Abstract
Background: Neonatal hypoxic-ischemic encephalopathy (HIE) is a clinical syndrome manifested by neurological symptoms in the first days of life in term infants. Purpose: To investigate the therapy effect of Buyanghuanwu Tang (BYHWT), a decoction with 7 herbal ingredients, on neonatal rats with hypoxic ischemic encephalopathy (HIE) and its mechanism. Methods: 50 3-week male Sprague-Dawley rats were divided into normal control group, model group, BYHWT 1d group, BYHWT 3d group and BYHWT 7d group, 10 rats in each group. The HIE model of was established in later 4 groups. The later 3 groups were treated with BYHWT for 1, 3 and 7 days, respectively, and the normal control group and model group were treated with PBS. The Morris water maze test and dynamic 18F-FDG-PET/CT imaging were performed. The changes of hippocampal tissue observed by histopathologic examination, and the expressions of JNK1/JNK2 and TNF-α protein were observed western blotting. Results: Compared with model group, the impaired performance on distance and latency parameters was mitigated in BYHWT 1d group, BYHWT 3d group and BYHWT 7d group (P < 0.01), the FDG uptake was decreased in BYHWT 3d group and BYHWT 7d group, the apoptotic cells and inflammatory cells were significantly decreased in BYHWT 3d group and BYHWT 7d group, and the expressions of JNK1/JNK2 and TNF-α protein were significantly decreased in BYHWT 7d group (P < 0.05). Conclusion: BYHWT can delay the HIE onset and preserve the motor function, primarily by regulating inflammation, apoptosis and inhibition by mediating JNK signaling.
Keywords: Buyanghuanwu Tang, hypoxic ischemic encephalopathy, PET/CT, JNK
Introduction
Neonatal encephalopathy is a clinical syndrome manifested by neurological symptoms in the first days of life in term infants. Several etiologies, including infections and metabolic and genetic disorders, can cause neonatal encephalopathy. However, when it is caused by perinatal asphyxia, as occurs in 30-60% of cases, the syndrome is called neonatal hypoxic-ischemic encephalopathy (HIE), which has an incidence of 1.5 per 1,000 live births [1]. HIE is a relatively common severe complication of perinatal asphyxia that can result in cerebral palsy, seizures, and mental retardation and causes a heavy burden on families and society [2]. HIE remains a major perinatal cause of neurological morbidity in full-term newborns [3]. And now, there are still no effective ways of repairing HI brain damage (HIBD). Therefore, it is necessary to find suitable therapies for HIBD.
Buyanghuanwutang (BYHWT) is a popular Traditional Chinese Medicine formula consisting of seven herbal medicines which include i) Radix Astragali (huangqi), the dried roots of Astragalus membranaceus (Fisch.) Bge.var. mongholicus (Bge.) Hsiao; ii) the carda part of Radix Angelicae Sinensis root (guiwei), the dried lateral roots of Angelica sinensis (Oliv.) Diels; iii) Radix Paeoniae Rubra (chishao), the dried roots of Paeonia lactiflora Pall.; iv) Rhizoma Chuanxiong (chuanxiong), the dried rhizomes of Ligusticum chuanxiong Hort; v) Flos Carthami (honghua), the dried flowers of Carthamus tinctorius L.; vi) Semen Persicae (taoren), the dried seeds of Amygdalus persica L.; and vii) Pheretima (dilong), the dried bodies of Pheretima aspergillum (E. Perrier), in the ratio of 120:6:4.5:3:3:3:3 on a dry weight basis. All of these components are recorded in the Chinese Pharmacopoeia (2005 Edition) [4]. Recent studies show that BYHWT also provides neuroprotective effects for conditions such as brain ischemia, stroke-induced disability [5] and act against cerebral ischaemia-reperfusion (CI/R) injury [6,7]. But its mechanism has not been clarified.
c-Jun N-terminal kinases (JNKs) belong to the super family of MAP-kinases involved in the regulation of cell proliferation, differentiation, and apoptosis. Analyses of pathways regulated by JNKs have shown that JNKs are indispensable for both cell proliferation and apoptosis [8]. Signaling pathways that initiate apoptosis has been broadly classified into two: i) extrinsic pathway initiated by death receptors such as those of TNF-α, TRAIL, and FAS-L and ii) intrinsic pathway initiated by mitochondrial events [9]. JNK has been observed to play a central role in both of these pathways. To date, multiple splice variants of JNKs encoded by three distinct genes, namely JNK1, JNK2, and JNK3, have been identified [10]. This study evaluated the therapeutic effect of BYHWT for neonatal rats with HIE, and explore the relationship between BYHWT and the JNK signaling pathway. The objective was to provided a reference for further application of BYHWT to treating HIE.
Materials and methods
Animals
The protocol listed below was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) by the Institutional Animal Experimentation Committee of the Southern Medical University. Postnatal 3-week male Sprague-Dawley rats (200 ± 15 g) were obtained from the Laboratory Animal Center of Southern Medical University. The animals had free access to food and water was allowed at all times. This study was approved by the supervising state agency (license number: Scxk Yue 2006-0015) and performed in full accordance with the state guidelines.
Animal grouping and establishment of HIE model
50 rats were divided into normal control group, model group, BYHWT 1d group, BYHWT 3d group and BYHWT 7d group, 10 rats in each group. For rats in later 4 groups, the HIE model was established by the same investigator who was blinded to treatment allocation. The rats were anesthetized with pentobarbital (50 mg/kg, i.p.), and the left carotid artery was permanently sutured between double ligatures with 4-0 sterile surgical silk. The rats were allowed to recover for 1-2 h, then exposed to 2 h hypoxia in a plastic container (Animal research center, Guangzhou, China) that was perfused with a mixture of humidified 8% oxygen balanced with nitrogen. The temperature inside the container was kept at 34°C. After surgery, the rats were placed in experimental cages and allowed to recuperate for one day. The rats were kept fasted for 12 h and water were available ad libitum.
Treatment methods
The dosage of BYHWT was purchased from the institution of Chinese Traditional Medical of Southern Medical University (Guangzhou, China). It was selected by the indication of concentrated herbal extracts for clinical application which was 3.0 g each time and 2-3 times daily. BYHWT was dissolved in normal saline at doses of 10 g/kg for oral administration and fed by gavages to rats, respectively. According to Lee-Hsin Shaw’ studies, the dose of BYHWT (10 g/kg) was appropriate for oral administration in rats [11]. The BYHWT 1d group, BYHWT 3d group and BYHWT 7d group were treated with BYHWT for 1, 3 and 7 days, respectively. The normal control group and model group were treated with phosphate buffered sodium (PBS) (Sigma-Aldrich Corp., MO, USA) replacing BYHWT for 7 days.
Behavioral test
The spatial working memory Morris water maze (MWM) test (RD1101-M, Shanghai Yishu Mdt InfoTech Ltd., Shanghai, China) was used to study spatial memory and learning for rats. Rats were trained for 3 trials per day for 4 consecutive days during the acquisition phase. The platform was kept in the same position throughout the test. Rats were subjected to a learning test in which the platform was located to the quadrant of the pool but all distal visual cues remained constant. Learning revealed whether animals could extinguish their initial learning of the platform’s position and acquire a direct path to the new goal position. Latency to the platform, swimming distance were automatically recorded by video tracking mounted on the ceiling, where after digital images were analyzed by water maze software (HVS image, Buckingham, UK).
18F-FDG-PET/CT imaging
The rats were scanned by 18F-FDG-PET/CT (Discovery-LS PET/CT scanner, GE Healthcare, Milwaukee, WI, USA) at the various observed time points. Certain measures were followed to ensure the objectivity and accuracy of the images: i) All rats were required to fast for at least 8 h before undergoing imaging, and the serum glucose level was kept under 7 to 11 mmol/L; ii) At 50-60 min after intravenous injection of the 18F-FDG (0.11-0.13 mCi/kg), a static whole-body emission PET/CT scan from head to pelvic floor was initiated; iii) All rats received an intravenous injection of furosemide (0.5 mg/kg) and a 250 mL infusion of the saline solution, initiated 30 min after the 18F-FDG injection; iv) Using the fused PET/CT image, a region of interest (ROI, 3-4 cm2 in size) was placed in brain. When a hypermetabolic SUV lesion was detected on the pre- and post-irradiation PET/CT images, the maximum SUV was prospectively calculated using following equation was utilized: SUV = tissue concentration/injected FDG dose/body weight. If focal uptake was observed, an ROI was placed on the most intense area visually. All of these images and data were processed by a Xeleris workstation system (GE Healthcare, WI, USA).
Histopathologic examination
The animals in each group were sacrificed by bloodletting after being anesthetized. The hippocampal tissue samples were taken and fixed in 10% phosphate-buffered formalin (Sigma-Aldrich Corp., MO, USA) for 48 h, then the tissues were dehydrated in various grade of ethyl-alcohol and embedded in paraffin, three-micrometer sections were cut and stained with hematoxylin-eosin, followed by observation under DVM6 optical microscope (Leica Science Lab, Berlin, Germany).
Western blotting
Animal brains were perfused with cold saline. Proteins were extracted from the ischemic cortex 24 h after reperfusion according to procedures. Protein concentration was measured with a Pierce BCA protein assay kit (23225, Thermo Scientific, MD, USA). The equivalent extracted protein was separated using SDS-PAGE and transferred onto a nitrocellulose filter membrane. Membranes for cytosolic fraction were probed for cytochrome C. The membranes were blocked in 5% non-fat milk containing 20 mm Tris-HCl, pH 7.6, 137 mm NaCl, 0.1% Tween-20 (TBS-T) and then incubated with primary antibodies against JNK1/JNK2 (1:1000, rabbit polyclonal, 27709, Abcam, CA, USA), TNF-α (1:1000, rabbit polyclonal, 1793, Abcam, CA, USA) and β-actin (1:1000, rabbit polyclonal, 6276, Abcam, CA, USA), overnight at 4°C. After washing with TBS-T, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies at room temperature for 60 min. Blots were subjected to gel formatting (BIO-RAD, CA, USA) and quantified through Quantity One analysis. β-actin was used as an internal loading control.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., IL, USA). The survival rates were analyzed using chi-square test with Fisher’s exact test when applicable. Quantitative values were expressed as mean ± SD and analyzed using two-way analysis of variance. For behavioral parameters, the data were analyzed by two-way repeated factor analysis of variance. When the analysis of variance showed significance, Student-Newman-Keul’s post hoc test was used. P < 0.05 in two-tailed testing was considered as statistically significant.
Results
MWM test results
All rats entered into the observation of MWM without sickness behavior (Figure 1A). Swimming data revealed significant effects among different groups (P < 0.01) on both latency and distance during the acquisition phase (Figure 1B and 1C). Compared with normal control group, the distance and latency to platform in model group were significantly affected (P < 0.01). Compared with HIE model group, the impaired performance on distance and latency parameters was mitigated in BYHWT 1d group, BYHWT 3d group and BYHWT 7d group (P < 0.01). These results demonstrated that, BYHWT could relieve the HIE induced spatial learning and memory impairment in rats.
Figure 1.
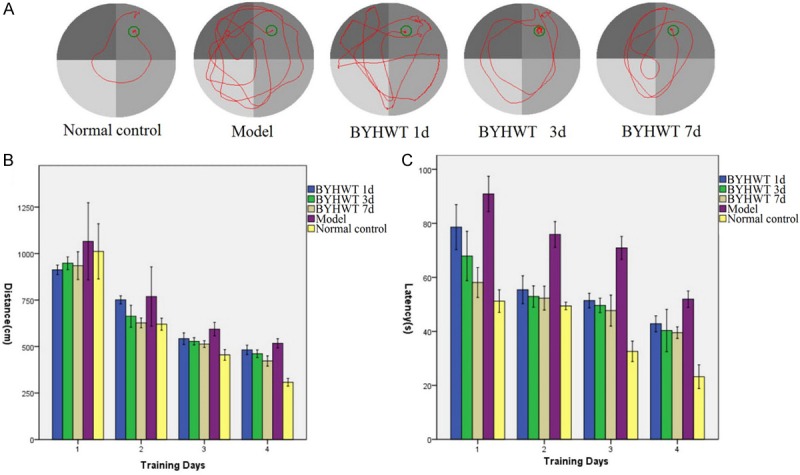
Cognitive impairment assessed by the Morris water maze test. A. Representative swim traces of different groups in reversal learning test. B. Swimming distance to platform during training days. C. Latency to the platform during training days.
Results of PET/CT imaging and histopathologic examination
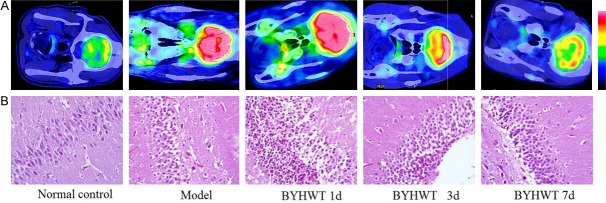
In normal control group, the 18F-FDG uptake was generally of a low grade, in addition to being diffuse. In HIE model group, the value of 18F-FDG uptake in hippocampal region increased more markedly, similar to the values in BYHWT 1d group. However, there was low FDG uptake in BYHWT 3d group, BYHWT 7d group and normal control group (Figure 2A). In model group and BYHWT 1d group, the necrosis of neuron cells and inflammatory cell infiltration in the hippocampal region were obvious, while in BYHWT 3d group and BYHWT 7d group, the apoptotic cells and inflammatory cells decreased significantly (Figure 2B).
Figure 2.
Changes in PET/CT images and histopathologic examination results. A. The value of 18F-FDG uptake in hippocampal region. B. The histopathologic examination results of hippocampal region (H&E stain, 10 × 40).
Changes in expression of JNK1/JNK2 and TNF-α protein
Western blotting showed that, compared with normal control group, the expressions of JNK1 and TNF-α protein in model group were significantly increased (P < 0.05). Compared with model group, the expressions of JNK1, JNK2 and TNF-α protein in BYHWT 7d group were significantly decreased (P < 0.05). These results indicated that, BYHWT treatment could reduce inflammatory response by antagonizing the protein expressions of JNK1/JNK2 and TNF-α (Figure 3).
Figure 3.
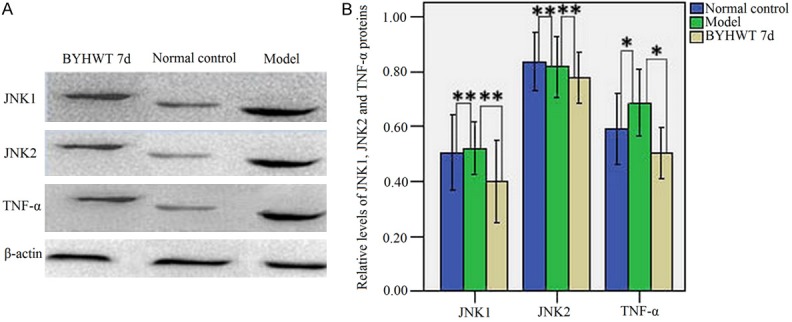
A. Western blot analysis of JNK1, JNK2 and TNF-α protein levels in the injured hemisphere from all groups. β-actin was used as a loading control. Lanes 1-3 denoted the BYHWT 7d, normal control and model group, respectively. B. Relative levels of JNK1, JNK2 and TNF-α proteins expressed as ratio of optical density to β-actin for each group. *P < 0.05, **P < 0.01.
Discussion
Brain injury in premature infants is a clinical syndrome associated with significant adverse outcomes [12]. Premature encephalopathy has multiple causes, including hypoxic-ischemia, infection, physical trauma, and so on [13]; Considerable evidence has implicated that administration of BYHWT may reduce spinal ischemia/reperfusion damage. The neuroprotective effect may be mediated, in part, by an increase in the transcription of thioredoxin [14]. In addition, accumulating evidence has suggested that the ameliorative effects of BYHWT on coronary heart disease with Qi deficiency and blood stasis syndrome in rats are mediated by the improvement of hemorheological disorders and energy metabolism [15]. A recent study has found that the BYHWT is able to protect mice against stroke and extend lifespan through the down-regulation of genes involved in inflammation, apoptosis, angiogenesis and blood coagulation, and an up-regulation of genes mediating neurogenesis and nervous system development. The changes in expression after treatment with BYHWT are beneficial after ischemic stroke [16].
In the present study, we have demonstrated that BYHWT treatment to newborn rats after HIE insult significantly reduced long-term HIE-induced brain damage and concomitantly alleviated behavioral dysfunction. To investigate the change of cognitive function after brain injury, we performed the MWM test, which is a standard measure of navigational memory and spatial orientation. Our results revealed that HIE animals had deficits of cognitive function, whereas the BYHWT treatment was shown to exert neuro-protective effects by improving cognitive impairments caused by HIE and some performance even returned to normal levels. However, the period of time at which acute injury becomes a chronic injury for the purpose of this treatment is not known. In our research, the administration treatment time was 1, 3 and 7 days, respectively. The results showed that the cognitive function was significantly improved after 3 days and 7 days of BYHWT treatment.
Glucose is the main energy resource for the brain [17], and PET with 18F-FDG has been used frequently as an in vivo measure of neuronal glucose metabolism in basic and clinical medicine [18]. The PET method provides functional analysis for multiple regions of the brain in an animal simultaneously. It has advantage over the traditional histological and biochemical analyses, including minimizing experimental animal sacrifice, and reducing workload [19]. The results combined with PET/CT and histopathologic showed that the value of 18F-FDG uptake increased more markedly in the hippocampal region and appeared neuron edema, vacuolization and the nuclear chromatin structure is not clear in HIE group. However, there was low FDG uptake in the 3d, 7d treatment group and the apoptotic cells and inflammatory cells decreased significantly.
Several studies have reported benefit with BYHWT treatment of experimental acute neonatal rodent HIE [11,20]. We supposed that BYHWT produced a suppression of proinflammatory cells during the process of infarction. To indentify this hypothesis, the effects of BYHWT treatment on JNK1/JNK2 and TNF-α protein are detected, the key proteins involved in the JNK signaling pathway and inflammation. Western blot analysis showed a lower concentration of JNK1/JNK2 and TNF-α proteins in BYHWT groups, compared to model group. This indicates that BYHWT treatment could down regulate JNK1/JNK2 and TNF-α protein expression in HI neonatal rats. Therefore, BYHWT treatment can reduce inflammatory response by antagonizing the expression of JNK and its down-stream protein JNK1/JNK2 and TNF-α.
In conclusion, BYHWT can delay the HIE onset, preserve the motor function and extend the lifespan, primarily by regulating inflammation, apoptosis and inhibition by mediating JNK signaling. The functional and mechanistic aspects of these changes in relation to specific neurobiological behaviors remains to be further studied.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81402625), Guangdong Natural Science Foundation (S2013010014720), China Postdoctoral Science Foundation (2014M550439), Southern Medical University Doctor Foundation (B1012041), and Key Program for International S&T Cooperation Projects of China (2011DFA33290).
Disclosure of conflict of interest
None.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, Cowan FM. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–2061. doi: 10.1212/WNL.0b013e31821f442d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White CR, Doherty DA, Henderson JJ, Kohan R, Newnham JP, Pennell CE. Accurate prediction of hypoxic-ischaemic encephalopathy at delivery: A cohort study. J Matern Fetal Neonatal Med. 2012;25:1653–1659. doi: 10.3109/14767058.2011.653421. [DOI] [PubMed] [Google Scholar]
- 4.Chinese Pharmacopoeia. 2005 edition. Beijing, China: Chinese Pharmacopeia Commission; 2005. [Google Scholar]
- 5.Li TF, Zhang J, Gulinuer M, Cai SQ, Lu JF. Inhibitory effects of BYHWD on ROS generation in stroke rat brain tissues. Acta Biophys Sin. 2003;19:441–447. [Google Scholar]
- 6.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 7.Li XM, Bai XC, Qin LN, Huang H, Xiao ZJ, Gao TM. Neuroprotective effects of Buyang Huanwu Decoction on neuronal injury in hippocampus after transient forebrain ischemia in rats. Neurosci Lett. 2003;346:29–32. doi: 10.1016/s0304-3940(03)00522-6. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 9.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 11.Shaw LH, Lin LC, Tsai TH. HPLC-MS/MS Analysis of a Traditional Chinese Medical Formulation of Bu-Yang-Huan-Wu-Tang and Its Pharmacokinetics after Oral Administration to Rats. PLoS One. 2012;7:e43848. doi: 10.1371/journal.pone.0043848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21. doi: 10.1016/j.brainres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Jiang DM. Neuroprotective effect of Buyang Huanwu Decoction on spinal ischemia/reperfusion injury in rats. J Ethnopharmacol. 2009;124:219–223. doi: 10.1016/j.jep.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 15.Wang WR, Lin R, Zhang H, Lin QQ, Yang LN, Zhang KF, Ren F. The effects of Buyang Huanwu Decoction on hemorheological disorders and energy metabolism in rats with coronary heart disease. J Ethnopharmacol. 2011;137:214–220. doi: 10.1016/j.jep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, Huang ST, Tsai YH, Cheng YC, Lin HJ, Shen YC. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011;138:22–33. doi: 10.1016/j.jep.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: Its constancy and functional basis. Am J Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 18.Nasrallah I, Dubroff J. An overview of PET neuroimaging. Semin Nucl Med. 2013;43:449–461. doi: 10.1053/j.semnuclmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Lancelot S, Zimmer L. Small-animal positron emission tomography as a tool for neuropharmacology. Trends Pharmacol Sci. 2010;31:411–417. doi: 10.1016/j.tips.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LH, Chen WM, Tsai TH. Identification of multiple ingredients for a Traditional Chinese Medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Molecules. 2013;18:11281–11298. doi: 10.3390/molecules180911281. [DOI] [PMC free article] [PubMed] [Google Scholar]