Abstract
Objective: The effect of miR-449 and miR-34 on the growth, cell cycle and target gene expressions of ovarian cancer cell line SKOV3 and SKOV3-ipl was discussed. Method: Real-time quantitative reverse transcription PCR was employed to detect the expressions of miR-449a/b and miR-34b, c in SKOV3 and SKOV3-ipl cells. The two miRNAs were successfully expressed in SKOV3-ipl cells by transfection. The variations in cell growth rate and cell cycle were determined by MTS assay and flow cytometry, respectively. The expressions of cell cycle-related proteins were detected by Western Blot. Results: miR-449b and miR-34c induced the decline of the adhesiveness of SKOV3-ipl cells by 20%-30%. The number of cells arrested in G1-phase increased and the number of cells arrested in S-phase decreased significantly. The cell cycle-related proteins CDK6 and CDC254 were downregulated. miR-449b caused the expression of CDK6 and CDC25A to decrease. After the co-transfection with miR-449b and miR-34c, the relevant proteins were downregulated more significantly. The expressions of CDK6, CDC25A and cyclin A were decreased significantly. Conclusion: miR-449b and miR-34c can induce cell cycle arrest in SKOV3-ipl cells and the downregulation of CDK6, CDC25A and cyclin A.
Keywords: Ovarian tumor, cell cycle-related proteins, microRNAs
Introduction
MicroRNA (miRNA) is a type of small, non-encoding RNA with the function of negatively regulating gene expression on both posttranscriptional and translational level. The abnormal expression of miRNA is associated with a variety of humor tumors, showing the effect similar to tumor suppressor genes or oncogenes. Recent studies indicate that miR-34 family members (including miR-34a, b, c) are involved in p53 signaling pathway. They have tumor-inhibitory effect in the downstream of p53 pathway [1-3]. In response to DNA damage or carcinogenic stress, miR-34 is upregulated in a p53-dependent manner [1,4]. The overexpression of miR-34 in p53-deficient cells can produce similar effect as p53. That is, the cell proliferation and the expression of anti-apoptotic genes are inhibited, and the cell cycle arrest, cell apoptosis or aging are induced [5,6]. miR-34a and miR-34b, c share some common functions but have different expression patterns in different tissues. miR-34a is expressed in many tissues, whereas miR-34b, c is mainly expressed in fallopian tubes, testes, lungs and trachea [7]. Their relative expressions also vary in different tumor cell lines. After the activation of p53, miR-34a and miR-34b, c can be significantly upregulated in different cells [8].
Advanced-stage serous ovarian cancer is the most common pathological type. The patients may already develop late-stage ovarian cancer when diagnosed. The mutation of p53 gene is the main form of molecular change in advanced-stage ovarian cancer. About 50%-80% of advanced-stage ovarian cancers are found to have p53 mutation [9-11], and miR-34b, c are downregulated in advanced-stage ovarian cancers [12,13]. There are increasing evidences to show that advanced-stage ovarian cancers may derive from the fallopian tubes. miR34b,c are involved in p53 signaling pathway and exhibit a high expression in the fallopian tubes, thus miR34b,c may play a key role in ovarian cancer.
The second to the eighth bases of miRNA (or seed sequence) have a decisive impact on the relevant target genes. miR-449a, b and miR-34a, b, c share the common seed sequences and are also expressed in fallopian tubes, testes, lungs and trachea like miR34b, c. It is inferred that they possess the same or similar gene-regulating function.
SKOV3 cell line, which is established in human ovarian serous cystadenocarcinoma, carries the mutation of p53 gene. SKOV3-ipl cell line is a cell subline that is isolated from the ascites of tumor-bearing nude mice seeded with SKOV3 cell line and has a greater invasiveness. By real-time quantitative PCR the expressions of miR-449a, b and miR-34b, c in the two cell lines were detected. The results showed that the expressions of miR-449a, b and miR34b, c were lower in SKOV3-ipl cell line than in SKOV3 cell line. Thus SKOV3-ipl cell line was chosen for subsequent experiment. Four pre-miRNA sequences of miR-449a, miR-449b, miR34b and miR-34c were used to transfect the cells separately or simultaneously. The morphology and proliferation of tumor cells and the expressions of cell cycle-related proteins were analyzed after miRNA transfection. The role of these miRNAs in the occurrence and progress of gastric cancer was investigated. Since no ovarian cancer cell line without p53 mutation had been established, we did not perform miR-499 or miR-34 knockout experiments.
Materials and methods
Materials
SKOV3 and SKOV3-ipl cell lines were purchased from Sichuan University. The pre-miRNA sequences and the transfection agent siPORT NeoFX were purchased from Ambion Company. Rat anti-human CDK6 and CDC25A antibodies and HRP-labeled secondary antibodies were purchased from Guangmai’er Company. Rabbit anti-human cyclin A was manufactured by Wuhan Boshide Biological Engineering Co., Ltd. The cell culture medium and fetal bovine serum (FBS) were manufactured by Invitrogen (USA).
Extraction and reverse transcription of miRNA and real-time quantitative PCR
McCoy’s 5A medium containing 10% FBS was used for the culture and passage of SKOV3 and SKOV3-ipl cells. miRNA extraction, tail adding, reverse transcription, primer design, real-time quantitative PCR detection and analysis were done according to the methods published previously [13,14].
Transfection of SKOV3-ipl cells by pre-miRNA sequences
In accordance with the manufacturer’s instruction of siPORT NeoFX, SKOV3-ipl cells were transfected with pre-miR-449a, pre-miR-449b, pre-miR-34b and pre-miR-34c separately and simultaneously at the concentration of 30-50 nmol/L. For the negative control group, CyTM3-labeled pre-miRNA transfection was conducted (Ambion). The transfection was confirmed as successful through the red fluorescence and real-time quantitative PCR. The cells obtained were named as SKOV3-ipl-neg, SKOV3-ip1-449a, SKOV3-ip1-449b, SKOV3-ipl-34b, SKOV3-ipl-34c, SKOV3-ip1-449a+449b and SKOV3-ip1-449b+34c, respectively.
Detection of cell proliferation and adhesion
The cells were seeded to 96-well plates. The miRNA-transfected cells were cultured for 24 h and 48 h, respectively. Then the culture medium was discarded, which was followed by the addition of 100 μL of fresh medium and 20 μL of MTS (Promega). After incubation at 37°C for 2 h, the optical density (OD) value was read at 490 nm. Cell adhesion experiment was carried out. The cells at 48 h after transfection were digested with trypsin to prepare cell suspension, which was seeded to 96-well plates (1×105 cells per well) for 1.5 h at 37°C. The non-adherent cells were discarded. The adherent cells were washed with PBS buffer for once and further incubated in 100 μL of fresh medium and 20 μL of MTS at 37°C for 2 h. The variations of the number of adherent cells were determined by OD value at 490 nm.
Cell cycle detection by flow cytometry
The cells were harvested at 24 h and 48 h after miRNA transfection at the amount of about 5×105, respectively. BD Cycletest PLUS DNA Reagent Kit was used in accordance with the instruction.
Detection of the cell cycle-related proteins
Given the results of the above experiments, only the joint effect of miR-449b and miR-34c or miR-449b and miR-34c was tested, with the miRNA concentration raised to 50 nmol/L [14]. Western Blot was conducted conventionally, and the antibodies were added according to the recommended concentration. The emitted fluorescence in the presence of horseradish peroxidase was captured by GE Image Quant 350 Digital Imaging System. The expressions of CDK6, CDC25A and cyclin A were analyzed by Image Quant TL 7.0 Image Analysis Software (relative content expressed as OD value).
Data analysis
Each experiment had at least 3 replicates, and the data were expressed as Mean ± SD. SPSS 13.0 software was used for the comparison of intergroup differences by analysis of variance. Pairwise intergroup difference was analyzed by S-N-K test.
Results
The expressions of miR-449a/b and miR-34a, b, c were much lower in SKOV3-ipl cells than in SKOV3 cells
Real-time quantitative PCR indicated that the expression levels of miR-449a/b and miR-34a, b, c were obviously lower in more malignant SKOV3-ipl cells than in SKOV3 cells (Figure 1). The sequences of miR-449a and miR-449b only differed in 2 bases at the head and the tail, so they were amplified by the same primers. The expression level detected was the sum of the two, represented as miR-449a/b.
Figure 1.
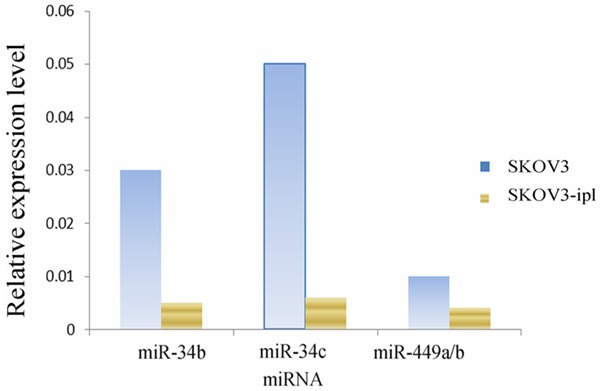
The expression of microRNA in SKOV3 and SKOV3-ipl.
Specific miRNA expressions after transfection of SKOV3-ipl cells
The transfection was proved to be successful through the red fluorescence emitted from the pre-miRNA sequences in the negative control and real-time quantitative PCR.
Effect of miR-449a, b and miR-34b, c on the proliferation and adhesion of SKOV3-ipl cell.
The SKOV3-ipl cells were transfected with miR-449a, 449b, 34b, 34c separately or simultaneously. MTS assay did not show obvious changes in proliferation rate. However, miR-449b and miR-34c caused the decrease of adhesiveness of SKOV3-ipl cells. The duration of trypsin digestion was shortened, and the number of adherent cells declined. No obvious changes were found in proliferation rate after co-transfection with miR-449b and miR-34c. The effect of co-transfection on the adhesion of cancer cells was comparable to that of transfection with either miR-449b or miR-34c alone.
miR-449b and miR-34c caused cell cycle arrest in SKOV3-ipl cells
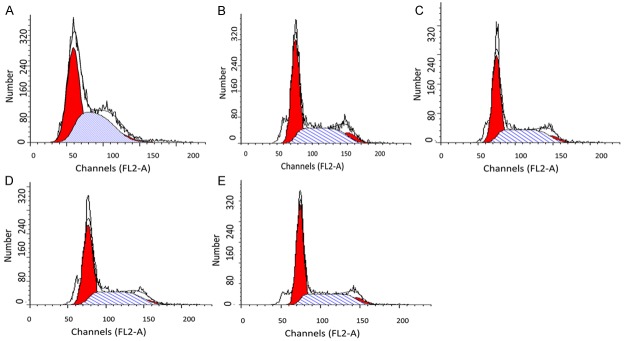
The SKOV3-ipl cells were transfected with miR-449a, 449b, 34b, 34c (30 nmol/L), respectively. Flow cytometry was conducted at 24 h and 48 h after transfection, and cell cycle arrest was observed at both time points (Table 1; Figure 2). miR-449b and miR-34c transfections showed differences of statistical significance from the control group (P<0.05). The number of cells arrested in G1 phase increased significantly. The number of cells arrested in S phase decreased significantly. The concentration of miR-449a was raised to 45 nmol/L (denoted as miR-449a+), and the co-transfections with miR-449a and 449b or miR-34b and 34c were performed at this concentration. However, the effect in cell cycle arrest did not exceed that of transfection with miR-449b or miR-34c alone. miR-449a+ transfection had a greater effect in cell cycle arrest than miR-449a tranfection, and was comparable to that of miR-449b or miR-34c transcription. The co-transfection with miR-449b and miR-34c at 45 nmol/L led to similar effect in cell cycle rest as that by miR-449b or miR-34c transfection.
Table 1.
Comparison of cell cycle changes
| Cell cycle | Control | miR-449a | miR-449b | miR-34b | miR-34c |
|---|---|---|---|---|---|
| G1 | 50.2±14.3 | 67.1±13.2 | 72.1±12.1* | 60.8±15.2 | 70.5±12.4* |
| S | 23.3±11.1 | 24.5±11.3 | 13.3±7.4* | 21.4±10.1 | 11.6±8.4* |
Compared to control group;
P<0.05.
Figure 2.
FCM results indicated the cell number increases in S of SKOV3-ipl transfected with miR-449a, miR-449b, and miR-34c, respectively. A. SKOV3-negative control; B. SKOV3-ipl-449a; C. SKOV3-ipl-449b; D. SKOV3-ipl-34b; E. SKOV3-ipl-34c.
miR-449b and miR-34c caused the downregulation of cell cycle-related proteins
SKOV3-ipl cells were co-transfected with miR-449b and miR-34c or miR-449b and miR-34c. The results of Western Blot indicated that the cell cycle-related proteins CDK6 and CDC25A were downregulated. However, the miR-449b and miR-34c transfections did not show obvious differences with respect to the downregulation of the proteins. miR-449b transfection caused the expression of CDK6 and CDC 25A to decrease. Also, miR-34c transfection caused the expression of CDK6 and CDC25A to decrease significantly. Cyclin A was obviously downregulated only under the joint effect of miR-449b and miR-34c (Figure 3). Real-time quantitative PCR did not reveal considerable changes of the mRNA levels of the cell cycle-related proteins after miR-449b or miR-34c transfection.
Figure 3.
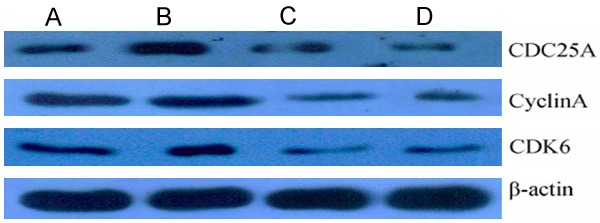
The result of western blot shows down-regulated CDK6, CyclinA, CDC25A 48 h after transfection of miR-449b, miR-34c, and miR-449b mixed with miR-34c. A. SKOV3-ipl-neg; B. SKOV3-ipl-449b; C. SKOV3-ipl-34c; D. SKOV3-ipl-449b+34c.
Discussion
SKOV3-ipl cell line is a mutant SKOV3 cell line isolated from ascites and has a greater invasiveness. In vitro culture it showed higher adhesiveness, faster adherence to wall and longer time needed for trypsin digestion. miR-449a/b and miR-34b, c showed an obviously decreased expression in SKOV3-ipl cells with higher malignancy than in SKOV3 cells. After transfection with miR-449b and miR-34c, the adhesiveness of SKOV3-ipl cells decreased, indicating the important role of the loss of function of miR-449 and miR-34 in the malignant progression of ovarian cancer cells. The p53 gene mutation in SKOV3 cells may cause the downregulation of miR-449a/b and miR-34b, c. During malignant progression, other gene mutations can lead to further downregulation of miR-449a/b and miR-34b, c.
miR-449x, b and miR-34a, b, c share common seed sequences. According to computer-based prediction, their common target genes amount to over 400 (Pictar. mdc-berlin). The target genes that have been experimentally confirmed in other cells include CDK6 and CDC25A. We demonstrated that the overexpression of miR-449b and miR-34 in SKOV3-ipl cells resulted in the downregulation of CDK6 and CDC25A. However, no obvious changes were detected on mRNA level, which conforms to the rule that miRNA regulation mainly occurs on the translational level rather than on the posttranscriptional level. Given the fact that serous ovarian cancer may derive from the fallopian tubes, it is speculated that the overexpression of miR-449a, b and miR-34b, c in the fallopian tubes has a close connection with ovarian cancer.
miR-449a, b genes are in tandem arrangement on No. 5 chromosome, while miR-34b, c are in tandem arrangement on No. 11 chromosome. They share the common target genes and seed sequences, so they may function in synergy in principle. Our results also indicated that the joint effect of miR-449b and miR-34c was greater than the effect of either miR-449b or miR-34c transfected alone in the downregulation of cell cycle-related proteins. However, flow cytometry detection did not reveal the synergistic effect between them. The reason may be that the higher miRNA concentration of the former enhanced the downregulation, or that some weak cells were lost due to trypsin digestion. But no relevant reports have been known so far.
We also found that the joint effect of miR-449b and miR-34c caused the obvious downregulation of cyclin A. However, sequence alignment or database searching failed to prove whether cyclin A was the direct target of miR-449b or miR-34c. Cyclin A is the protein mainly produced in the S phase of cells [15]. We speculated that the reduced number of cells arrested in S phase was caused by the overexpression of miR-449b and miR-34c. The latter indirectly led to the downregulation of cyclin A.
miR-34b and miR-34c are involved in p53 pathway, which are the direct target of p53. No reports have confirmed whether miR-449a/b is directly regulated by p53. Since p53 plays a crucial role in the complex molecular network related to tumor formation, p53 mutation may be associated with tumor progression or poor prognosis. Mutant p53 is the major molecular change in advanced-stage serous ovarian cancer [9]. The overexpression of miR-34b, c and miR-449a, b can lead to similar effect as p53 mutation, including cell cycle arrest and inhibition of cancer cells. miRNA has a small molecular weight and is easy to manipulate. All these features of miRNA can be exploited for the treatment of ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Lai M, Du G, Shi R, Yao J, Yang G, Wei Y, Zhang D, Xu Z, Zhang R, Li Y, Li Z, Wang L. MiR-34a inhibits migration and invasion by regulating the SIRT1/p53 pathway in human SW480 cells. Mol Med Rep. 2015;11:3301–7. doi: 10.3892/mmr.2015.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollock A, Bian S, Zhang C, Chen Z, Sun T. Growth of the developing cerebral cortex is controlled by microRNA-7 through the p53 pathway. Cell Rep. 2014;7:1184–96. doi: 10.1016/j.celrep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dar AA, Majid S, Rittsteuer C, de Semir D, Bezrookove V, Tong S, Nosrati M, Sagebiel R, Miller JR 3rd, Kashani-Sabet M. The role of miR-18b in MDM2-p53 pathway signaling and melanoma progression. J Natl Cancer Inst. 2013;105:433–42. doi: 10.1093/jnci/djt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, He XJ, Ma LP, Li N, Yang J, Cheng YX, Cui H. Expression and significance of microRNAs in the p53 pathway in ovarian cancer cells and serous ovarian cancer tissues. Zhonghua Zhong Liu Za Zhi. 2011;33:885–90. [PubMed] [Google Scholar]
- 5.He X, He L, Hannon GJ. The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 6.Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–43. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Nadal E, Chen G, Gallegos M, Lin L, Ferrer-Torres D, Truini A, Wang Z, Lin J, Reddy RM, Llatjos R, Escobar I, Moya J, Chang AC, Cardenal F, Capellà G, Beer DG. Epigenetic inactivation of microRNA-34b/c predicts poor disease-free survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2013;19:6842–52. doi: 10.1158/1078-0432.CCR-13-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–30. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 9.Erickson BK, Kinde I, Dobbin ZC, Wang Y, Martin JY, Alvarez RD, Conner MG, Huh WK, Roden RB, Kinzler KW, Papadopoulos N, Vogelstein B, Diaz LA Jr, Landen CN Jr. Detection of somatic TP53 mutations in tampons of patients with high-grade serous ovarian cancer. Obstet Gynecol. 2014;124:881–5. doi: 10.1097/AOG.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brachova P, Mueting SR, Carlson MJ, Goodheart MJ, Button AM, Mott SL, Dai D, Thiel KW, Devor EJ, Leslie KK. TP53 oncomorphic mutations predict resistance to platinum- and taxane-based standard chemotherapy in patients diagnosed with advanced serous ovarian carcinoma. Int J Oncol. 2015;46:607–18. doi: 10.3892/ijo.2014.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett. 2014;588:2622–7. doi: 10.1016/j.febslet.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH, Hermeking H, Nikitin AY. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2010;16:1119–28. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogt M, Munding J, Grüner M, Liffers ST, Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A, Hermeking H. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–22. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M, Yu Q. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 2009;23:2388–93. doi: 10.1101/gad.1819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Zheng Y, Deng H, Liang L, Peng J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int J Mol Med. 2014;33:1613–20. doi: 10.3892/ijmm.2014.1718. [DOI] [PubMed] [Google Scholar]