Abstract
Long non-coding RNAs (lncRNAs) have emerged as major players in governing fundamental biological processes, and play a functional role in tumorigenesis. Prostate cancer-associated transcript1 (PCAT-1) is a novel lncRNA that promotes cell proliferation in prostate cancer. However, the role of PCAT-1 on non-small cell lung cancer (NSCLC) remains unclear. In the present study, we firstly investigated PCAT-1 expression in NSCLC tissues and cell lines by using quantitative real-time PCR (QRT-PCR). Our results indicated that PCAT-1 was increased in NSCLC tissues and cell lines. PCAT-1 suppression using PCAT-1 small hairpin RNA (shRNA) with A549 cells inhibited cell proliferation, migration and invasion, while over-expression of PCAT-1 by synthetic plasmid vectors was shown to promote cell proliferation, migration and invasion. Our data suggested that PCAT-1 could play an oncogenic role in NSCLC progression. Silencing PCAT-1 is a potential novel therapeutic approach for lung cancer.
Keywords: Long non-coding RNAs, non-small cell lung cancer, PCAT-1
Introduction
Lung cancer is the most common cause of cancer death worldwide, in which non-small cell lung cancer (NSCLC) accounts for about 80-85% of all lung cancer cases [1,2]. Although in recent years there has been some progress in the treatment of NSCLC, the overall 5-year survival rate is still about 16% due to the late stage at diagnosis [3]. Thus, it is important to investigate the molecular mechanisms involved in lung cancer carcinogenesis and identify diagnostic markers for early detection and targeted treatment of lung cancer.
According to the size, non-coding RNAs (ncRNAs) are sub-divided into two major classes: small ncRNAs (< 200 nt) and long ncRNAs (lncRNAs > 200 NT) [4]. In recent years, microRNAs have been characterized as oncogenes or tumor suppressor genes to influence biological function of cancer cells through post-transcriptional regulation of protein expression [5,6]. In contrast, lncRNAs were once considered to be transcriptional noise [7]. However, it has become increasingly clear that lncRNAs execute important functions at various levels, including X chromosomal inactivation, chromatin remodeling, and transcriptional repression [8-10]. With the development of deep sequencing technologies, lncRNAs have increasingly been linked to many human diseases, especially in cancers [11].
Prostate cancer-associated ncRNA transcripts 1 (PCAT-1) is a long non-coding RNA that was originally identified as a biomarker for prostate cancer [12]. Ge et al found that PCAT-1 was up regulated in colorectal cancer and associated with tumor distant metastasis, furthermore, they suggested that PCAT-1 over expression as an independent prognostic factor for colorectal cancer [13]. Yan et al showed that high expression of PCAT-1 was involved in the progression of hepatocellular carcinoma [14]. Shi et al reported that High expression of PCAT-1 was correlated with advanced clinical stage and poor prognosis of esophageal squamous carcinoma [15]. However, the biological roles and underlying mechanism of PCAT-1 in NSCLC remains unclear. In this study, we investigated the roles of PCAT-1 on the proliferation, migration and invasion of NSCLC cells.
In the present study, our results showed that PCAT-1 was up regulated in NSCLC tissues and cell lines. Decreased expression of PCAT-1 could suppress NSCLC cell proliferation, migration and invasion, while up regulated expression of PCAT-1 could promote NSCLC cells proliferation, migration and invasion. Therefore, our results provided a new therapeutic target for the treatment of NSCLC.
Materials and methods
Clinical sample collection
This study included 36 primary NSCLC patients who had undergone surgeries at Department of Surgical Oncology, Xinxiang Central Hospital between 2010 and 2011. All patients did not receive chemotherapy or radiotherapy before surgery. All collected tissue samples were immediately snap-frozen in liquid nitrogen and stored until required. The study was approved by the Ethics Committee of Xinxiang Central Hospital, and it was performed in compliance with the Declaration of Helsinki Principles, and each patient participated after providing informed consent.
Cell culture and transfection
Human NSCLC cell lines (A549, SPC-A-1, and H460) and a normal bronchial epithelial cell line (16HBE) were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in RPMI-1640 medium (HyClone) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin sodium, and 100 mg/ml streptomycin sulfate at 37°C in a humidified air atmosphere containing 5% CO2. In all experiments, exponentially growing cells were used.
The cells were cultured in a 6-well for 24 hours and then were transfected with over expression plasmid vector or shRNAs. All transfections were performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. PCAT-1 small hairpin RNA (shR-PCAT1) and negative control shRNA (shR-NC) were ordered from GenePharma. The sequence of shR-PCAT1 was 5’-GAGAAAGCAUCUGUACCCUUACAAU-3’. The overexpression plasmid vector of PCAT-1 (PCAT-1-pcDNA3.1 + vector) was synthesized in Life Technology (Invitrogen). The pcDNA3.1 + empty vector was used as a negative control.
RNA isolation and quantitative real-time PCR (QRT-PCR)
Total RNA was isolated from NSCLC tissues and cell lines using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The expression level of PCAT-1 in NSCLC tissues and cell lines was measured by QRT-PCR using the SYBR-Green method (Takara) according to the manufacturer’s protocol and normalized using GAPDH. The primers were as follows: PCAT-1 sense: 5’-AATGGCATGAACCTGGGAGGCG-3’; PCAT-1 antisense: 5’-GGCTTTGGGAAGTGCTTTGGAG-3’; GAPDH sense: 5’-AGAAGGCTGGGGCTCATTTG-3’; GAPDH antisense: 5’-AGGGGCCATCCACAGTCTTC-3’. All experiments were performed using the 2-ΔΔCt method. Each experiment was repeated three times.
Cell proliferation assay
NSCLC cells A549 had been transfected with overexpression plasmid vector or shRNAs, and then was reseeded into 96-well plates. Cell density was adjusted to 5×103/well, and the final volume was 150 μl/well. MTT solution (20 μL) was added to the plates 24, 48, 72 and 96 hours later. The cells were cultured for 4 hours at 37°C. Then, the medium was discarded and 150 μL DMSO was added and oscillated for 15 minutes. Optical density (OD) was detected at a wavelength of 490 nm using an enzyme-labeled analyzer. Each experiment was repeated three times.
Cell migration and invasion assay
Migration and invasion assays were performed using transwell chambers. For migration assay, 5×104 cells were seeded into the upper chamber of transwells (BD Bioscience). For invasion assay, 1×105 cells were added into the upper chamber precoated with matrigel (BD Bioscience). In both assays, cells were maintained in medium without serum in the upper chamber, and medium containing 10% FBS was added to the lower chamber as chemoattractant. After 24 hours incubation, cells that did not migrate or invade through the membrane were wiped out. Then the membranes were fixed and stained with 0.5% crystal violet. Three random fields were counted per chamber using an inverted microscope (Olympus), and each experiment was repeated three times.
Statistical analysis
All statistical analyses were performed using SPSS version 18.0 software (IBM). All data are presented as the mean ± SD and differences between groups were analyzed using Student’s t test or chi-square test analysis. Results were considered significant when P value less than 0.05.
Results
PCAT-1 expression level was increased in NSCLC tissues and cell lines
The expression of PCAT-1 in 36 paired NSCLC tissues and adjacent non-tumor tissues from NSCLC patients were detected by QRT-PCR. Compared with the levels of the adjacent non-tumor tissues, a significant upregulated of PCAT-1 was observed in NSCLC patients (Figure 1A, P < 0.05). Furthermore, NSCLC cell lines (A549, SPC-A-1, and H460) were also tested, compared with human normal bronchial epithelial cell line 16HBE, PCAT-1 expression level was increased in all three NSCLC cell lines (Figure 1B, P < 0.05). These results suggested that lncRNA PCAT-1 might play critical roles in the progression of NSCLC.
Figure 1.
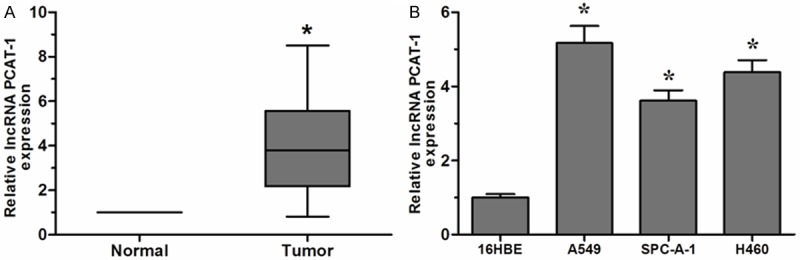
Expression of lncRNA PCAT-1 was increased in NSCLC tissues and cell lines. A. Expression of lncRNA PCAT-1 in 36 NSCLC tissues and adjacent non-tumor tissues was determined by qRT-PCR. B. Expression of lncRNA PCAT-1 in three NSCLC cell lines (A549, SPC-A-1, and H460) and human bronchial epithelial cell line (16HBE) was measured by QRT-PCR. Data are presented as means ± SD from three independent experiments. *P < 0.05.
PCAT-1 promoted NSCLC cell proliferation
To investigate the effect of lncRNA PCAT-1 on NSCLC cell proliferation, we selected A549 cells to perform knockdown and over-expression experiments. The successful knockdown and overexpression experiments of PCAT-1 in the cells were confirmed by qRT-PCR (Figure 2A, 2B, P < 0.05). Next, we explored the effect of lncRNA PCAT-1 on NSCLC cell proliferation. Our data showed that the proliferation rate of NSCLC cells transfected with shR-PCAT1 was significantly decreased compared with negative control (shR-NC) (Figure 2C, P < 0.05). In contrast, the proliferation rate of NSCLC cells transfected with PCAT-1 overexpression plasmid (PCAT-1-pcDNA3.1 + vector) was significantly increased compared with negative control (pcDNA3.1 + empty vector) group (Figure 2D, P < 0.05).
Figure 2.
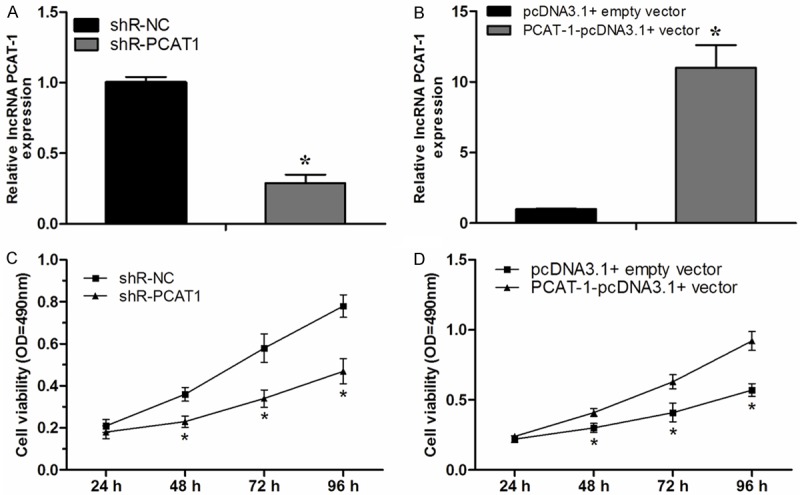
PCAT-1 promoted NSCLC cell proliferation. A. Downregulation of PCAT-1 by transfection with shR-PCAT1 in A549 cells. B. Over expression of PCAT-1 by transfection with PCAT-1-pcDNA3.1 + vector in A549 cells. C, D. Analysis of the effect of PCAT-1 on the proliferation of A549 cells by MTT assay. Data are presented as means ± SD from three independent experiments. *P < 0.05.
PCAT-1 promoted NSCLC cell migration and invasion
We further investigated the effect of lncRNA PCAT-1 on NSCLC cell migration and invasion. Transwell migration assay showed that decreased expression of PCAT-1 dramatically suppressed NSCLC cell migration compared with negative control (Figure 3A, P < 0.05). Consistent with this result, over expression of PCAT-1 resulted in a significant increase in the capability of NSCLC cell migration (Figure 3B, P < 0.05). Moreover, Trans well invasion assay revealed that the invasion potential of NSCLC cells transfected with shR-PCAT1 was significantly decreased (Figure 3C, P < 0.05), whereas over expression of PCAT1 significantly increase the the capability of NSCLC cell invasion (Figure 3D, P < 0.05). These findings indicated that PCAT-1 could promote the development and progression of NSCLC.
Figure 3.
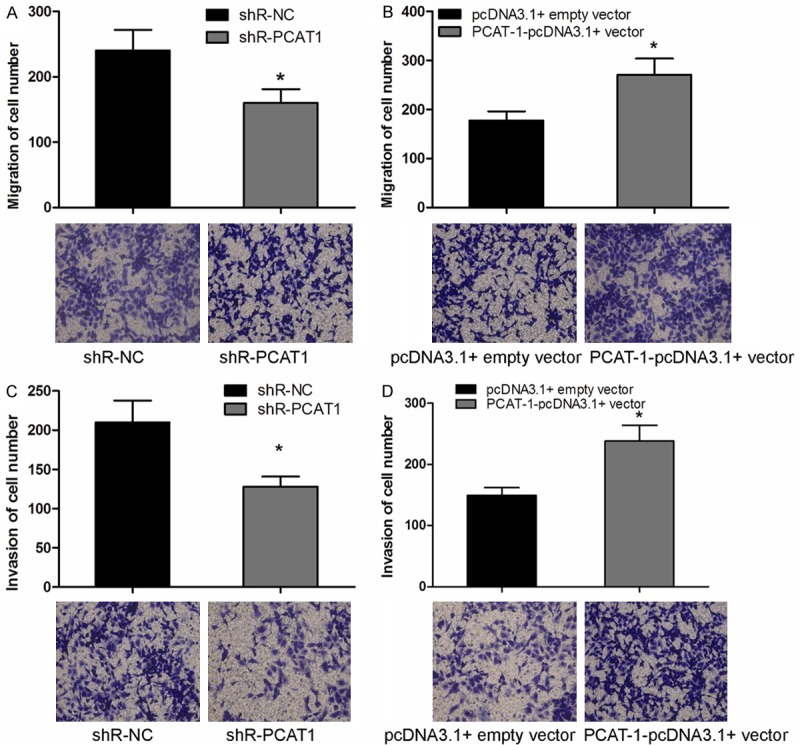
PCAT-1 promoted NSCLC cell migration and invasion. A, B. Analysis the effect of PCAT-1 on the migration of A549 cells by Transwell migration assay. C, D. Transwell invasion assay was utilized to analyze the effect of PCAT-1 on the invasion of A549 cells. Data are presented as means ± SD from three independent experiments. *P < 0.05.
Discussion
lncRNAs represent an emerging player in non-small cell lung cancer, contributing to tumor proliferation, migration and invasion [16-18]. As a new identified lncRNA, PCAT-1 is located in the chromosome 8q24 gene desert and contributes to cell proliferation in prostate cancer [12]. Liu et al showed that decrease expression of PCAT-1 could inhibit cell proliferation and induce cell apoptosis in human bladder cancer [19]. Prensner et al showed that PCAT-1 could promote prostate cancer cell proliferation through CMYC [20], and they also suggested that PCAT-1 expression produced a functional deficiency in homologous recombination through its repression of the BRCA2 tumor suppressor in sporadic cancers [21].
In the present study, we explored the role of lncRNA PCAT-1 in NSCLC progression. Our results revealed that PCAT-1 was significantly increased in NSCLC tissues compared to adjacent non-tumor tissues. Furthermore, PCAT-1 was also increased in NSCLC cell lines (A549, SPC-A-1, and H460) compared with human normal bronchial epithelial cell line 16HBE. These finding indicated that PCAT-1 could play an oncogenic roles in NSCLC progression.
To better understand the biological functions of PCAT-1 in NSCLC, we detected the cell proliferation, migration and invasion by knockdown and overexpression of PCAT-1 in NSCLC cells A549. Our data showed that decreased expression of PCAT-1 in A549 cells could significantly inhibit NSCLC cell proliferation, migration and invasion. In contrast, overexpression of PCAT-1 could markedly promote NSCLC cell proliferation, migration and invasion. These data demonstrated that lncRNA PCAT-1 could play an oncogenic role in the progression of NSCLC.
In conclusion, this study is the first time to examine the function of PCAT-1 in NSCLC progression. Our work suggested that blocking lncRNA PCAT-1 activity in NSCLC is a potential novel therapeutic approach. lncRNA PCAT-1 is involved in many areas of tumor progression, including cell proliferation, migration and invasion. Intervention of PCAT-1 function by agents, may have potential therapeutic value in the prevention of lung cancer. Thus, our findings may not only provide a molecular basis for the role of PCAT-1 in NSCLC but also suggest a novel therapeutic target for the treatment of NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Dempke WC, Suto T, Reck M. Targeted therapies for non-small cell lung cancer. Lung Cancer. 2010;67:257–274. doi: 10.1016/j.lungcan.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Somaiah N, Simon GR. Molecular targeted agents and biologic therapies for non-small cell lung cancer. J Thorac Oncol. 2010;5:S434–454. doi: 10.1097/01.JTO.0000391362.10517.1f. [DOI] [PubMed] [Google Scholar]
- 4.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Dutta A. MicroRNAs: small but potent oncogenes or tumor suppressors. Curr Opin Investig Drugs. 2006;7:560–564. [PubMed] [Google Scholar]
- 6.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 7.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gayen S, Maclary E, Buttigieg E, Hinten M, Kalantry S. A Primary Role for the Tsix lncRNA in Maintaining Random X-Chromosome Inactivation. Cell Rep. 2015;11:1251–1265. doi: 10.1016/j.celrep.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Tanigawa A, Naganuma T, Ohkawa Y, Souquere S, Pierron G, Hirose T. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc Natl Acad Sci U S A. 2015;112:4304–4309. doi: 10.1073/pnas.1423819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang W, Ge X, Yang S, Huang M, Chen P, Zhang X, Fu J, Qu J, Li B. Upregulation of lncRNA MEG3 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells From Multiple Myeloma Patients By Targeting BMP4 Transcription. Stem Cells. 2015;33:1985–1997. doi: 10.1002/stem.1989. [DOI] [PubMed] [Google Scholar]
- 11.Fatima R, Akhade VS, Pal D, Rao SM. Long noncoding RNAs in development and cancer: potential biomarkers and therapeutic targets. Mol Cell Ther. 2015;3:5. doi: 10.1186/s40591-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD, Cao X, Jing X, Wang X, Siddiqui J, Wei JT, Robinson D, Iyer HK, Palanisamy N, Maher CA, Chinnaiyan AM. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W. Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol. 2013;30:588. doi: 10.1007/s12032-013-0588-6. [DOI] [PubMed] [Google Scholar]
- 14.Yan TH, Yang H, Jiang JH, Lu SW, Peng CX, Que HX, Lu WL, Mao JF. Prognostic significance of long non-coding RNA PCAT-1 expression in human hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:4126–4131. [PMC free article] [PubMed] [Google Scholar]
- 15.Shi WH, Wu QQ, Li SQ, Yang TX, Liu ZH, Tong YS, Tuo L, Wang S, Cao XF. Upregulation of the long noncoding RNA PCAT-1 correlates with advanced clinical stage and poor prognosis in esophageal squamous carcinoma. Tumour Biol. 2015;36:2501–2507. doi: 10.1007/s13277-014-2863-3. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Liu H, Shi X, Yao Y, Yang W, Song Y. The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma. Oncotarget. 2015;6:9160–72. doi: 10.18632/oncotarget.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, Gu ZT, Chen WH, Cao KJ. Increased expression of the long non-coding RNA ANRIL promotes lung cancer cell metastasis and correlates with poor prognosis. Diagn Pathol. 2015;10:14. doi: 10.1186/s13000-015-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P, Lu B, Liu G, Wang Z. The Long Noncoding RNA MEG3 Contributes to Cisplatin Resistance of Human Lung Adenocarcinoma. PLoS One. 2015;10:e0114586. doi: 10.1371/journal.pone.0114586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Liu Y, Zhuang C, Xu W, Fu X, Lv Z, Wu H, Mou L, Zhao G, Cai Z, Huang W. Inducing cell growth arrest and apoptosis by silencing long non-coding RNA PCAT-1 in human bladder cancer. Tumour Biol. 2015;36:7685–9. doi: 10.1007/s13277-015-3490-3. [DOI] [PubMed] [Google Scholar]
- 20.Prensner JR, Chen W, Han S, Iyer MK, Cao Q, Kothari V, Evans JR, Knudsen KE, Paulsen MT, Ljungman M, Lawrence TS, Chinnaiyan AM, Feng FY. The long non-coding RNA PCAT-1 promotes prostate cancer cell proliferation through CMYC. Neoplasia. 2014;16:900–8. doi: 10.1016/j.neo.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, Sahu A, Malik R, Wilder-Romans K, Navone N, Logothetis CJ, Araujo JC, Pisters LL, Tewari AK, Canman CE, Knudsen KE, Kitabayashi N, Rubin MA, Demichelis F, Lawrence TS, Chinnaiyan AM, Feng FY. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74:1651–1660. doi: 10.1158/0008-5472.CAN-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]


