Abstract
MicroRNAs (miRNAs) are involved in cancer biology, and some distinctive serum miRNAs could be useful for cancer diagnosis and prognosis. However, little is known about whether serum miR-96 is a satisfactory biomarker for hepatocellular carcinoma (HCC). Four hundreds and fourteen participants were enrolled in this study, and they were divided into four age- and gender-matched groups, including the HCC group (n = 104), liver cirrhosis (LC) group (n = 90), chronic hepatitis B (CHB) group (n = 100) and healthy control group (n = 120). Serum miR-96 was measured by real-time PCR, the levels of which were calculated by the 2-ΔCt method. Serum miR-96 levels in the HCC patients were remarkably higher than in the other groups (P < 0.01), and the serum miR-96 levels discriminated HCC patients from CHB patients with an area under the ROC curve (AUC) of 0.803 (77.9% sensitivity and 75.3% specificity). Furthermore, the AUC for combined miR-96 and α-fetoprotein (AFP) was 0.889 (83.6% sensitivity and 82.4% specificity). High serum miR-96 levels in HCC patients were associated with larger tumor size, higher prevalence of lymph node metastasis, higher TNM stage and worse overall survival (OS) (P < 0.05). Our findings suggest that serum miR-96 is a promising biomarker for HCC patients with chronic HBV infection.
Keywords: Hepatocellular carcinoma, liver cirrhosis, chronic hepatitis B, biomarker, microRNA-96
Introduction
Liver cancer is the second and sixth most frequent cause of cancer-related mortality in males and females, respectively [1]. Hepatocellular carcinoma (HCC) accounts for 85% to 90% of primary liver cancers worldwide, with an epidemiological assessment of increasing incidence and higher trends in the coming decades. The major etiological factors for HCC are chronic hepatitis B and C virus (HBV, HCV) infection and alcoholic cirrhosis. HBV infection, with and without aflatoxin exposure, contributes to the vast majority of cases of liver cancer in developing countries [2]. The poor clinical outcome is due to its late stage diagnosis and limited treatment options. Serum α-fetoprotein (AFP) is the serological tumor marker for HCC and is the most widely applied marker at present. However, clinical studies have shown that AFP determination lacks adequate sensitivity and specificity for effective surveillance [3,4]. Earlier detection of HCC has been a critical challenge for clinicians and clinical researchers for years.
MicroRNAs (miRNAs) are a class of small, noncoding, regulatory RNAs that contain 20-24 nucleotides, which bind to the 3’-untranslated region (UTR) of the target mRNA and bring about its degradation or translational inhibition [5]. Deregulations of some miRNAs have been revealed to be involved in human carcinogenesis [6-8]. Recently, it has been demonstrated that miRNAs are present in the plasma or serum of animals and humans, as well as in both healthy subjects and cancer patients [9,10]. Importantly, circulating miRNAs can be sampled non-invasively, and they have a remarkable resistance to ribonuclease activity, extreme variations in pH, freeze-thaw cycles and boiling. Therefore, the study of miRNAs is becoming a field of key interest for the molecular diagnosis of cancer.
Some high-throughput technical approaches have shown the differential expression of miRNAs in HCC in contrast to normal liver tissue [11-13]. Of these miRNAs, it has been observed that miR-96, which is considered a genuine oncomiR, was up-regulated during the disease progression from a normal liver through cirrhosis to full-blown HCC [13]. In vitro, the suppression of miR-96 expression inhibits the migration and invasion of HCC cells [14], which implies that there is a correlation between miR-96 expression and the clinicopathological characteristics of HCC. To the best of our knowledge, there are limited reports on the detection of circulating miR-96 for HCC diagnosis, and it is not understood whether circulating miR-96 alteration could have clinicopathological implications.
The aim of this study was to explore whether miR-96 is detectable and altered in the serum of patients with HBV-related HCC compared with age- and gender-matched patients with chronic hepatitis B (CHB), liver cirrhosis (LC) and healthy individuals. In addition, we evaluated the potential relationship between serum miR-96 levels and the clinicopathological features and overall survival (OS) of HCC patients. Finally, we assessed whether serum miR-96 was a satisfactory biomarker for HCC patients with chronic HBV infection.
Material and methods
Ethics statement
This study was approved by the Medical Ethics Committee of Hangzhou First People’s Hospital, and all participants completed an informed consent process.
Patients
A total of 414 participants who visited Hangzhou First People’s Hospital from June 2011 to June 2014 were enrolled in this study. The patients were divided into four age- and gender-matched groups (HCC, LC, CHB patients and healthy control subjects). One hundred and four HCC patients were examined using serum α-fetoprotein (AFP), liver ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI), which revealed that they met the diagnostic criteria for HCC, and this was confirmed by histopathological examination. The HCC patients had not received any preoperative radiotherapy or chemotherapy. Ninety LC patients were diagnosed by liver ultrasound, CT and MRI, and they exhibited accompanying portal hypertension and hypersplenism. One hundred CHB patients met the diagnostic criteria based on the guidelines of prevention and treatment for CHB (2010 version) by the Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association and were without LC and HCC, as determined by liver ultrasound. One hundred and twenty healthy individuals were collected from the physical examination center of Hangzhou First People’s Hospital. In addition, HCC, LC and CHB patients were HBV surface antigen (HBsAg)-positive in serum. Patients who presented with other liver diseases, such as autoimmune hepatitis, alcoholic hepatitis and other types of hepatitis virus infection, were excluded from this study.
Serum collection and miRNA extraction
The serum samples of all participants were collected in the fasting state on the first visiting day. The separated sera were stored at -80°C before analysis. To each 250 μL of serum, 750 μL of TRIzol LS (Invitrogen, Carlsbad, California, USA) was added, followed by 200 μL chloroform. The mixture was then centrifuged. After supernatant separation and precipitation with isopropanol, the pellet was washed twice with 75% diethylpyrocarbonate (DEPC)-treated ethanol, air-dried, and dissolved in 20 μL RNase-free water. DNase (Qiagen, Hilden, Germany) was added to remove the residual DNA.
Serum miR-96 detection by quantitative real-time PCR
Complement DNA was synthesized using the miRCURY LNA™ Universal RT miRNA PCR kit (Exiqon, Vedbaek, Denmark). Reverse transcriptase products were used as templates for the next PCR process after a 1:5 dilution and were detected in 10 μL PCR reactions according to the protocol for miRCURY LNA™ Universal RT miRNA PCR kit. The primers of miR-96 and miR-16 for PCR were designed and synthesized commercially (Exiqon, Vedbaek, Denmark). Deionized water, as the negative control, was treated similarly to the samples. All amplifications were assayed with SYBR Green on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, California, USA) under the following cycling conditions: 95°C for 1 min, followed by 45 cycles of 95°C for 10 s and 60°C for 1 min. All reactions were run in triplicate, including no-template controls.
The cycle threshold (CT) is defined as the number of cycles required for the SYBR Green signal to cross the threshold in real-time PCR. The relative expression levels of miR-96 were calculated by the 2-ΔCt method, as previously described [15]. MiR-16 was used as the endogenous control to normalize the data [16].
Statistical analysis
Non-parametric variables are presented as the median and interquartile ranges (IQR) and were analyzed using the Mann-Whitney test. Categorical variables were compared by the X2 test. Receiver-operating-characteristic (ROC) curves were constructed, and the areas under the ROC curves (AUCs) were estimated to determine the feasibility of using serum miR-96 as a biomarker for HCC. OS curves were calculated using the Kaplan-Meier method. A value of P < 0.05 was considered statistically significant. Data analyses were performed using SPSS software (version 15; SPSS Inc., Chicago, USA).
Results
Clinical characteristics of study cohort
A total of 414 participants, including 104 HCC patients, 90 LC patients, 100 CHB patients and 120 healthy subjects, were recruited. Their baseline clinical features are listed in Table 1. The HCC group had significantly different laboratory results from the other three groups with respect to ALT, albumin, TBIL and blood platelet count (P < 0.05). In addition, there were no differences in age, gender and the other laboratory results among the 4 groups.
Table 1.
Baseline clinical characteristics of the study cohort
| Parameters | Healthy subjects (n = 120) | CHB patients (n = 100) | LC patients (n = 90) | HCC patients (n = 104) |
|---|---|---|---|---|
| Age (yr) | 47.9 (34, 59) | 48.9 (35, 62) | 46.1 (36, 60) | 47.6 (33, 64) |
| Gender (Male %) | 80 (66.7) | 66 (66.0) | 58 (64.4) | 68 (65.4) |
| ALT (U/L) | 25 (8, 41) | 71 (30, 151) | 97 (39, 203) | 63 (16, 358) |
| Albumin (g/L) | 40.1 (35.2, 49.8) | 38.1 (30.6, 47.1) | 28.1 (18.4, 31.7) | 33.3 (18.8, 35.8) |
| TBIL (μmol/L) | 9.8 (3.1, 16.5) | 50.2 (9.2, 96.8) | 76.4 (9.2, 153.6) | 26.5 (5.3, 137.2) |
| Blood platelet count (×109/μL) | 19.2 (9.6, 35.7) | 15.8 (5.4, 23.1) | 5.3 (1.2, 12.8) | 9.6 (3.7, 19.5) |
| HBeAg (+) | 0 (0) | 56 (56.0) | 50 (55.6) | 54 (51.9) |
| HBV DNA (log10 copies/mL) | 0 | 5.9 (4.8, 7.1) | 5.8 (4.5, 6.8) | 5.7 (3.8, 7.5) |
Abbreviations: CHB, chronic hepatitis B; LC, liver cirrhosis; HCC, hepatocellular carcinoma; ALT, alanine aminotransferase; TBIL, total bilirubin; HBeAg, HBV e-antigen. There were significantly different levels of ALT, albumin, TBIL and blood platelet count among the 4 groups (P < 0.05). All data are presented as the median (IQR) or n (%).
The levels of serum miR-96
MiR-96 levels were investigated in the 4 groups, which included 414 serum samples, by real-time PCR. The levels of serum miR-96 were 0.194 (0.072, 0.436) in HCC patients (n = 104); 0.105 (0.053, 0.249) in LC patients (n = 90); 0.063 (0.044, 0.120) in CHB patients (n = 100); and 0.058 (0.012, 0.071) in healthy subjects (n = 120). The serum miR-96 levels in HCC patients were remarkably higher than in the other groups (P < 0.01), and those levels in the LC patients were clearly higher than in both the CHB patients and the healthy individuals (P < 0.05). However, no differences were found between the CHB patients and the healthy individuals (P > 0.05) (Figure 1).
Figure 1.
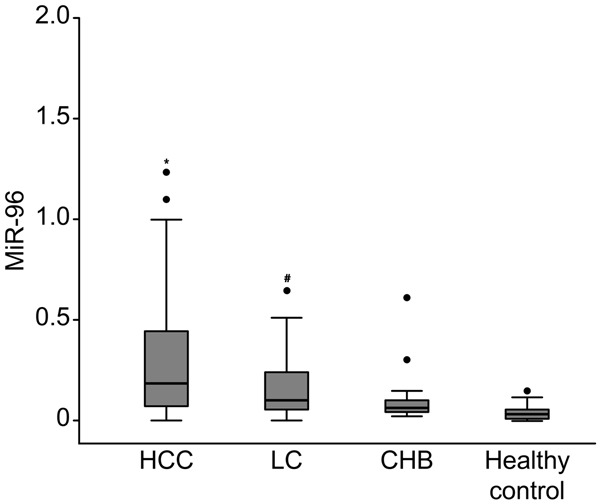
The differential serum levels of miR-96 in the HCC group (n =104), LC group (n = 90), CHB group (n = 100) and healthy control group (n = 120). P* < 0.01 versus LC, CHB and healthy control group; P# < 0.05 versus CHB and healthy control group.
Evaluation of serum miR-96 as a potential HCC diagnostic marker
To explore whether serum miR-96 could be a potential diagnostic marker for HCC, ROC curve analyses were performed (Figure 2). Serum miR-96 discriminated HCC patients from CHB patients with an AUC of 0.803 (95% CI: 0.751-0.894). At the cut-off value of 0.138, the sensitivity and specificity for serum miR-96 was 77.9% and 75.3%, respectively. Serum AFP at the cut-off value of 20 ng/mL yielded an AUC of 0.713 (95% CI: 0.572-0.795) with 73.2% sensitivity and 61.3% specificity. Furthermore, an AUC for the combination of serum miR-96 and AFP was 0.889 (95% CI: 0.761-0.913) with 83.6% sensitivity and 82.4% specificity.
Figure 2.
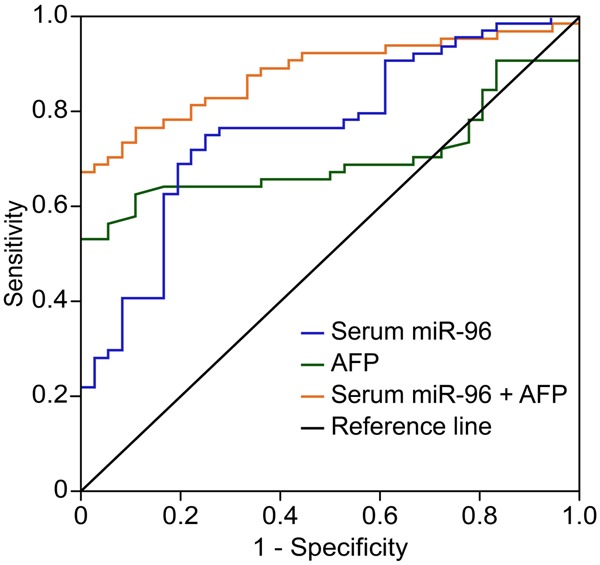
Receiver operating characteristics (ROC) curve analyses using serum miR-96 for discriminating HCC patients from CHB patients. Serum miR-96 at the cut-off value of 0.138 yielded an AUC (the area under the ROC curve) of 0.803 (95% CI: 0.751-0.894) with 77.9% sensitivity and 75.3% specificity for discriminating HCC patients from CHB patients. Serum AFP at the cut-off value (≥ 20 ng/mL) yielded an AUC of 0.713 (95% CI: 0.572-0.795) with 73.2% sensitivity and 61.3% specificity. Combined serum miR-96 and AFP yielded an AUC of 0.889 (95% CI: 0.761-0.913) with 83.6% sensitivity and 82.4% specificity.
Relationship between serum miR-96 levels and clinicopathological features in HCC patients
We examined the relationship between serum miR-96 levels and clinicopathological parameters in 104 HCC patients. No significant associations were found between serum miR-96 levels and gender, age, tumor number, histological grade and cirrhosis (P > 0.05), while the levels of serum miR-96 showed associations with tumor size, lymph node metastasis and TNM stage (P < 0.05) (Table 2).
Table 2.
Relationship between serum miR-96 levels and clinicopathological characteristics of HCC patients
| Characteristics | n | Serum miR-96 level | P value |
|---|---|---|---|
| Gender | |||
| Male | 68 | 0.186 (0.065, 0.425) | 0.203 |
| Female | 36 | 0.199 (0.073, 0.476) | |
| Age (yr) | |||
| ≥ 50 | 64 | 0.196 (0.082, 0.429) | 0.357 |
| < 50 | 40 | 0.189 (0.064, 0.452) | |
| Tumor number | |||
| Single | 38 | 0.183 (0.071, 0.402) | 0.188 |
| Multiple | 66 | 0.197 (0.077, 0.461) | |
| Tumor size (cm) | |||
| < 5 | 56 | 0.117 (0.057, 0.365) | 0.036 |
| ≥ 5 | 48 | 0.206 (0.079, 0.516) | |
| Histological grade | |||
| I+II | 26 | 0.188 (0.066, 0.411) | 0.115 |
| III+IV | 78 | 0.201 (0.087, 0.506) | |
| Lymph node metastasis | |||
| Present | 62 | 0.204 (0.091, 0.504) | 0.043 |
| Absent | 42 | 0.167 (0.063, 0.385) | |
| TNM stage | |||
| I+II | 34 | 0.172 (0.061, 0.404) | 0.027 |
| III+IV | 70 | 0.224 (0.095, 0.529) | |
| Cirrhosis | |||
| Present | 76 | 0.199 (0.063, 0.486) | 0.131 |
| Absent | 28 | 0.175 (0.075, 0.423) |
Abbreviations: HCC, hepatocellular carcinoma. The histological examination was conducted by referencing the standard of Edmondson grade. Tumor stage was classified according to the TNM criteria of the International Union Against Cancer. The levels of serum miR-96 are presented as median (IQR).
Association between serum miR-96 levels and HCC survival
In this study, the clinical cases for 49 HCC patients were followed for 30 months. According to the cut-off value of miR-96 (0.138), HCC patients were divided into two subsets. Thirty patients whose serum miR-96 levels were higher than 0.138 were classified into the high-level subset, and 19 patients whose serum miR-96 levels were lower than 0.138 were classified into the low-level subset. Using the Kaplan-Meier analysis, our results demonstrated that the serum miR-96 level was a prognostic factor in 49 HCC patients. High serum miR-96 levels were associated with worse OS in HCC patients (P < 0.05) (Figure 3).
Figure 3.
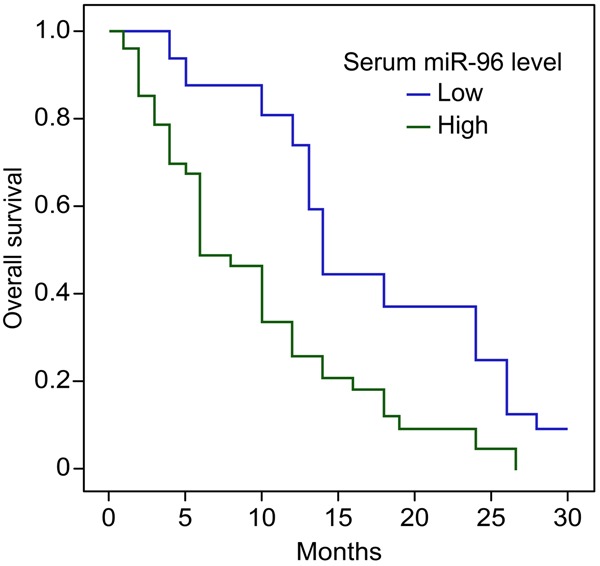
Kaplan-Meier analysis of overall survival (OS) of 49 HCC patients. Forty-nine HCC patients were followed for 30 months. According to the serum miR-96 levels, 30 HCC patients were stratified to serum high-level subset (more than 0.138), and 19 HCC patients to serum low-level subset. Kaplan-Meier analysis showed high serum miR-96 levels were associated with worse OS (Log Rank = 4.347, P = 0.038).
Discussion
HCC remains one of the most common and malignant tumors in humans worldwide, and it has a 5-year survival rate of approximately 10% [17]. HCC occurs mainly in patients with chronic HBV infection in certain areas, including Southeast Asia and Sub-Saharan Africa. These high-risk patients should have regular clinical exams. Satisfactory diagnostic methods for HCC have not been achieved, which contributes to the low survival rate of HCC. AFP, as a serological biomarker, is the most commonly used biomarker for screening HCC. However, the sensitivity of HCC diagnosis is 60-80% with a 20 ng/mL AFP cut-off value, and the sensitivity decreases to 20-40% for the detection of small tumors [18]. Over the last several years, an increased understanding of the molecular mechanisms underlying HCC initiation and progression has provided useful clinical data, not only to its pathogenesis but also to its prognosis and treatment efficacy. Moreover, some novel biomarkers, including miRNAs, have been suggested to be useful for HCC diagnosis and prognosis [19].
MiR-96, together with miR-182 and miR-183, belongs to the miR-183-96-182 cluster. Their seed regions (UUGGCA, nucleotides 2-7) are identical, which suggests potential general properties in mRNA target recognition and cellular functions [20]. Recently, some studies have demonstrated that the aberrant expression of miR-96 could play important roles in tumorigenesis and tumor progression, including hepatoma, gastric cancer, breast cancer and glioma [21-24]. In HepG2 hepatoma cells [21], the overexpression of miR-96 induces cell proliferation and clonogenicity. In addition, miR-96 promotes the migration and invasion of HCC cells (HCCLM6) via the modulation of osteopontin expression in vitro [14]. The majority of the differential expression of miRNAs in serum can reflect the changes in the corresponding tumor tissues [25], which implies that miRNAs should be widely applicable in clinical noninvasive diagnoses.
In this study, we detected the levels of serum miR-96 in 104 patients with HCC, 90 patients with LC, 100 patients with CHB and 120 healthy subjects, and we found that the levels of miR-96 in the HCC group were significantly higher than those in the other groups, which is consistent with the results of liver tissues [13]. In addition, we found that the levels of miR-96 in the LC patients were clearly higher than those in the CHB patients and healthy individuals. This finding suggests that the overexpression of miR-96 may be an early event during HCC development. We also tested whether serum miR-96 could be a powerful predictive tool for distinguishing patients with HCC from those with CHB. Our study showed that an AUC (0.803) of serum miR-96 at the cut-off value of 0.138 was superior to the AUC (0.713) of AFP at the cut-off value of 20 ng/mL. Combined serum miR-96 and AFP analysis yielded an AUC up to 0.889. Therefore, our results suggest the potential clinical value of serum miR-96 in the diagnosis of HCC.
Some distinctive miRNA expressions have been found to be associated with clinical progression and prognostic factors in several cancers [9,10,16]. In the present study, we analyzed the relationship between serum miR-96 levels and the clinicopathological characteristics in HCC. Our results indicate that a high level of serum miR-96 is associated with higher tumor size, higher prevalence of lymph node metastasis and higher TNM stage, which represent more aggressive clinical phenotypes and tend to have a larger tumor burden. Finally, the correlation of serum miR-96 levels with the prognosis of HCC patients was evaluated, and we found that patients with high serum miR-96 levels showed worse OS than those with low miR-96 levels, which revealed that the level of serum miR-96 has an important predictive value in HCC prognosis classification.
In conclusion, this study demonstrated that serum miR-96 levels were increased and had a satisfactory diagnosis performance in HCC patients with chronic HBV infection. Furthermore, a high serum miR-96 level was closely correlated with more aggressive clinical characteristics and poorer prognosis of HCC. Our findings suggest that serum miR-96 may be a novel biomarker for HCC patients with chronic HBV infection.
Acknowledgements
The authors acknowledge that this work was supported by a grant from the Hangzhou board of health, Zhejiang province (2012Z004), and the authors are thankful for this grant.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339–346. doi: 10.3748/wjg.v19.i3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Salvi A, Sabelli C, Moncini S, Venturin M, Arici B, Riva P, Portolani N, Giulini SM, De Petro G, Barlati S. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J. 2009;276:2966–2982. doi: 10.1111/j.1742-4658.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 8.Elyakim E, Sitbon E, Faerman A, Tabak S, Montia E, Belanis L, Dov A, Marcusson EG, Bennett CF, Chajut A, Cohen D, Yerushalmi N. hsa-miR-191 is a candidate oncogene target for hepatocellular carcinoma therapy. Cancer Res. 2010;70:8077–8087. doi: 10.1158/0008-5472.CAN-10-1313. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, Tian W, Zhang Q, Wang C, Zhang Q, Zhuang SM, Zheng L, Liang A, Tao W, Cao X. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, Pan Z, Hu X, Zhao Y, Xie H, Jiang G, Chen T, Wang J, Zheng S, Cheng J, Wan D, Yang S, Li Y, Gu J. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 13.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen RX, Xia YH, Xue TC, Ye SL. Suppression of microRNA-96 expression inhibits the invasion of hepatocellular carcinoma cells. Mol Med Rep. 2012;5:800–804. doi: 10.3892/mmr.2011.695. [DOI] [PubMed] [Google Scholar]
- 15.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 16.Qi P, Cheng SQ, Wang H, Li N, Chen YF, Gao CF. Serum microRNAs as biomarkers for hepatocellular carcinoma in Chinese patients with chronic hepatitis B virus infection. PLoS One. 2011;6:e28486. doi: 10.1371/journal.pone.0028486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Ho DW, Lee NP, Sun S, Lam B, Wong KF, Yi X, Lau GK, Ng EW, Poon TC, Lai PB, Cai Z, Peng J, Leng X, Poon RT, Luk JM. Enhanced detection of early hepatocellular carcinoma by serum SELDI-TOF proteomic signature combined with alpha-fetoprotein marker. Ann Surg Oncol. 2010;17:2518–2525. doi: 10.1245/s10434-010-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramantieri L, Fornari F, Callegari E, Sabbioni S, Lanza G, Croce CM, Bolondi L, Negrini M. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12:2189–2204. doi: 10.1111/j.1582-4934.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalvy-Delvaille S, Maurel M, Majo V, Pierre N, Chabas S, Combe C, Rosenbaum J, Sagliocco F, Grosset CF. Molecular basis of differential target regulation by miR-96 and miR-182: the Glypican-3 as a model. Nucleic Acids Res. 2012;40:1356–1365. doi: 10.1093/nar/gkr843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C, Jiang X, Sun S, Lin J. Inhibition of miR-96 expression reduces cell proliferation and clonogenicity of HepG2 hepatoma cells. Oncol Rep. 2013;29:653–661. doi: 10.3892/or.2012.2138. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Zheng D, Hu P, Zeng Z, Li M, Tucker L, Monahan R, Resnick MB, Liu M, Ramratnam B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988–2998. doi: 10.1093/nar/gkt1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu C, Li J, Wang X, Song L. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS One. 2010;5:e15797. doi: 10.1371/journal.pone.0015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang H, Bian Y, Tu C, Wang Z, Yu Z, Liu Q, Xu G, Wu M, Li G. The miR-183/96/182 cluster regulates oxidative apoptosis and sensitizes cells to chemotherapy in gliomas. Curr Cancer Drug Targets. 2013;13:221–231. doi: 10.2174/1568009611313020010. [DOI] [PubMed] [Google Scholar]
- 25.Le HB, Zhu WY, Chen DD, He JY, Huang YY, Liu XG, Zhang YK. Evaluation of dynamic change of serum miR-21 and miR-24 in pre- and post-operative lung carcinoma patients. Med Oncol. 2012;29:3190–3197. doi: 10.1007/s12032-012-0303-z. [DOI] [PubMed] [Google Scholar]


