highly expressed in CFs, PRNK inhibits myocardial fibrosis by inhibiting the phosphorylation of Pyk2 through competitive binding. We preliminarily demonstrate the feasibility of using adenoviral vector carrying PRNK gene for targeted inhibition of Pyk2 to treat myocardial fibrosis.
Keywords: Pyk2-related non-kinase (PRNK), myocardial fibrosis, adenoviral vector
Introduction
Myocardial fibrosis is one form of myocardial remodelling that is related to rheumatic heart disease, hypertension, cardiac insufficiency, arrhythmia and sudden cardiac death. Recent studies showed that the severity of myocardial interstitial fibrosis may be one important factor affecting the survival of patients with rheumatic heart disease. Therefore, the prevention and control of myocardial fibrosis is a research hotspot in the field of heart-related diseases. Some progress has been achieved with gene therapy.
Adenovirus is considered to be the most appropriate for gene transfer in the treatment of cardiovascular system due to its high efficiency and stable expression of genes carried. At present, adenovirus has been applied to treat stage III cardiovascular diseases [1].
Proline-rich tyrosine kinase2 (Pyk2) is a new member of focal adhesion kinase (FAK) family and has a high homology with FAK. Pyk2 plays a crucial role in intracellular signal transduction. PRNK, a homologous isomer of Pyk2, only encodes the C-terminal domain of Pyk2. Like Pyk2, PRNK also contains focal adhesion targeting sequence, but lacks N-terminal kinase-activating domain as in Pyk2. With this structural feature, PRNK may selectively regulate the functions of Pyk2 in specific cells [2]. It is uncertain whether PRNK competes with Pyk2 for focal adhesions, by which the phosphorylation of Pyk2 is inhibited. Besides, little is known concerning the role of PRNK in the prevention of myocardial interstitial fibrosis induced by high stress. We established the rat model of myocardial interstitial fibrosis induced by abdominal aortic stenosis. Transfection was performed using adenoviral vector carrying PRNK gene to study the preventive effect against myocardial fibrosis and to explore the feasibility of adenoviral vector in targeted inhibition of Pyk2.
Materials and methods
The gene-encoding cDNA of PRNK was synthesized by whole gene synthesis and then inserted into the adenoviral vector. PCR was performed to confirm whether the adenoviral vector expressing PRNK was constructed successfully.
Rat cardiac fibroblasts (CFs) induced by Ang II were transfected with the adenoviral vector carrying PRNK gene. The CFs were divided into different groups: blank group where no intervention was performed; Ang II group where Ang II (10-6 mol/L) was added at the same time point as in PRNK group and empty vector group to treat the cells for 48 h; empty vector group + Ang II group where Ang II (10-6 mol/L) was added to treat the cells for 48 h after transfection of CFs with empty vector for 24 h; PRNK + Ang II group where Ang II (10-6 mol/L) was added to treat the cells for 48 h after transfection of CFs with adenoviral vector carrying PRNK gene for 24 h. Expressions of PRNK and phosphoryated Pyk2 in CFs were detected by Western Blot.
Rat model of abdominal aortic stenosis was prepared and 4 groups were set up, with 10 rats in each group: sham operation group; PRNK group where 1 ml of adenoviral vector carrying PRNK was injected via the tail vein one week after modeling; drug intervention group where 100 mg·kg-1·d-1 RSG was intraperitoneally injected one week after modeling; operation group where abdominal aortic stenosis was achieved through operation. Left ventricular hypertrophy index (LVHI) was measured 5 weeks after operation; CVF and PVCA were detected through VG staining; the content of blue-stained collagen was detected by Masson’s trichrome staining. TUNEL method was used to detect myocardial cell apoptosis; secretions of type I and IV collagen in myocardial tissues were detected by ELISA; expressions of PRNK and phosphorylated Pyk2 proteins after transfection with adenoviral vector carrying PRNK were detected by Western Blot.
Results
Construction of adenoviral vector carrying PRNK gene
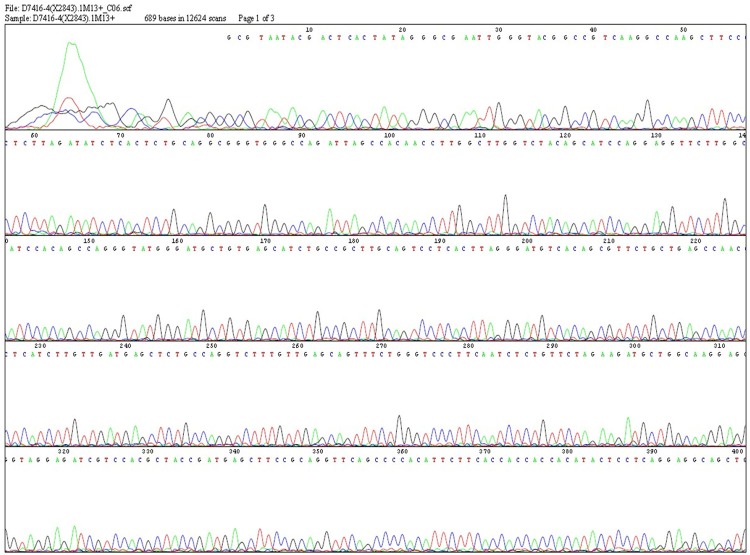
With PRNK gene sequence as primers, PCR was performed for cDNA in recombinant adenovirus that was reversely transcribed. The amplified products were subjected to bidirectional sequencing. Homology analysis showed that the amplified fragment was consistent with gene-encoding cDNA sequence of PRNK gene reported in literature [3]. Thus recombinant virus carrying PRNK was successfully constructed (Figure 1).
Figure 1.
cDNA sequencing of PRNK.
Detection of expressions of PRNK and phosphorylated Pyk2 proteins in each group of cells by western blot
Expression of PRNK protein in CFs in vitro
After Western Blot, the grayscale values of the target bands and the bands of the internal reference were calculated using Quantity One software along with the ratio between the two. One-way ANOVA was performed. It was found that Ang II group and empty vector group were not significantly different in PRNK expression (P>0.05); the expression in PRNK group was much higher than that in other groups (P<0.01) (Table 2), indicating the high expression of PRNK after transfection.
Table 2.
Comparison of LVHI between the groups (x̅±s)
| Group | n | LVHI (mg/g) |
|---|---|---|
| Sham operation group | 8 | 1.718±0.094 |
| Operation group | 6 | 2.945±0.198a |
| RSG group | 8 | 2.252±0.137b |
| PRNK group | 7 | 2.124±0.143b,c |
P<0.01 compared with sham operation group;
P<0.05 compared with operation group;
No statistically significant difference compared with RSG group.
Expression of phosphorylated Pyk2 protein in CFs in vitro
As shown by grayscale analysis, the expressions of phosphorylated Pyk2 protein in Ang II group and empty vector group were considerably higher than that of the blank group (P<0.01); however, the expression of phosphorylated Pyk2 protein in PRNK group was much lower than that of Ang II group and empty vector group (P<0.01) (Table 1). It was obvious that high expression of PRNK inhibited the phosphorylation of Pyk2 induced by Ang II.
Table 1.
Comparison of expressions of PRNK and phosphorylated Pyk2 protein between the groups (target protein/β-actin; x̅±s)
| Group | n | PRNK/β-actin | pPyk2/β-actin |
|---|---|---|---|
| Blank group | 6 | 0.00361±0.00028 | 0.09337±0.00201 |
| Ang II group | 6 | 0.01956±0.00197 | 0.53628±0.06376a |
| Empty vector group | 6 | 0.01862±0.00147 | 0.60673±0.06720a |
| PRNK group | 6 | 0.12445±0.00203a,b,c | 0.14824±0.01052b,c |
Compared with blank group;
Compared with Ang II group;
Compared with empty vector group (P<0.05).
Measurement of LVHI
Compared with the sham operation group, the operation group showed a substantial increase of LVHI (P<0.01), indicating successful modeling. Compared with the operation group, LVHI of PRNK group and RSG group was decreased, but no significant difference was found between PRNK group and RSG group (Table 2). Abdominal aortic stenosis remarkably facilitated myocardial hypertrophy and fibrosis, which further led to the rise of LVHI; in contrast, PRNK and RSG inhibited myocardial hypertrophy and fibrosis.
Comparison of CVF, PVCA and grayscale of blue-stained collagen
Under the inverted microscope, the myocardial interstitial space was filled with a small amount of collagen in the sham operation group; a small amount of collagen was deposited in the outer membrane of small artery within a restricted scope. The fibrous connective tissues in the operation group were stained reddish orange by VG staining and presented a wave-like appearance; the content of collagen in myocardial interstitial space was increased; at some positions, the fibrous connective tissues had branching fibers that penetrated into the myocardial tissues; fibrous septa were formed between the muscle bundles and tightly enveloped the myocardial cells, with the manifestation of reactive fibrosis (Figure 2). CVF and PVCA of the operation group were both significantly higher compared to the sham operation group (P<0.01). Collagen deposition in PRNK group and RSG group revealed by VG staining was less severe compared with the operation group (Figure 2); CVF and PVCA of these two groups were significantly lower than those of the operation group (P<0.05) (Table 3).
Figure 2.
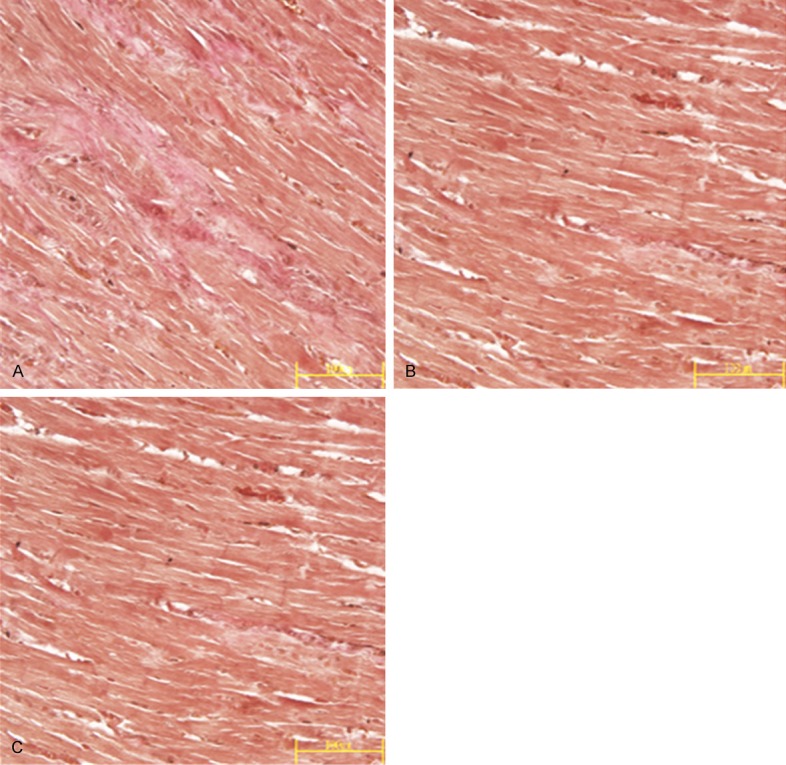
Comparison of content of red-stained collagen during VG staining (VG×400) in myocardial tissues in operation group (A), PRNK group (B) and RSG group (C).
Table 3.
CVF, PVCA and content of blue-stained collagen in myocardial tissues (x̅±S)
| Group | n | CVF (%) | PVCA | Blue-stained collagen |
|---|---|---|---|---|
| Sham operation | 8 | 2.18±0.14 | 0.76±0.06 | 10.14±2.26 |
| Operation group | 6 | 11.36±0.41a | 2.75±0.16a | 29.08±7.75a |
| RSG group | 8 | 8.43±0.84b | 1.74±0.62b | 17.61±1.92b |
| PRNK group | 7 | 6.72±0.38b,c | 1.21±0.51b,c | 13.04±1.86b,c |
P<0.01 compared with sham operation group;
P<0.05 compared with operation group;
No statistically significant difference compared with RSG group.
After Masson’s trichrome staining, the myocardial tissues were stained red, the collagen fibers blue green, and the nuclei blue black. The content of collagen in the operation group was increased to a large extent (Figure 3), and the grayscale of the blue-stained collagen was much higher than that of the sham operation group (P<0.01). The grayscale values of PRNK group and RSG group had a marked decrease compared with the operation group (P<0.05) (Table 3).
Figure 3.
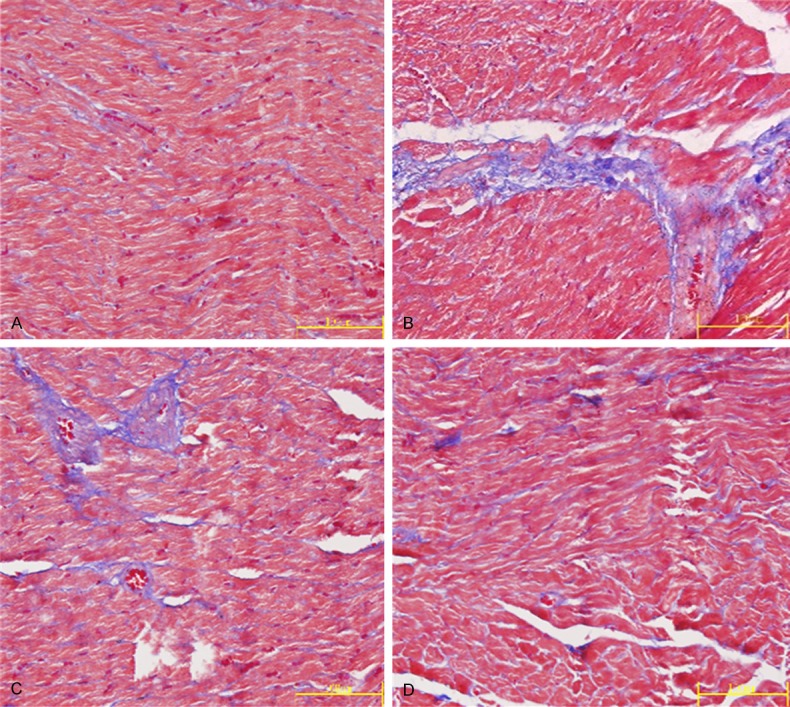
Comparison of content of blue-stained collagen during Masson’s trichrome staining (Masson×400) in myocardial tissues in sham operation group (A), operation group (B), PRNK group (C) and RSG group (D).
Comparison of content of hydroxyproline, and type I and IV collagen in myocardial tissues of each group
The contents of hydroxyproline, type I and IV collagen in myocardial tissues were detected. The content of type I and IV collagen in PRNK group was significantly lower than that of the operation group at the same time point (P<0.05). The contents of hydroxyproline, type I and IV collagen in the operation group had an obvious increase compared with the control (P<0.01). Thus abdominal aortic stenosis caused myocardial fibrosis and the increase of hydroxyproline, type I and IV collagen, and this trend was inhibited by intervention with PRNK. Similar antifibrotic effect was achieved as with RSG (Table 4).
Table 4.
Comparison of secretions of hydroxyproline, type I and IV collagen in culture supernatant (x̅±S)
| Group | n | Hydroxyproline (μg/g.pr) | Type I collagen (pg/ml) | Type IV collagen (pg/ml) |
|---|---|---|---|---|
| Sham operation group | 8 | 326.21±19.51 | 247.82±13.52 | 154.31±16.83 |
| Operation group | 6 | 589.82±21.83a | 718.52±21.43a | 638.63±21.37a |
| RSG group | 8 | 471.09±15.42b | 462.61±20.41b | 469.83±20.93b |
| PRNK group | 7 | 452.68±17.42b,c | 483.71±18.65b,c | 487.72±19.04b,c |
P<0.01 compared with sham operation group;
P<0.05 compared with operation group;
No statistically significant difference compared with RSG group.
Immunohistochemical detection of phosphorylated Pyk2 protein
Immunohistochemical detection revealed that there were only a few brown particles in the myocardial cytoplasm and interstitial space in the sham operation group, indicating weak expression of phosphorylated Pyk2 protein. In the operation group, the myocardial cytoplasm and interstitial space were filled with brown particles, indicating strong expression of phosphorylated Pyk2. The intensity of expression of phosphorylated Pyk2 in PRNK group lied between that of the two groups, and the integral optical density showed statistically significant difference (P<0.05) (Table 5).
Table 5.
Immunohistochemical detection of phosphorylated Pyk2 protein
| Group | n | A value of pPyk2 |
|---|---|---|
| Sham operation group | 8 | 0.106±0.013 |
| Operation group | 6 | 0.325±0.032a |
| PRNK group | 7 | 0.207±0.027b |
P<0.01 compared with sham operation group;
P<0.05 compared with operation group.
Apoptosis of CFs detected by TUNEL method
Fields of view were randomly selected to count the apoptotic CFs stained with TUNEL in the operation group and PRNK group. As shown in Table 6, the apoptosis rate of CFs in PRNK group was considerably higher than that of the operation group and the sham operation group, indicating the induction of apoptosis of CFs by PRNK.
Table 6.
Influence of PRNK on apoptosis of rat CFs (x̅±s)
| Group | n | Apoptosis index (%) |
|---|---|---|
| Sham operation group | 8 | 13.31±2.07 |
| Operation group | 6 | 15.62±1.91 |
| PRNK group | 7 | 27.16±2.33a,b |
Compared with sham operation group;
P<0.01 compared with operation group.
Expression of PRNK and phosphorylated Pyk2 protein detected by western blot
Expression of PRNK
Grayscale values were analyzed after Western Blot detection. The grayscale of PRNK group was considerably higher than that of the sham operation group and the operation group (P<0.05). It was indicated that PRNK gene was highly expressed in CFs after transfection (Table 7).
Table 7.
Comparison of expression of PRNK and phosphorylated Pyk2 protein between the groups (target protein/β-actin; x̅±s)
| Group | n | PRNK/β-actin | Phosphorylated Pyk2 PRNK/ β-actin |
|---|---|---|---|
| Sham operation group | 6 | 0.00872±0.00201 | 0.01518±0.00340 |
| Operation group | 6 | 0.02177±0.00334 | 0.19854±0.03317a |
| PRNK group | 6 | 0.09280±0.00950a,b | 0.08470±0.00813b |
Compared with sham operation group;
P<0.01 compared with operation group.
Expression of phosphorylated Pyk2
As indicated by the grayscale, the expression of phosphorylated Pyk2 in PRNK group was much lower than that of the operation group (P<0.05), while the expression level of the operation group was significantly higher than that of the sham operation group (P<0.01). It was indicated that the transfection with adenoviral vector carrying PRNK gene inhibited overphosphorylation of Pyk2 induced by abdominal aortic stenosis (Table 7).
Discussion
Evaluation of the constructed adenoviral vector
We used pAd Easy Adenoviral Vector to construct the recombinant adenovirus carrying the coding region of PRNK gene from rats. According to unpublished results, this adenovirus can proliferate stably in 293T cells and achieve a high titer through repeated transfection. Therefore pAd Easy Adenoviral Vector is suitable to be applied to analyze the mechanism of action of PRNK on Pyk2 and its antifibrotic effect.
Expression of PRNK in CFs in vitro
CFs are easy to culture in vitro and can be transfused back into the body after gene transfer, or the gene transfer can be directly done within the cardiac chamber using a catheter. When carrying the target gene, CFs may secrete the desired proteins into the cardiac chamber, which then enter blood circulation to perform systemic or local function. CFs which is the target cells in gene therapy offer new hope in targeted therapy for cardiovascular diseases [4]. In our experiment, PRNK was highly expressed after transfection into CFs using the adenoviral vector. By detecting the phosphorylation level of Pyk2 at Tyr402, it was found that the high expression of PRNK significantly inhibited the phosphorylation of full-length Pyk2. The focal adhesion targeting domain in PRNK gene may facilitate the binding of PRNK to focal adhesions, which reduced the binding by full-length Pyk2 that contains N-terminal kinase-activating domain. As a result, the phosphorylation level was decreased, which had an influence on downstream signaling. This was probably the mechanism of the action of PRNK on Pyk2. It was inferred that PRNK may be an endogenous dominant negative mutant of Pyk2, which competed with Pyk2.
Evaluation of rat model of myocardial fibrosis
Myocardial fibrosis is a type of pathological changes found in the model of hypertension and heart failure. It is highly important to choose a rat model of myocardial fibrosis showing effective and consistent PRNK expression. Using the method by Zhao et al. [5], abdominal aortic stenosis was simulated by operation to construct the rat model of myocardial fibrosis. According to Stilli D et al. [6], detections were performed 5 weeks after modeling. Results showed that left ventricle mass/body weight ratio was increased obviously compared with normal rats; CVF, PVCA, grayscale of blue-stained collagen, and content of type I and IV collagen all had a significant increase compared with the sham operation group. Moreover, the data of each parameter were consistent with those in the operation group. This proved that rat model of myocardial fibrosis was constructed successfully 5 weeks after modeling.
PRNK carried by adenoviral vector was stably expressed in CFs after injection into rats via the tail vein
There are many ways to deliver the adenoviral vectors. Though intramyocardial injection of adenoviral vectors has the highest infection rate [7], the mortality caused by injection is high and it is difficult to perform repeated intramyocardial injections. In the present study, myocardial fibrosis was modeled through abdominal aortic stenosis and adenoviral vector was injected via the tail vein. By this method, a lower stress was caused by second injection. One week after operation, 1 ml of adenoviral vector was injected via the tail vein. Western Blot was performed 4 weeks later, which found that PRNK expression in CFs showed a marked increase compared with other groups. This indicated PRNK gene introduced using the adenoviral vector was stably expressed for at least 4 weeks in CFs, conforming to the need of clinical treatment for myocardial fibrosis as a chronic disease. Moreover, the phosphorylation level of Pyk2 in CFs was increased significantly in the rat model of myocardial fibrosis. After the introduction of PRNK gene, the phosphorylation level of Pyk2 decreased. This result confirmed the role of Pyk2 in myocardial fibrosis and even in myocardial remodeling. The overexpression of PRNK effectively inhibited overphosphorylation of Pyk2 in CFs caused by abdominal aortic stenosis in rats. So this mechanism may come into effect in the inhibition of myocardial fibrosis or even cardiac hypertrophy.
Treatment effect of PRNK on myocardial fibrosis in rats
After intervention with PRNK, CVF, PVCA and grayscale of blue-stained collagen were all decreased compared with the operation group. The remodeling of collagen network of the heart can increase myocardial stiffness and directly affect diastolic function. The increase of apoptosis rate of CFs and decrease of LVHI compared with the operation group also demonstrated the inhibition of myocardial fibrosis and even cardiac hypertrophy after introduction of PRNK gene.
We found through preliminary experiments that PPARγ antagonist RSG had an inhibitory effect on myocardial fibrosis [8]. PPARγ prevents the transcription of TGF-β and CTGF and inhibits the high expression of MMP-9 induced by interleukin-1 (IL-1) by inhibiting activator protein-1 or tumor necrosis factors and the activation of NF-κB induced by IL-1. Such mechanism is also inhibitory on PI3k/Akt signaling pathway [9,10]. We injected RSG for a comparison, and as shown by the changes of hydroxyproline, type I and IV collagen in myocardial tissues, the introduction of PRNK gene achieved similar effect. Hence the delivery of PRNK gene using adenoviral vectors has a great application prospect in the treatment of myocardial fibrosis.
We proved through animal experiment that PRNK inhibited the phosphorylation of Pyk2 via competitive binding. Besides, high expression of PRNK could effectively inhibit myocardial fibrosis. But the parameters of different groups did not differ by a large margin. The reason may be that high stress, which was the root cause of myocardial fibrosis, was not precluded during the experiment and myocardial fibrosis was the result of multiple factors.
Acknowledgements
This work was supported by National Natural Science Foundation of China (30700822).
Disclosure of conflict of interest
None.
References
- 1.Gray SJ, Samulski RJ. Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin Biol Ther. 2008;8:911–922. doi: 10.1517/14712598.8.7.911. [DOI] [PubMed] [Google Scholar]
- 2.Sun CK, Man K, Ng KT, Ho JW, Lim ZX, Cheng Q, Lo CM, Poon RT, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes proliferation and invasiveness of hepatocellular carcinoma cells through c-Src/ERK activation. Carcinogenesis. 2008;29:2096–2105. doi: 10.1093/carcin/bgn203. [DOI] [PubMed] [Google Scholar]
- 3.Xiong WC, Macklem M, Parsons JT. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci. 1998;111:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]
- 4.Kane NM, Nowrouzi A, Mukherjee S, Blundell MP, Greig JA, Lee WK, Houslay MD, Milligan G, Mountford JC, von Kalle C, Schmidt M, Thrasher AJ, Baker AH. Lentivirus-mediated reprogramming of somatic cells in the absence of transgenic transcription factors. Mol Ther. 2010;18:2139–2145. doi: 10.1038/mt.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DL, Heidkamp MC, Iyengar R, Vijayan K, Szotek EL, Barakat JA, Leya M, Henze M, Scrogin K, Henderson KK, Samarel AM. CRNK gene transfer improves function and reverses the myosin heavy chain isoenzyme switch during post-myocardial infarction left ventricular remodeling. J Mol Cell Cardiol. 2008;45:93–105. doi: 10.1016/j.yjmcc.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ihm SH, Chang K, Kim HY, Baek SH, Youn HJ, Seung KB, Kim JH. Peroxisome proliferator-activated receptor-gamma activation attenuates cardiac fibrosis in type 2 diabetic rats: the effect of rosiglitazone on myocardial expression of receptor for advanced glycation end products and of connective tissue growth factor. Basic Res Cardiol. 2010;105:399–407. doi: 10.1007/s00395-009-0071-x. [DOI] [PubMed] [Google Scholar]
- 7.You K, Hao J, Chen L, et al. Peroxisome proliferator-activated receptor γ and its mechanism in myocardial interstitial fibrosis. Guangdong Yi Xue. 2010:1084–1087. [Google Scholar]
- 8.Zhao LJ, Jiao DD, Zhao ZM. Abdominal aortic coarctation-induced rat model of myocardial fibrosis. Chinese Microcirculation. 2009;5:402–404. [Google Scholar]
- 9.Stilli D, Bocchi L, Berni R, Zaniboni M, Cacciani F, Chaponnier C, Musso E, Gabbiani G, Clément S. Correlation of alpha-skeletal actin expression, ventricular fibrosis and heart function with the degree of pressure overload cardiac hypertrophy in rats. Exp Physiol. 2006;91:571–580. doi: 10.1113/expphysiol.2005.032607. [DOI] [PubMed] [Google Scholar]
- 10.Honda A, Matsuura K, Fukushima N, Tsurumi Y, Kasanuki H, Hagiwara N. Telmisartan induces proliferation of human endothelial progenitor cells via PPARgamma-dependent PI3K/Akt pathway. Atherosclerosis. 2009;205:376–38. doi: 10.1016/j.atherosclerosis.2008.12.036. [DOI] [PubMed] [Google Scholar]