Abstract
The aim of the present study was to ascertain the effect of Porphyromonas gingivalis cysteine protease gingipain on the proliferation of rat aortic smooth muscle cells (RASMCs). Gingipains were isolated and purified from the supernatant of P. gingivalis W83, which was cultured under standard anaerobic conditions; primary RASMCs were also cultured. RASMCs were exposed to 200, 100, 50, 25, 12, 6, 3, 1, and 0 μg/mL activated gingipains and the proliferation was evaluated using a cell counting kit-8 (CCK-8) assay after 48 h. α-Smooth muscle actin (α-SMA) and osteopontin (OPN) expression were measured by immunohistochemical staining. In addition, RASMCs were stimulated with 5, 10, 20, and 40 μM KYT-1 (arg-gingipain inhibitor) and KYT-36 (lys-gingipain inhibitor) in combination with the gingipain extracts. Different concentrations of gingipains significantly promoted the proliferation of RASMCs, except those treated with 1 μg/mL, compared to the untreated controls. The proliferation was sustained at a concentration above 12 μg/mL. Immunohistochemical staining showed OPN expression after gingipain stimulation. The proliferative effects of gingipains on RASMCs were blocked after treatment with 10 μM KYT-1 or 10 μM KYT-36 (P < 0.0001); however, the difference between KYT-1 and KYT-36 groups was not statistically significant. These results demonstrated that gingipains can promote phenotypic transformation and proliferation of RASMCs and their effects were blocked by KYT-1 and KYT-36, which help us to ascertain whether Rgp or Kgp contributes to the development of atherosclerosis.
Keywords: Gingipains, smooth muscle cell, proliferation, phenotype transformation
Introduction
Porphyromonas gingivalis (Pg), a black-pigmented, gram-negative anaerobe, has proved to be a major etiologic agent for the development and progression of chronic periodontitis. Several epidemiological surveys have revealed that periodontitis is one of the important risk factors of atherosclerosis [1-3]. Since Pg was detected in atherosclerosis plaques, we hypothesized that there may be correlation between atherosclerosis and Pg [4]. After ApoE-/-mice were vaccinated with PG, the Pg antibody was detected in the serum, and interleukin (IL)-6 and IL-1β levels as well as the atherosclerosis plaque area increased, so as to the C57BL/6 mice [5,6]. Therefore, Pg was thought to induce atherosclerosis and promote the development of atherosclerosis in association with hyperlipidemia.
During the course of the atherosclerotic growth, as vascular smooth muscle cells (VSMCs) are the sole cell type in the medial layer of the vascular wall, they undergo phenotypic transformation and transmigrate from the media to the intima followed by proliferation and production of extracellular matrix and apoptosis accompanied by release of lipids, which leads to the development of a typical lipid core of atherosclerotic lesions [7]. Pg adheres to the surface of smooth muscle cells, invades the cells, induces cell phenotypic transformation from contractile to synthetic, and induces endothelia proliferation [8,9].
Gingipains, which exist in two forms i.e., arginine-gingipains (Rgps) and lysine-gingipain (Kgp), are important virulence factors of Pg and are found on the outer membrane, vesicles, or in the extracellular matrix. Gingipain helps in the uptake of iron and hemoglobin, thereby maintaining an anaerobic environment and aiding in the attachment of Pg to the host and invasion of the host, thus promoting the development of periodontitis [10]. In the vascular system, gingipains induce arterial endothelial cell apoptosis, cell-adhesive molecule cleavage, cytokines synthesis, aid in selective proteolysis of apolipoprotein B-100, and trigger a proinflammatory response in human monocyte-derived macrophages [11-14]. In the present study, we aimed to study the role of gingipains in the induction of VSMC transformation from contractile to synthetic phenotype and ascertain whether gingipain promotes cell proliferation.
Materials and methods
Materials
Pg W83 was obtained from Beijing Stomatological Attached Hospital of Capital Medical University. N-Benzoyl-d-l-arginine-4-nitroanilide hydrochloride (BAPNA), was obtained from Sigma-Aldrich (St. Louis, MO, USA). KYT-1 and KYT-36, which are specific inhibitors of gingipains, were purchased from a peptide institute (Osaka, Japan).
Pg strains and culturing conditions
Pg W83 strains were cultured in brain heart infusion broth (BD, Franklin, USA) supplemented with 0.5% yeast extract (Difco Laboratories), hemin (5 μg/mL), vitamin K (0.5 μg/mL), and cysteine (0.1%) (Sigma-Aldrich, St. Louis, MO, USA). All cultures were incubated at 37°C in an anaerobic chamber (10% H2, 10% CO2, 80% N2).
Gingipain extract preparation
Pg W83 strains were cultured as previously described [12]. Bacterial cultures were centrifuged (12,000 × g, 45 min, 4°C) to remove the cells and then filtered through a 0.45-μm pore filter (Millipore, Bellerica, Mass.). The extracellular culture fluid was precipitated with acetone at -20°C in a 60:40 ratio of acetone to cell-free medium, with constant stirring. This precipitate was centrifuged (12,000 × g, 30 min, 4°C), and the pellet was resuspended in a solution containing 150 mM NaCl, 20 mM Bis-Tris, and 5 mM CaCl2. The resuspended pellets were dialyzed at 4°C in a Spectrapor 12,000- to 14,000-molecular-weight cut-off dialysis tubing versus 4 L of the same buffer with Aldrithiol-4 (Sigma-Aldrich) overnight, to stabilize the gingipains, with three more changes of the dialysis buffer without Aldrithiol-4. After dialysis, the sample was centrifuged (34,000 × g, 1 h, 4°C), and the resulting supernatant was concentrated by ultrafiltration (Ultra-15 10 K, Amicon) at 4°C. This concentrated gingipain-active extract was clarified by centrifugation (192,000 × g, 1 h, 4°C) and stored in aliquots at -80°C.
Gingipain concentration and protease assay
The concentration of the gingipain extracts was determined by the Pierce Protein Assay (Pierce, Rockford, USA) following the manufacturer’s instructions.
To determine gingipain activity, 5 μL (for Rgp) and 15 μL (for Kgp) of the concentrated extract was preincubated in a final volume of 180 μL activated assay buffer, pH 7.6, containing 0.2 M Tris-HCl, 0.15 M NaCl, and 20 mM L-cysteine. The reaction was initiated by the addition of 20 μL of 10 mM Bz-L-arginine-p-nitroanilide (L-BAPNA) (Sigma) for Rgp activity, or acetyl-lysine-p-nitroanilide (ALNA) (Bachem, King of Prussia, Pa.) for Kgp activity, which was added to the 150 μL reaction mixture, and the rate of enzymatic substrate hydrolysis was read with a microplate reader (Bio-Rad, Hercules, Calif) at 405 nm using a kinetic measurement software program. One unit of gingipain activity is defined as the amount of enzyme releasing 1 pmol of p-nitroanilide per minute as calculated based on the maximum velocity and extinction coefficient of p-nitroanilide of 9,200 at 405 nm. The gingipain extract was activated in 10 mM L-cysteine at 37°C for 10 min, followed by dilution with smooth muscle cell growth medium. To block the enzymatic activity, the activated extracts were incubated with KYT-1 as well as KYT-36 for 10 min at room temperature.
Rat aortic smooth muscle cell isolation and culture
Primary cultures of RASMCs were prepared from the thoracic aortas of male Sprague-Dawley rats (weight, 150 g). The rats were euthanized with pentobarbital sodium. The thoracic aortas were isolated after opening the thoracic cavity and rinsed three times with a D-Hands buffer. The adventitia was avulsed with ophthalmic forceps in a “cuffing” manner, and the endothelium was mechanically removed by scraping with a sterile gauze after opening the media longitudinally. The separated rat aortic media was excised and cut into small pieces (about 1 mm2) with ophthalmic scissors. The pieces were then planted on 25 cm2 culture flasks (Corning) with the luminal side down and incubated in DMEM medium (Gibco, Carlsbad, CA) containing 10% fetal calf serum (Gibco, Carlsbad, CA). When primary cultures reached confluence of 90%, they were redistributed by trypsinization and fresh cultures were initiated. Cultured smooth muscle cells after 3-6 passages were used for the following experiments.
The experimental procedures and the animal use and care protocols were approved by the Committee on Ethical Use of Animals of Hangzhou Normal University.
Proliferation assay
RASMCs proliferative activity was assessed using a Cell Counting Kit-8 (CCK-8) assay. The smooth muscle cells were seeded at a density of 5,000 cells per well in 96-well plates and were serum starved for 20 h using DMEM medium with antibiotics (Gibco, Carlsbad, CA). After the 20-h serum starvation, the culture medium was discarded and replaced with DMEM medium supplemented with 10% fetal calf serum containing activated or inactivated gingipains. After 48 h, the medium was discarded and replaced with 100 µL DMED medium containing 10% CCK-8 solution, according to the manufacturer’s instructions and incubated at 37°C for 3 h in a humidified 5% CO2 atmosphere. The light absorbance at 450 nm was then measured with a microplate reader.
Immunocytochemistry
Before and after incubation for 48 h, immunohistochemical staining of osteopontin (OPN) protein (Santa Cruz Biotechnology, Santa Cruz, USA) and the α-smooth muscle actin (α-SMA) were performed. For immunocytochemical analysis, 1 × 106 cells were seeded per well in 6-well plates with a slide in each well. After incubation for 24 h, the cells were fixed with 4% paraformaldehyde for 15 min, incubated with 1% TritonX-100 at 37°C for 30 min, and then washed with phosphate-buffered saline (PBS). A mouse monoclonal antibody against OPN or α-SMA was used at a dilution of 1:200 as the primary antibody, overnight at 4°C. Biotinylated goat anti-mouse immunoglobulin G (IgG) secondary antibody was used to react with the primary antibody and incubated for 45 min at room temperature, developed with DAB, counterstained with hematoxylin, and dehydrated in 70%, 80%, and 100% ethanol. The coverslip was then with neutral balsam.
Statistical analysis
All results are expressed as mean ± SD. A one-way analysis of variance (ANOVA) and a post hoc analysis (Turkey’s test) was used for more than two groups, and Student’s t-test was used for statistical comparisons of the two groups. A P value < 0.01 was considered to be statistically significant.
Results
Concentration and activity of gingipain extracts
A protein concentration of 4,848 µg/mL was reached by gingipain extraction procedures from whole cells of Pg cultures. The Rgp enzymatic activity of gingipain-active extracts was found to be 280 U/L, while the Kgp enzymatic activity was found to be 84 U/L.
Effects of gingipains on RASMC proliferation
Different concentrations of activated gingipain extracts were used as stimulants for cell proliferation and showed positive effects, except the 1 μg/mL groups. A gradual increase was observed in the proliferation of RASMCs with an increase in the concentration of gingipains, where the increase was significant at a concentration of 3 μg/mL and showed exponential growth. However, the proliferation gradually decreased and reached a plateau after attaining a concentration of 12 μg/mL (Figure 1).
Figure 1.
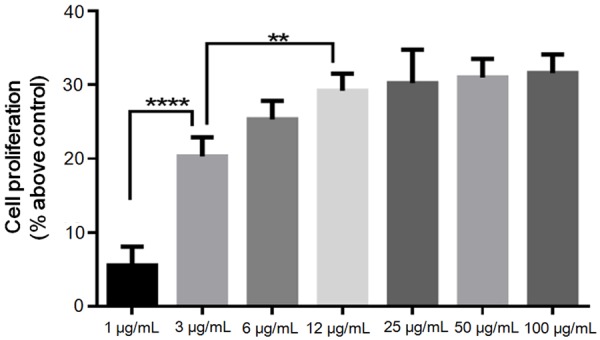
Role of gingipains with RASMC proliferation. Quantitative analysis of effects of different concentrations of gingipains on RASMC proliferation. The cell proliferation was calculated at 48 h as percent above control (no gingipains). Data are shown as the mean ± SD (n = 4). **P < 0.01, ****P < 0.0001.
Effects of KYT-1 and KYT-36 on RASMCs proliferation
In order to evaluate whether Rgp and Kgp played a role in RASMCs proliferation, we evaluated the effects of gingipain-specific inhibitors KYT-1 (arginine-specific gingipain inhibitor) and KYT-36 (lysine-specific gingipain inhibitor) on gingipain-induced proliferation. As shown in Figure 2, pretreatment of gingipains with KYT-1 or KYT-36 blocked the gingipain-induced RASMCs proliferation in a dose-dependent manner; the inhibitory effects at 10 µM were the most significant. However, there was no difference between the effects of KYT-1 and KYT-36, and no synergistic action was observed with the combination.
Figure 2.
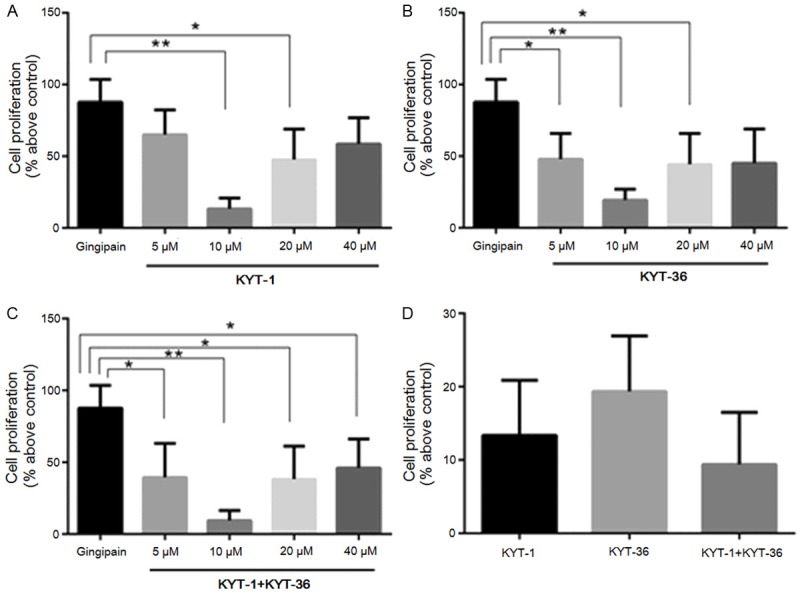
Effects of KYT-1 and KYT-36 on RASMC proliferation. After incubating with various concentrations of KYT-1 (A), KYT-36 (B), and KYT-1 combination with KYT-36 (C), the 12 μg/ml gingipains were used as stimulants for cell proliferation. Inhibitory effects of 10 µM KYT-1, KYT-36, and KYT-1 combination with KYT-36 were compared in (D). Data are shown as the mean ± SD (n = 5). *P < 0.01, **P < 0.0001.
Gingipains promote OPN expression in RASMCs
Immunohistochemical staining demonstrated that smooth muscle cells express abundant α-SMA (brown in Figure 3), without OPN expression under an electron microscope before gingipain stimulation. Alternatively, after gingipain stimulation, immunohistochemical studies showed positive OPN staining in smooth muscle cells (brown).
Figure 3.
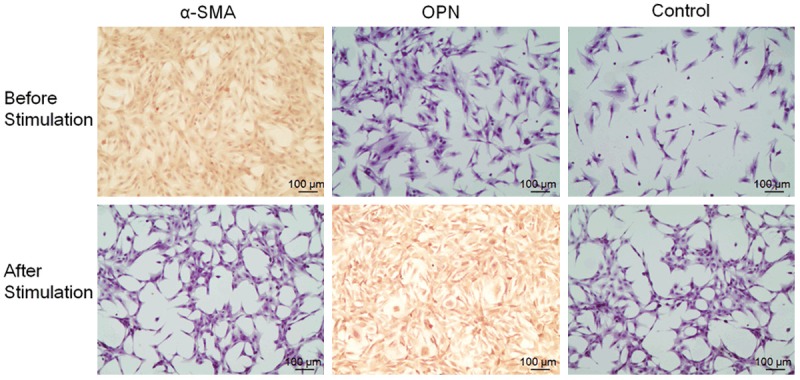
The immunohistochemical staining for α-SMA and OPN in RASMCs at 48 h before and after the 12 μg/ml gingipains stimulation (× 100).
Discussion
VSMCs exhibited a high rate of proliferation and synthetic ability during vascular development and a number of proliferative vascular diseases such as atherosclerosis, hypertension, and restenosis after angioplasty [15]. Pg can invade arterial smooth muscle cells and stimulate proliferation through activation of TGF-beta and Notch signaling pathways [16]. The present study demonstrates that the proliferation of rat smooth muscle cells significantly increased after gingipain stimulation. It has been reported that the gingipains activate protease-activated receptors (PARs) located on various cells, including epithelial cells, human gingival endothelial cells, and platelets, leading to the release of proinflammatory cytokines IL-6, the expression and release of human growth factor (HGF), and platelet aggregation [10]. Moreover, gingipains and toll-like receptor (TLR) on the human arterial endothelial cells synergistically induce the synthesis of the cytokine IL-8 [12]. After incubation of Pg with plasma and addition of supernatants to the VSMC medium, Inaba et al. [17] hypothesized that the indirect effects of gingipains on promotion of VSMCs proliferation occur via cleavage of plasma proteins at the lysine and arginine residues and conversion of the factor X to Xa thereby leading to blood coagulation, which induces proliferation. In our present study, we used gingipain extracts to directly stimulate RASMCs. Therefore, gingipain itself may promote VSMC proliferation. Gingipains, cysteine proteases, are essential, and therefore, owing to some enzyme characteristics, the substrate was consumed gradually with an increase in the gingipain concentration and not enough substrate was remaining above 12 μg/mL.
KYT-1 and KYT-36, which are inhibitors of Rgp and Kgp, respectively, strongly inhibited the virulence induced by the Pg culture supernatant [18]. The effects differ due to differences in the structure. Our study showed that the proliferation reduced after the gingipain extracts were incubated with KYT-1 or KYT-36. In the study, the inhibitory effects of KYT-1 and KYT-36 on the proliferative effects of gingipains showed a U-shape curved with an increase in the concentration of KYT-1 and KYT-36. KYT-1 and KYT-36 were observed to promote RASMCs proliferation (data not shown), which may explain why KYT-1 and KYT-36 failed to block the effects of gingipains at concentrations higher than 10 µM. Rgp and Kgp, which have different gene structures, play different roles in the development of the periodontitis [19-21]. However, in our study, no difference was observed between KYT-1 and KYT-36 groups, which indicate that Kgp and Rgp activities were responsible for gingipains-mediated RASMCs proliferation. In addition, although the proliferation in response to their combination is higher than that with Rgp and Kgp, no synergistic action was observed with the combination; these results were consistent with those of Inaba et al. [18].
VSMCs exhibits special phenotypic plasticity in response to fluctuating environmental cues. VSMCs within adult normal blood vessels show contractile function with reduced proliferation rate and synthetic activity, high levels of contractile gene expression, such as α-SMA, SM22α, caldesmon, and calponin, among which α-SMA is the most classical one [22,23]. During the development and progression of atherosclerosis, the VSMCs switch from contractile phenotype to synthetic, which is characterized by increased rates of proliferation, migration, and production of extracellular matrix, as well as reduced expression of contractile genes. OPN, a soluble secreted phosphoprotein, integrin-binding ligand, is expressed by various cells including some epithelia, macrophages, T cells, and VSMCs. We demonstrated that VSMCs expressed OPN during the proliferative phase, synthetic phase, and OPN promoted atherosclerosis and inhibitory effect during vascular calcification [24].
As a marked protein of synthetic VSMCs, OPN play an important role in the development of atherosclerosis and acts as an enhancer of atherosclerosis. OPN promotes VSMCs phenotypic switching and induces medial thickening and neointimal formation [25,26]. It was reported that oxLDL promoted the proliferation of human coronary artery smooth muscle cells in a dose-dependent manner via induction of the activation of OPN followed by upregulation of expression levels of MMP-9 [27]. To explore the possible effects of gingipain on VSMC proliferation, we observed the expression of α-SMA and OPN using immunohistochemical staining before and after gingipain stimulation. In the present study, the expression of α-SMA was high before gingipain stimulation and low after stimulation. However, the expression of OPN was opposite, was expressed after gingipain stimulation. The OPN level was related with the severities of coronary artery disease [28], in our study, the OPN level was not analyzed. Although the mechanism of SMC proliferation mediated by gingipains is unclear, a plausible explanation can be speculated. Gingipains stimulate VSMCs to express OPN and induce phenotype transformation, followed by acceleration of the proliferation of VSMCs.
Conclusion
The present findings suggest that Pg gingipains are involved in VSMC phenotypic transformation and induce the proliferation of VSMCs and Rgp or Kgp may contribute to the development of atherosclerosis. However, the present study only focused on the in vitro effects of gingipains on the proliferation of cultured RASMCs, and further genetic studies are warranted to understand the mechanism of action of gingipains on the proliferation of RASMCs.
Acknowledgements
This research was supported by grants from Public Projects Foundation of Zhejiang Province (Grant No. 2012C33100), the National Natural Science Foundation of China (Grant NO. 81570989) and Xinjiang Province Science Foundation for Youths (Grant NO. 2014211C074). The authors report no conflicts of interest related to this study.
Disclosure of conflict of interest
None.
References
- 1.Ford PJ, Gemmell E, Hamlet SM, Hasan A, Walker PJ, West MJ, Cullinan MP, Seymour GJ. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol Immunol. 2005;20:296–302. doi: 10.1111/j.1399-302X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 2.Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15:403–413. doi: 10.1177/154411130401500606. [DOI] [PubMed] [Google Scholar]
- 3.Sanz M, D’Aiuto F, Deanfield J, Fernandez-Avile’s F. European workshop in periodontal health and cardiovascular disease-scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Suppl. 2010;12:B3–12. [Google Scholar]
- 4.Yakob M, Söder B, Meurman JH, Jogestrand T, Nowak J, Söder PÖ. Prevotella nigrescens and Porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontitis. J Periodont Res. 2011;46:749–755. doi: 10.1111/j.1600-0765.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 5.Velsko IM, Chukkapalli SS, Rivera MF, Lee JY, Chen H, Zheng DH, Bhattacharyya I, Gangula PR, Lucas AR, Kesavalu L. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukasawa A, Kurita-Ochiai T, Hashizume T, Kobayashi R, Akimoto Y, Yamamoto M. Porphyromonas gingivalis accelerates atherosclerosis in C57BL/6 mice fed a high-fat diet. Immunopharmacol Immunotoxicol. 2012;34:470–476. doi: 10.3109/08923973.2011.627866. [DOI] [PubMed] [Google Scholar]
- 7.Burnier L, Fontana P, Angelillo-Scherrer A, Kwak BR. Intercellular communication in atherosclerosis. Physiology (Bethesda) 2009;24:36–44. doi: 10.1152/physiol.00036.2008. [DOI] [PubMed] [Google Scholar]
- 8.Wada K, Kamisaki Y. Roles of oral bacterial in cardiovascular diseases-from molecular mechanisms to clinical cases: Involvement of Porphyromonas gingivalis in the development of human aortic aneurysm. J Pharmacol Sci. 2010;113:115–119. doi: 10.1254/jphs.09r22fm. [DOI] [PubMed] [Google Scholar]
- 9.Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, Yoshioka H, Taniguchi K, Kamisaki Y, Ooshima T, Umemura K, Murad F, Wada K, Amano A. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett. 2009;583:128–134. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick RE, Wijeyewickrema LC, Pike RN. The gingipains: scissors and glue of the periodontal pathogen, Porphyromonas gingivalis. Future Microbiol. 2009;4:471–487. doi: 10.2217/fmb.09.18. [DOI] [PubMed] [Google Scholar]
- 11.Sheets SM, Potempa J, Travis J, Casiano CA, Fletcher HM. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun. 2005;73:1543–1552. doi: 10.1128/IAI.73.3.1543-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng S, Jepsen S, Dommisch H, Stiesch M, Fickenscher H, Maser E, Chen H, Eberhard J. Cysteine proteases from Porphyromonas gingivalis and TLR ligands synergistically induce the synthesis of the cytokine IL-8 in human artery endothelial cells. Arch Oral Biol. 2011;56:1583–1591. doi: 10.1016/j.archoralbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto M, Kadowaki T, Tsukuba T, Yamamoto K. Selective proteolysis of apolipoprotein B-100 by Arg-gingipain mediates atherosclerosis progression accelerated by bacterial exposure. J Biochem. 2006;140:713–723. doi: 10.1093/jb/mvj202. [DOI] [PubMed] [Google Scholar]
- 14.Grenier D, Tanabe S. Porphyromonas gingivalis gingipains trigger a proinflammatory response in human monocyte-derived macrophages through the p38α mitogen-activated protein kinase signal transduction pathway. Toxins (Basel) 2010;2:341–352. doi: 10.3390/toxins2030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. doi: 10.1161/ATVBAHA.111.226670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Elmabsout AA, Khalaf H, Basic VT, Jayaprakash K, Kruse R, Bengtsson T, Sirsjö A. The periodontal pathogen Porphyromonas gingivalis changes the gene expression in vascular smooth muscle cells involving the TGFbeta/Notch signalling pathway and increased cell proliferation. BMC Genomics. 2013;14:770. doi: 10.1186/1471-2164-14-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inaba H, Tagashira M, Kanda T, Amano A. Proliferation of smooth muscle cells stimulated by Porphyromonas gingivalis is inhibited by apple polyphenol. J Periodontol. 2011;82:1616–1622. doi: 10.1902/jop.2011.100785. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Baba A, Abe N, Takii R, Hashimoto M, Tsukuba T, Okazaki S, Suda Y, Asao T, Yamamoto K. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol Pharmacol. 2004;66:1599–1606. doi: 10.1124/mol.104.004366. [DOI] [PubMed] [Google Scholar]
- 19.Brien-Simpson NM, Veith PD, Dashper SG, Reynolds EC. Porphyromonas gingivalis Gingipains: the molecular teeth of a microbial vampire. Curr Protein Pep Sci. 2003;4:409–426. doi: 10.2174/1389203033487009. [DOI] [PubMed] [Google Scholar]
- 20.Wilensky A, Polak D, Houri-Haddad Y, Shapira L. The role of RgpA in the pathogenicity of Porphyromonas gingivalis in the murine periodontitis model. J Clin Periodontol. 2013;40:924–932. doi: 10.1111/jcpe.12139. [DOI] [PubMed] [Google Scholar]
- 21.Kinane JA, Benakanakere MR, Zhao J, Hosur KB, Kinane DF. Porphyromonas gingivalis influnences actin degradation within epithelial cells during invasion and apoptosis. Cell Microbiol. 2012;14:1085–1096. doi: 10.1111/j.1462-5822.2012.01780.x. [DOI] [PubMed] [Google Scholar]
- 22.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 24.Wolak T. Osteopontin-a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis. 2014;236:327–337. doi: 10.1016/j.atherosclerosis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, Lun Y, Wu X, Xia Q, Zhang X, Xin S, Zhang J. Association between the hypomethylation of osteopontin and integrin β3 promoters and vascular smooth muscle cell phenotype switching in great saphenous varicose veins. Int J Mol Sci. 2014;15:18747–18761. doi: 10.3390/ijms151018747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Sun Y, Wang T, Liu G. Prevention of neointimal hyperplasia in balloon-injured rat carotid artery via small interference RNA mediated downregulation of osteopontin gene. Mol Cell Biochem. 2013;377:1–10. doi: 10.1007/s11010-012-1554-x. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Ren Y, Kang L, Zhang LH. Oxidized low-density lipoprotein increases the proliferation and migration of human coronary artery smooth muscle cells through the upregulation of osteopontin. Int J Mol Med. 2014;33:1341–1347. doi: 10.3892/ijmm.2014.1681. [DOI] [PubMed] [Google Scholar]
- 28.Mohamadpour AH, Abdolrahmani L, Mirzaei H, Sahebkar A, Moohebati M, Ghorbani M, Ferns GA, Ghayour-Mobarhan M. Serum Osteopontin Concentrations in Relation to Coronary Artery Disease. Arch Med Res. 2015;46:112–117. doi: 10.1016/j.arcmed.2015.02.005. [DOI] [PubMed] [Google Scholar]


