Abstract
To summarize the clinical characteristics of intracranial arachnoid cysts (IACs) in pediatric cases. A retrospective analysis was carried out on clinical characteristics of IACs in 488 pediatric cases who were treated at our hospital from January 2003 to September 2013. There were 342 males and 146 females (male-to-female ratio, 2.34:1), aged 5.61±3.25 years on average. 221 cases (45.29%) were diagnosed accidentally, 267 cases had clinical complaints (54.71%), among which relationships between clinical complaints and IACs were identified in 123 (46.07%). Simple IACs occurred in 364 cases (4.59%), and concurrent congenital abnormalities occurred in 124 cases (4.59%). In terms of location, 355 had IACs in middle cranial fossa (72.75%), 82 cases in posterior cranial fossa (16.80%), 20 cases in anterior cranial fossa (4.10%), 12 cases in dorsolateral surface (2.46%), 7 cases in suprasellar cistern (1.43%), 5 cases in cerebral ventricle (1.02%), 5 cases in quadrigeminal cistern (1.02%), and 2 cases in interhemispheric region (0.41%). There were 449 cases with single IAC (92.01%) and 39 cases with multiple IACs (7.99%). On MRI, the cysts produced tension in 127 cases (26.02%), but not in the remaining 361 cases (73.98%). Surgery was performed on 76 of 488 cases (15.57%), while conservative observation was accepted in 412 cases (84.43%). For the former, the symptoms and the cyst volume were improved to varying extent; for the latter, the follow-up lasting for 3-72 months (average 32.43±8.92 months) showed that the cyst volume remained stable in 407 cases (98.78%), enlarged with aggravated symptoms in 3 cases (0.73%), and shrank in 2 cases (0.49%). Clinical complaints of IACs varied in pediatric cases, and the relationships between clinical complaints and IACs were established only partially. Some pediatric cases were combined with other congenital abnormalities. The cyst volume largely remained stable during the disease course, and surgery was required for only a few IACs.
Keywords: Intracranial arachnoid cysts (IACs), pediatric, natural history, clinical symptoms, treatment
Introduction
Arachnoid cysts are pouch-like intraarachnoid masses filled with cerebrospinal fluid (CSF). As a congenital benign space-occupying lesion, arachnoid cysts are divided into intracranial arachnoid cysts (IACs) and spinal arachnoid cysts (SACs). IACs account for 1% among all intracranial space-occupying lesions, and 2.6% among the population aged below 18 years [1]. It is difficult to predict the biological behaviors of IACs, and diversified clinical manifestations are hardly consistent with the imaging findings. For these reasons, the diagnosis of IACs is not easy. We reviewed the clinical characteristics and treatments of IACs in 488 pediatric cases (0-14 years) treated at the both outpatient and inpatient department in First Affiliated Hospital of Fujian Medical University from January 2003 to September 2013.
Materials and methods
Clinical data
General data
The subjects reviewed included 342 males and 146 females (male-to-female ratio, 2.31:1), with an average age of 5.61±3.25 years.
Diagnostic criteria
All cases received cranial CT scan and/or MRI. Inclusion criteria were as follows: Plain CT scan revealed signal intensity of cysts was lower than that of CSF, without enhancement in contrast-enhanced CT (in cases with differentiation difficulty). Plain MRI scan revealed tumors that had CSF-like signal intensity combined with cranial cavity enlargement, skull thinning, displacement of surrounding brain tissues, displacement of midline structure and hydrocephalus, without enhancement in contrast-enhanced CT (in cases with differentiation difficulty).
Method
Indicators and method
Clinical complaints, imaging findings and concurrent congenital abnormalities were reviewed for 488 pediatric cases. The locations of IACs, compression of surrounding brain tissues, skull invasion and CSF circulation were observed by imaging. The phenomenon that cysts caused obvious compression of surrounding brain tissues and skull and displacement of brain tissues (midline structure) was defined as tension.
Treatment method
Surgical approach was selected based on symptoms, size of IACs and tension on imaging findings. Symptoms of headache, hydrocephalus and skull invasion were clearly attributed to IACs. Surgery was given to those with the presence of tension or cyst diameter larger than 3 cm after obtaining informed consent. Surgical approaches adopted were cystoperitoneal shunting, microscopic fenestration and endoscopic fenestration. Conservative observation was adopted if it was unclear whether the symptoms arose from cysts, or the tension was not obvious, or the relatives did not support the surgery. In the present study, 412 cases were kept in conservative observation (84.43%). During follow-up, 6 cases had rupture of cysts with bleeding, who received drainage of subdural hematoma. Among 76 surgical cases (15.57%), 40 cases received cystoperitoneal shunting, 31 received microscopic fenestration, and 5 cases received endoscopic fenestration.
The procedures of microscopically assisted fistulization were as follows
For supratentorial IACs, the supine position was taken, and prone position was taken for infratentorial IACs. Cysts were approached differently depending on location. For suprasellar cysts, interhemispheric fissure-corpus callosum approach was taken, and infratentorial supracerebellar approach was taken for quadrigeminal cysts. The surgical procedures for supratentorial cyst of the middle cranial fossa were illustrated in details: endotracheal intubation and general anesthesia were performed. After conventional disinfection and draping, the scalp in the temporal region was cut open. The bone flap was lifted, with the lower margin of bone window kept as close to the skull base as possible, and thorough hemostasis was done. A cross-shaped incision was made on the dura mater so as to open the dura mater and the lateral wall of the cyst. Hemostasis was performed for the small veins within the wall. Meanwhile, suprasellar cistern and basal cistern were opened to make them communicated. The cyst wall surrounding the blood vessels was stripped with attention given to avoid accidental bleeding or damage to the temporal lobe. After thorough hemostasis, flushing and suture of dura mater, the bone flap was restored and the scalp was sutured layer by layer.
Endoscopic fistualization
For supratentorial IACs, the supine position was taken, and prone position was taken for infratentorial IACs. The surgical procedures for supratentorial cyst of the middle cranial fossa were introduced in details. Endotracheal intubation and general anesthesia were performed. After conventional disinfection and draping, the scalp in the temporal region was cut open. A hole was drilled on the skull, with thorough hemostasis. A cross-shaped incision was made on the dura mater so as to open the dura mater and the lateral wall of the cyst. Once the cyst fluid or CSF flowed out, neuroendoscope was inserted for thorough hemostasis. A fistula was made under the endoscopce, and the cyst was fully communicated with the cisterns via the fistula. The diameter of the fistula was 10-15 mm. After thorough hemostasis, flushing and suture of dura mater, the bone flap was restored and the scalp was sutured layer by layer.
Cystoperitoneal shunting
Antibiotics were administered 1 h before surgery. The supine position was taken with a roll pillow placed beneath the neck. The head or the abdomen was supported with towels so that the abdomen, occiput and top of the occipital bone were on the same line. Conventional disinfection and draping were performed for head, neck, chest and abdomen. A half-moon-shaped incision was made on the head. The shunt tube was kept away from the site below the incision. A hole was drilled on the skull, and the dura mater was cauterized with an electrocoagulator. The incision was covered with iodophor gauzes. The abdominal incision was located below xiphoid process. The peritoneal cavity was accessed by cutting open the abdominal wall. The shunt was inserted via the abdominal incision and reached the cranial incision through the subcutaneous tunnel. Using the shunt, one end of the tube was delivered to the abdomen from the cranial incision. The opening of the tube was covered with iodophor gauzes. Dura mater was excised with No. 11 scalpel. The tube was placed inside the cyst according to the predetermined length, which was about 1/3-2/3 of the diameter of the cyst. The shunt device was a subcutaneous sneak device fixed with threads and filled with cyst fluid. The incision was covered with iodophor gauzes. After the placement of abdominal drainage tube, the shunt devise was examined regularly to ensure a smooth drainage. The abdomen was excised to reach rectus sheath with blunt separation. After the excision of peritoneum, the tube was directly inserted into the peritoneal cavity. The abdominal incision and incision in the head were sutured layer by layer.
Results
Clinical characteristics
Clinical complaints
IACs were accidentally diagnosed in 221 cases who had traumatic brain injury, meningoencephalitis and stunting (45.29%). The possible or definite relationships between clinical complaints and IACs were established in 267 cases (54.71%), among which the definite relationships were established in 123 cases (46.07%, Tables 1 and 2).
Table 1.
Clinical complaints of pediatric IACs
| Clinical complaints | IACs cases (n=267) |
|---|---|
| Headache | 101 |
| Onset of epilepsy | 69 |
| Hearing impairment | 32 |
| Dyskinesia | 16 |
| Abnormal increase of head circumference | 13 |
| Nausea and vomiting | 10 |
| Dizziness | 10 |
| Behavioral problems and psychiatric disorders | 8 |
| Skull protrusion | 5 |
| Visual disorders | 2 |
| Sensory disturbance | 1 |
Table 2.
Clinical complaints of pediatric IACs in the responsibility
| Clinical complaints | IACs cases (n=123) |
|---|---|
| Headache | 60 |
| Onset of epilepsy | 29 |
| Nausea and vomiting | 10 |
| Abnormal increase of head circumference | 9 |
| Behavioral problems and psychiatric disorders | 8 |
| Skull protrusion | 5 |
| Visual disorders | 2 |
Other concurrent congenital abnormalities
Of 488 cases, 364 cases had simple IACs (74.59%) and 124 cases were combined with other congenital abnormalities (25.41%, Table 3).
Table 3.
Other congenital abnormalities complicated with pediatric IACs
| Congenital abnormalities | Cases (n=124) |
|---|---|
| Stunting | 36 |
| Autism | 24 |
| Dystonia | 14 |
| Leukodystrophy | 9 |
| Microcephaly | 6 |
| Glutaric aciduria type I | 4 |
| Dysplasia of corpus callosum | 4 |
| Cerebrovascular malformation | 4 |
| Inner ear malformations | 3 |
| Facial vascular malformations | 3 |
| Tuberous sclerosis | 2 |
| Gray matter heterotopia | 2 |
| Schizencephaly | 2 |
| Gyrus deformation | 2 |
| Hepatolenticular degeneration | 2 |
| Ossicle dysplasia | 1 |
| Bilateral cochlear dysplasia | 1 |
| Hypophysial duct tumor | 1 |
| Suprasellar pilocytic astrocytoma | 1 |
| Cerebella tonsil herniation | 1 |
| Sellar glioma | 1 |
| Schistorachis | 1 |
Locations of IACs (Table 4).
Table 4.
Location distribution of pediatric IACs
| Location | Cases (n=488) |
|---|---|
| Middle cranial fossa | |
| Left middle cranial fossa base | 222 |
| Temporal region/lateral fissure cistern | 73 |
| Right middle cranial fossa base | 36 |
| Bilateral middle cranial fossa base | 23 |
| Medial side of hippocampus | 1 |
| Posterior cranial fossa | |
| Vermiform process of cerebellum | 60 |
| Cerebellopontine angle cistern | 17 |
| Convex side of cerebellar hemisphere | 5 |
| Anterior cranial fossa | 20 |
| Dorsolateral surface | 12 |
| Suprasellar cistern | 7 |
| Cerebral ventricle | 5 |
| Quadrigeminal region | 5 |
| Interhemispheric region | 2 |
MIR characteristics
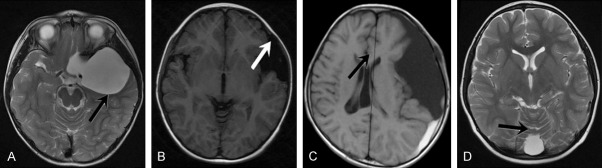
On MRI, 449 cases had single IACs (92.01%) and 39 cases had multiple IACs (7.99%). IACs were located in various parts of the brain, most commonly in the middle cranial fossa, followed by posterior cranial fossa (Table 3). Obvious tension was formed in 127 cases (26.02%), among which 89 cases had compression of gyres (18.24%) and 38 cases had obvious impression of the skull (7.79%). Obvious tension was absent in 361 cases (73.98%, Figures 1, 2 and 3).
Figure 1.

MRI characteristics of single and multiple pediatric IACs. A. IACs in the right temporal region; B. IACs in bilateral temporal region.
Figure 2.

Common sites of pediatric IACs. Middle cranial fossa (A), suprasellar cistern (B), Cerebellopontine angle cistern (C), quadrigeminal cistern (D), dorsolateral surface (E), posterior cranial fossa (F), lateral ventricle (G), vermiform process of cerebellum (H).
Figure 3.
Tension characteristics of pediatric IACs on MRI. Tension-type IACs: A. Displacement of adjacent brain tissues (indicated by the arrow); B. Compression and deformation of the skull (indicated by the arrow); C. Midline structure displacement under compression (indicated by the arrow); D. Non-tension-type IACs, with clearly visualized sulcus (indicated by the arrow) and no obvious compression of adjacent brain tissues.
Outcome and effect of intervention
Conservative treatment was adopted in 412 cases. The follow-up for 3-27 months (average, 32.43±8.92 months) indicated that the cyst volume remained stable in 407 cases (98.78%), and 6 cases (1.23%) had rupture of cyst with bleeding which received drainage of subdural hematoma with a good effect achieved. The cyst was enlarged with aggravated symptoms in 3 cases (0.73%) and shrank in 2 cases (0.49%). After surgical treatment (fenestration and shunting) in 76 cases, the symptoms were improved and the cyst volume shrank to varying extent.
Discussion
Although pediatric IACs are common, there are few large-sample-size systemic reviews of its clinical characteristics. After analysis on IACs in 488 pediatric cases, we found that IACs had complex clinical manifestations including epilepsy and intracranial hypertension. Some IACs cases were complicated by other congenital abnormalities such as schizencephaly, chiari malformation and schistorachis. The imaging findings were also diversified in location of IACs, cyst number and volume, and presence or absence of tension. Long-term follow-up showed that 98.78% of IACs had stable volume, and a few were enlarged or shrank. We carried out the following discussion based on the findings of the present study.
Pathogenesis, mechanism and natural history of IACs
IACs are divided into primary and acquired IACs. It is believed that primary IACs are due to abnormal development of the arachnoid in embryonic stages such defective development of mesenchymal cells or abnormalities of CSF flow [2]. Acquired IACs are probably related to hemosiderin and inflammatory cyst fluid [3]. During natural course of disease, cyst volume may remain stable, increase or decrease, and the mechanism of cyst volume enlargement is unknown. The hypotheses of active secretion of CSF by the cyst wall [4] and ball valve mechanism [5] cannot perfectly explain. It is hard to predict the natural disease course of IACs, but Lee et al. [6] believe that young age is the only factor contributing to the enlargement of IACs, and location and initial volume of IACs or the follow-up period are not relevant factors in cyst volume enlargement. In the present study, 407 pediatric cases of IACs (98.78%) had stable cyst volume over tine, 3 cases had enlargement with aggravated symptoms, and 2 cases had shrinkage of cyst volume. Another major event during the natural disease course of IACs is rupture of cyst, leading to subdural effusion or hematoma. It is traditionally believed that the cross-sectional diameter of IACs above 3 cm is one surgical indication for pediatric cases and rupture of cyst can be prevented by surgery. Cress M et al. [7] analyzed the risk of rupture of IACs and bleeding after trauma for population aged below 18 years. They found that IACs with cross-sectional diameter above 5 cm were more likely to rupture (9/13 cases), while those below 5 cm were more stable (5/29 cases). It was also pointed out that sagittal-plane height was not the high-risk factor for rupture of cyst and bleeding after trauma. In our group, 6 cases had rupture of cyst with bleeding, among which one had cross-sectional diameter of IACs of about 3.5 cm, and the remaining 5 cases had cross-sectional diameter above 5 cm. Thus cross-sectional diameter of IACs above 3 cm, especially 5 cm, indicated a higher risk of rupture after trauma for those who received conservative treatment.
Clinical manifestations of IACs
Clinical characteristics of IACs depend on age, location, cyst volume and complication by other congenital abnormalities. Typical symptoms include headache, epilepsy, skull protrusion and hemiplegia. However, we are not certain as to which specific clinical complaints are directly attributed to IACs. For example, IACs in the temporal poles are responsible for psychiatric disorders and neurophysiological defects [8-10]. IACs in fetal period can be found by ultrasonography or cranial MRI. Young infants with IACs may present no obvious clinical symptoms, making early diagnosis difficult. For these cases, IACs are usually diagnosed accidentally by cranial CT/MRI for other symptoms. Older children may have clinical complaints. IACs may affect supratentorial or infratentorial regions, especially supratentorial region. IACs in middle cranial fossa are the most common, and headache, epilepsy, dyskinesia and stunted growth are the common symptoms. IACs in sellar region and suprasellar cistern may cause hydrocephalus, visual impairment and pituitary function impairment. A few IACs compress the third ventricle and thalamus, leading to bobble-head doll syndrome. IACs in cerebellopontine angle cistern have complex clinical manifestations, including hydrocephalus, cerebellar ataxia and cranial nerve involvement. The latter may lead to hearing impairment, facial paralysis, ambiopia and difficult swallowing. IACs in cerebral ventricles are less common and the typical symptoms are headache and vomiting, which are due to acute hydrocephalus caused by obstruction of CSF channel by cysts. Besides, rupture of cyst and bleeding after trauma may lead to subdural hematoma or effusion and the symptoms of intracranial hypertension and local nerve dysfunction. The situation becomes more complex when complicated by other congenital abnormalities. The clinical complaints of cases in our group were greatly diversified: 45.29% of the cases were diagnosed accidentally, and the possible or definite relationships between complaints and IACs were established in 54.71% of the cases among which the definite relationships were established in 46.07% of the cases. Clinically, IACs are usually divided into symptomatic IACs and asymptomatic IACs. In the study, IACs were divided according to the relationships between clinical complaints and IACs, as was advantageous in guiding clinical treatment.
Other concurrent congenital abnormalities
IACs may be complicated by other congenital abnormalities, such as malformations of corpus callosum, Chiari malformation, glutaric aciduria type I (GA-I), multiple sclerosis and neurofibromatosis. Most of these congenital abnormalities are independent from IACs, but some of them cannot be neglected during the treatment for IACs. For example, IACs in bilateral lateral fissure cisterns are usually complicated by GA-I, and if left untreated, it may cause metabolic disorders and aggravate brain damage [11]. In our group, 25.41% of IACs were combined with other congenital abnormalities, indicating the combination with other congenital abnormalities was not rare. Therefore IACs can be divided into simple IACs and complex IACs by whether complicated with other congenital abnormalities or not. The medical history should be carefully inquired with attention to the suspected symptoms. Plasma or urine organic acid analysis should be combined with EEG and chromosomal analysis for classification of IACs.
Disease evaluation
Evaluation of IACs in pediatric cases depends on not only clinical symptoms, but also imaging and electrophysiological tests. Cranial MRI reveals the position of cysts as well as CSF flow pattern and relationship between cyst and brain tissues. CT cisternography further shows communications in IACs and divides IACs into communicated (filled within 1 h), partially communicated (filled within 3 h) and non-communicated (not filled or little filled after 24 h) types [12]. IACs can be found by conventional ultrasonography in fetal period, and then chromosomal evaluation and fetal MRI may be carried out to exclude other malformations. For older children, EEG applies to supratentorial IACs so as to assess the need for antiepileptics. Some scholars contend that IACs may be combined with cognitive deficit and recommend electrophysiological tests for older children in order to find clinical syndromes that are otherwise negligible [9]. Cranial CT/MRI is the most important examination technique as it can directly or indirectly reveal the presence of tension and the communications with subarachnoid cavity and provide clues for surgery.
Treatments
Clinical symptoms provide important basis for the treatment of IACs. Conservative treatment is considered appropriate for asymptomatic IACs, but the surgical indications for symptomatic IACs are still disputed. Many scholars [13-15] agree on the following: Subdural effusion or hematoma caused by rupture of cyst; Significantly increased intracranial hypertension; Local neurosigns, including facial paralysis, vision loss and local cranial nerve dysfunction; Refractory epilepsy; Cysts with expansive growth, such as skull protrusion, compression of adjacent brain tissues or complicated by hydrocephalus. Others believe that diameter of IACs, age, and communications with adjacent cisterns are among the surgical indications [14-16]. Based on clinical symptoms, presence of tension and viewpoints of the guardians, only 76 cases received surgical treatments in our study.
Common surgical methods for IACs are fenestration and shunting, and the controversy is still going on as to the optimal approach [16,17]. The selection of surgical methods mainly depends on personal choice and disease conditions, such as clinical symptoms, location and shape of cysts, intra-cyst septa, adjacent nerves and vessels, as well as surgeon’s experience, among which location of cysts and age are the most important. According to the existing studies, fenestration and shunting have comparable long-term effect. However, shunting requires the use of shunt tube and may cause intracranial infections. Some recommend the use of fenestration first and shunting may be adopted secondarily if the preliminary treatment effect is less satisfactory [18-20]. For IACs in suprasellar cistern, cerebellopontine angle cistern, and posterior cranial fossa, some researchers recommend neuroendoscopic fenestration, and fenestration or shunting may be used for IACs in dorsolateral surface [20].
Summary
To conclude, 488 pediatric cases of IACs were diversified in clinical manifestations, among which 25.41% cases were complicated by other congenital abnormalities, and 26.02% cases showed tension on MRI. During the disease course, 98.78% of IACs had basically stable volume, and only a few were enlarged or shrank. We believed that clinical classification should be based on the relationships between IACs and clinical complaints, conditions of other concurrent congenital abnormalities and presence or absence of tension. The evaluation of IACs should be carried out in combination with various examination methods. A good treatment effect was achieved in 84.43% of the cases, but the risk of rupture of cyst deserved our attention. Surgery was required for only a few IACs.
Acknowledgements
This work was supported by the Key Clinical Subject Construction Project of Fujian Province.
Disclosure of conflict of interest
None.
References
- 1.Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in children. J Neurosurg Pediatr. 2010;5:578–585. doi: 10.3171/2010.2.PEDS09464. [DOI] [PubMed] [Google Scholar]
- 2.Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40:61–83. [PubMed] [Google Scholar]
- 3.Cagnoni G, Fonda C, Pancani S, Pampaloni A, Mugnaini L. [Intracranial arachnoid cyst in pediatric age] . Pediatr Med Chir. 1996;18:85–90. [PubMed] [Google Scholar]
- 4.Helland CA, Aarhus M, Knappskog P, Olsson LK, Lund-Johansen M, Amiry-Moghaddam M, Wester K. Increased NKCC1 expression in arachnoid cysts supports secretory basis for cyst formation. Exp Neurol. 2010;224:424–428. doi: 10.1016/j.expneurol.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Halani SH, Safain MG, Heilman CB. Arachnoid cyst slit valves: the mechanism for arachnoid cyst enlargement. J Neurosurg Pediatr. 2013;12:62–66. doi: 10.3171/2013.4.PEDS12609. [DOI] [PubMed] [Google Scholar]
- 6.Lee JY, Kim JW, Phi JH, Kim SK, Cho BK, Wang KC. Enlarging arachnoid cyst: a false alarm for infants. Childs Nerv Syst. 2012;28:1203–1211. doi: 10.1007/s00381-012-1722-z. [DOI] [PubMed] [Google Scholar]
- 7.Cress M, Kestle JR, Holubkov R, Riva-Cambrin J. Risk factors for pediatric arachnoid cyst rupture/hemorrhage: a case-control study. Neurosurgery. 2013;72:716–722. doi: 10.1227/NEU.0b013e318285b3a4. [DOI] [PubMed] [Google Scholar]
- 8.Gjerde PB, Schmid M, Hammar A, Wester K. Intracranial arachnoid cysts: impairment of higher cognitive functions and postoperative improvement. J Neurodev Disord. 2013;5:21. doi: 10.1186/1866-1955-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wester K. Intracranial arachnoid cysts--do they impair mental functions? J Neurol. 2008;255:1113–1120. doi: 10.1007/s00415-008-0011-y. [DOI] [PubMed] [Google Scholar]
- 10.Di Rocco C. Sylvian fissure arachnoid cysts: we do operate on them but should it be done? Childs Nerv Syst. 2010;26:173–175. doi: 10.1007/s00381-009-1041-1. [DOI] [PubMed] [Google Scholar]
- 11.Lutcherath V, Waaler PE, Jellum E, Wester K. Children with bilateral temporal arachnoid cysts may have glutaric aciduria type 1 (GAT1); operation without knowing that may be harmful. Acta Neurochir (Wien) 2000;142:1025–1030. doi: 10.1007/s007010070058. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Chen JX, You C, Jiang S. CT cisternography in intracranial symptomatic arachnoid cysts: classification and treatment. J Neurol Sci. 2012;318:125–130. doi: 10.1016/j.jns.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Gui SB, Wang XS, Zong XY, Li CZ, Li B, Zhang YZ. Assessment of endoscopic treatment for middle cranial fossa arachnoid cysts. Childs Nerv Syst. 2011;27:1121–1128. doi: 10.1007/s00381-011-1399-8. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Xi YQ, Tang S. Excision of intracranial cysts with cyst wall excision plus cyst. Chin J Clin Neurosurg. 2008;13:688–690. [Google Scholar]
- 15.Xue CJ, Hu XB, Zhao HY, Zhao AH, Zhao JS, Zhu XL. Clinical Analysis of Intracranial Arachnoid Cysts Treatment. Chin J Clin Neurosurg. 2006;11:487–488. [Google Scholar]
- 16.Duz B, Kaya S, Daneyemez M, Gonul E. Surgical management strategies of intracranial arachnoid cysts: a single institution experience of 75 cases. Turk Neurosurg. 2012;22:591–598. doi: 10.5137/1019-5149.JTN.5616-11.0. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson DH, Vogel TD, Baker AA, Patel NB, Ackerman LL, Smith JL, Boaz JC. Cyst-ventricle stent as primary or salvage treatment for posterior fossa arachnoid cysts. J Neurosurg Pediatr. 2011;7:549–556. doi: 10.3171/2011.2.PEDS10457. [DOI] [PubMed] [Google Scholar]
- 18.Oertel JM, Wagner W, Mondorf Y, Baldauf J, Schroeder HW, Gaab MR. Endoscopic treatment of arachnoid cysts: a detailed account of surgical techniques and results. Neurosurgery. 2010;67:824–836. doi: 10.1227/01.NEU.0000377852.75544.E4. [DOI] [PubMed] [Google Scholar]
- 19.Spacca B, Kandasamy J, Mallucci CL, Genitori L. Endoscopic treatment of middle fossa arachnoid cysts: a series of 40 patients treated endoscopically in two centres. Childs Nerv Syst. 2010;26:163–172. doi: 10.1007/s00381-009-0952-1. [DOI] [PubMed] [Google Scholar]
- 20.Gangemi M, Seneca V, Colella G, Cioffi V, Imperato A, Maiuri F. Endoscopy versus microsurgical cyst excision and shunting for treating intracranial arachnoid cysts. J Neurosurg Pediatr. 2011;8:158–164. doi: 10.3171/2011.5.PEDS1152. [DOI] [PubMed] [Google Scholar]