Abstract
Aims: The present study is to detect the expression of cyclin D1 in different clinical molecular subtypes in breast cancer, and to analyze its relationship to the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (Her-2), tumor size, clinical stages, histological grades, age of menarche, and prognosis. Methods: In the present study, we retrospectively reviewed the clinical information of 226 patients with breast cancer who were hospitalized at The First Affiliated Hospital of Xinjiang Medical University between January 2000 and December 2012. Immunohistochemical method was used to detect the expression of cyclin D1 in breast cancer tissues. Pearson’s Chi-square test was performed to compare the expression of cyclin D1 under different clinical indicators, and under different immune indexes and subtypes. Spearman rank correlation method was used to analyze the correlation between cyclin D1 expression and ER, PR, Her-2, tumor size, clinical stages, histological grades and age of menarche. Kaplan-Meier was employed to calculate the survival time of tumor-free survival time. Log-rank method was used to analyze the survival curves. Results: The expression of cyclin D1 was not significantly correlated to tumor size, clinical stages, histological grades, age of menarche, or PR, but was correlated to ER. Higher cyclin D1 positive rate corresponded to higher ER positive rate. The expression of cyclin D1 was negatively correlated to Her-2 expression (P < 0.05). Higher cyclin D1 positive rate corresponded to lower Her-2 positive rate. In cyclin D1 positive group, the percentage of Luminal A type was the highest. In cyclin D1 negative group, the percentage of Luminal B type was the highest. Higher cyclin D1 positive rate led to longer tumor-free survival time. Conclusions: The expression of cyclinD1 is significantly correlated to ER and Her-2. Positive expression of cyclin D1 suggests good prognosis, and can be used as an indicator for the evaluation of the prognosis of breast cancer.
Keywords: Breast cancer, cyclin D1, molecular subtype, prognosis
Introduction
Breast cancer is regulated and modulated by multiple factors and genes. As an important regulator of cell cycle, cyclins become a focus in cancer researches [1]. It is reported that one of the main reasons for uncontrolled cell proliferation is cell cycle regulation disorder, in which cyclins play important roles as key regulators [2]. Cyclins are a member of protein family with molecular weights of over 5000, and are originally named because their concentrations vary in a cyclical pattern in cell cycle [3]. Cyclin family includes cyclin A, B, D, E, G and H. They control the progression of cells through cell cycles by exhibiting enzymatic activities through binding to important protein kinases [4]. Recently, the expression of cyclin D1 is found to shorten the G1 phase of cell cycle, reduce cell volume, and lower the dependence on mitogen. As a result, the cells enter S phase at an earlier time, leading to uncontrolled cell proliferation, further transformation, and the occurrence of canceration. Some studies demonstrate that cell cycle regulatory gene cyclin D1 is an oncogene that is directly related to the occurrence of tumors. The overexpression of cyclin D1 gene is observed in many types of tumor tissues [5]. Cell transformation requires the overexpression or gene amplification of cyclin D1, which is an important molecular event in canceration.
Many studies show that expression of cyclin D1 in about 50% invasive breast cancer cases is higher than that in normal epithelium, but the gene amplification rate is only about 13% of that in normal epithelium [6]. Experiments on human beings and mice show that cyclin D1 is related to mammary epithelial cell proliferation induced by steroid [7]. In addition, cyclin D1 may be an independent activator of estrogen receptor (ER) [8]. Some studies demonstrate that cyclin D1 expression is up-regulated in breast cancer tissues [9,10], suggesting that levels of cyclin D1 in breast cancer can be used for the early diagnosis and therapy for breast cancer. Cai et al. report that multiple examinations on genes related to breast cancer such as cyclin D1 can help identify breast cancer at early stage, and provide important basis on its pathological diagnosis [11]. In the present study, we investigate how the expression of cyclin D1 is correlated to the clinical indicators and prognosis of breast cancer.
Materials and methods
Patients
In the present study, we retrospectively reviewed the clinical information of 226 patients with breast cancer who were hospitalized at The First Affiliated Hospital of Xinjiang Medical University between January 2000 and December 2012. All procedures were approved by the Ethics Committee of Xinjiang Medical University. Written informed consents were obtained from all patients or their families.
Immunohistochemistry
Tissue sample slices (3 μm) were fixed with formalin and embedded with paraffin before incubation at 70°C overnight. The paraffin sections were soaked in dimethyl benzene for 20 min, 100% ethanol for 1 min and 3% hydrogen peroxide solution for 10 min. After soaking in 90%-70% ethanol for 3 min each, the sections were rinsed with distilled water. After boiling in EDTA repair solution for 20 min, the sections were cooled to room temperature, followed by washing with phosphate-buffered saline (PBS). Human anti-rabbit polyclonal ERβ primary antibody (BY-02101, Yueyan Biotechnology, Shanghai, China) was dripped on the slices, followed by incubation in dark at 37°C for 1 h. After washing with PBS, the sections were incubated with horseradish peroxidase-labeled human anti-rabbit polycolonal secondary antibody (K500711, Gene Biotechnology, Shanghai, China) in dark at 37°C for 30 min. For control, PBS was added instead of the secondary antibody. After washing with PBS, DAB color development reagent was added. After 5 min, the sections were soaked in distilled water to stop color development. The sections were then soaked in hematoxylin, and hydrochloric acid and alcohol. After washing with distilled water, the sections were soaked in hot water. The sections were then dehydrated in 75%, 80%, 95%, 100% and 100% ethanol for 3 min each, followed by drying and mounting.
Determination of immunohistochemical results
Cyclin D1 positive results were indicated by brown granules in the nucleus. Immunohistochemical staining scores were evaluated according to staining intensity and positive cell number [12]. For the scoring of positive cell number, the number of positive cells among 1000 breast cancer cells was calculated. In cases with no positive cells, 0 score was achieved; for 1-10%, 1 point was scored; for 11-50%, 2 points were scored; for > 50%, 3 points were scored. The final score of each sample was calculated by multiplying the percentage of positive cells with staining intensity, with 0 point being indicated by “-”, 1 point being indicated by “+”, 2-3 points being indicated by “++”, and > 3 points being indicated by “+++” (Figure 1).
Figure 1.
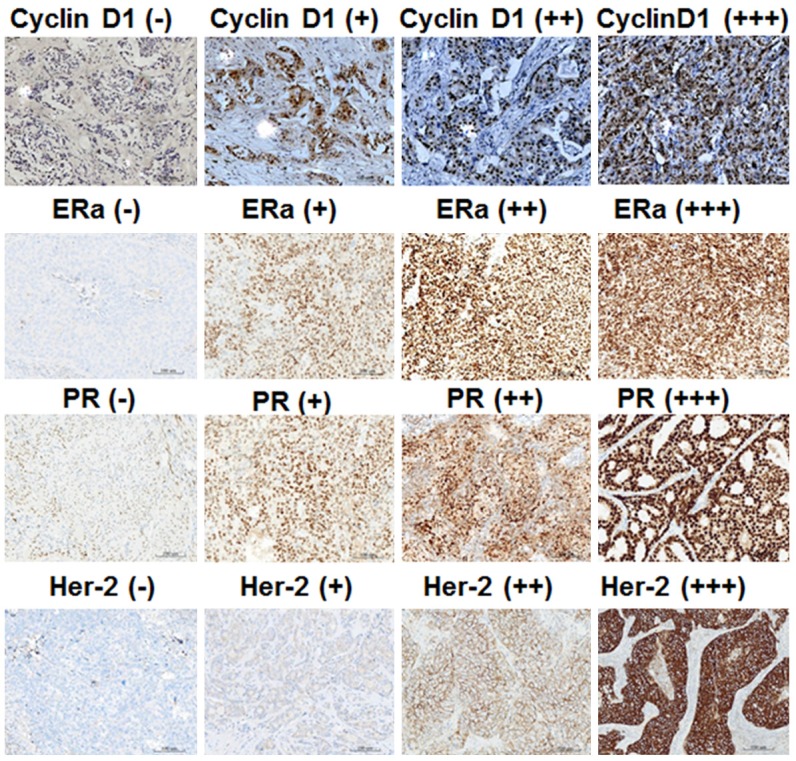
Expression of cyclin D1, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (Her-2) in breast cancer tissues. Immunohistochemical staining was performed to detect cyclin D1, ER, PR and Her-2 expression. The magnification was 100×.
ERα and PR positive results were indicated by brown granules. In cases with no positive cells, the grade was “-”; for cases with < 30% positive cells, the grade was “+”; for cases with 30-50% positive cells, the grade was “+”; for cases with 50-70% positive cells, the grade was “++”; for cases with > 70% positive cells, the grade was “+++”. In the present study, we classified “+” and “++” as weak positive, and “+++” as strong positive (Figure 1).
Human epidermal growth factor receptor-2 (Her-2) positive results were evaluated as follows. In cases with no positive staining, the score was “0”; for cases with any percentage of invasive cells with weak brown cell membrane, the grade was “1+”; for cases where more than 10% invasive cells have weak to medium intensity or complete and uneven brown color, or less than 30% invasive cancer cells exhibit strong, complete and even membrane coloring, the grade was “2+”; for cases where more than 30% invasive cancer cells show strong, complete and even membrane coloring, the grade was “3+”. For easy analysis of data, we classify “0” as negative, “1+” to “2+” as weak positive, and “3+” as strong positive (Figure 1).
Statistical analysis
The results were analyzed using SPSS 17.0 (IBM, Armonk, NY, USA). Pearson’s Chi-square test was performed to compare the expression of cyclin D1 under different clinical indicators, and under different immune indexes and subtypes. Spearman rank correlation method was used to analyze the correlation between cyclin D1 expression and ER, PR, Her-2, tumor size, clinical stages, histological grades and age of menarche. Kaplan-Meier was employed to calculate the survival time of tumor-free survival time. Log-rank method was used to analyze the survival curves.
Results
Expression of cyclin D1 is dependent on tumor size, lymph node metastasis, chemotherapy and radiotherapy
To study the expression of cyclin D1 under each clinical indicator, tissues were stained using immunohistochemical staining. The data showed that cyclin D1 expression was not significantly different among different age groups (χ2 = 5.329, P > 0.05). However, expression of cyclin D1 was significantly different among tumors with different sizes (χ2 = 16.526, P < 0.05). In addition, expression of cyclin D1 was significantly different among patients with different degrees of lymph node metastasis (χ2 = 13.543, P < 0.05). Furthermore, expression of cyclin D1 was significantly different between patients with or without chemotherapy (χ2 = 9.952, P < 0.05), or between patients with or without radiotherapy (χ2 = 7.075, P < 0.05) (Table 1). These results suggest that the expression of cyclin D1 is dependent on tumor size, lymph node metastasis, chemotherapy and radiotherapy.
Table 1.
Relationship between the expression of Cyclin D1 and clinical indicators of breast cancer (%)
| Clinical indicators | N | Cyclin D1 | χ2 | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Negative | Weak positive | Strong positive | ||||
| Age | ||||||
| ≥ 60 | 46 | 14 (30.4) | 20 (43.5) | 12 (26.1) | 5.329 | 0.255 |
| ≥ 40-59 | 117 | 21 (17.9) | 51 (43.6) | 45 (38.5) | ||
| ≤ 39 | 63 | 52 (23.0) | 100 (44.2) | 74 (32.7) | ||
| Tumor diameter | ||||||
| ≤ 1 | 141 | 27 (19.1) | 61 (43.3) | 53 (37.6) | 16.526 | 0.002 |
| ≤ 2 | 23 | 8 (34.8) | 4 (17.4) | 11 (47.8) | ||
| ≤ 3 | 62 | 17 (27.4) | 35 (56.5) | 10 (16.1) | ||
| Total lymphoid | ||||||
| L (-) | 169 | 44 (26.0) | 78 (46.2) | 47 (27.8) | 13.543 | 0.009 |
| L (0-4) | 37 | 5 (13.5) | 18 (48.6) | 14 (37.8) | ||
| L > 4 | 20 | 3 (15.0) | 4 (20.0) | 13 (65.0) | ||
| Chemotherapy | ||||||
| Yes | 199 | 49 (24.6) | 92 (46.2) | 58 (29.1) | 9.952 | 0.007 |
| No | 27 | 3 (11.1) | 8 (29.6) | 16 (59.3) | ||
| Radiotherapy | ||||||
| Yes | 159 | 29 (18.2) | 76 (47.8) | 54 (34.0) | 7.075 | 0.029 |
| No | 67 | 23 (34.3) | 24 (35.8) | 20 (29.9) | ||
Expression of cyclin D1 is dependent on immune indicators and molecular subtypes
To investigate the expression of cyclin D1 under different immune indicators and four different molecular subtypes, immunohistochemical staining was performed. The data showed that expression of cyclin D1 is significantly different among patients with different levels of ERα expression (χ2 = 15.207, P < 0.05). In addition, expression of cyclin D1 was significantly different among patients with different levels of PR expression (χ2 = 12.987, P < 0.05). Furthermore, expression of cyclin D1 was also significantly different among patients with different levels of Her-2 expression (χ2 = 12.286, P < 0.05). Regarding the four subtypes, the expression of cyclin D1 was significantly different among the four different molecular subtypes (χ2 = 13.437, P = 0.037). The percentage of Lumina A type was the highest among cyclin D1 positive expression groups, but the percentage of Lumina B type was the highest among cyclin D1 negative expression groups (Table 2). These results indicate that the expression of cyclin D1 is dependent on immune indicators and molecular subtypes.
Table 2.
Relationship between the expression of Cyclin D1 and immune indicators of breast cancer (%)
| N | Cyclin D1 (negative) | Cyclin D1 (weak positive) | Cyclin D1 (strong positive) | χ2 | P | |
|---|---|---|---|---|---|---|
| ERα | ||||||
| Strong positive | 77 | 12 (15.6) | 28 (36.4) | 37 (48.1) | 15.207 | 0.004 |
| Weak positive | 80 | 21 (26.3) | 43 (53.8) | 16 (20.0) | ||
| Negative | 69 | 19 (27.5) | 29 (42.0) | 21 (30.4) | ||
| PR | ||||||
| Strong positive | 51 | 15 (29.4) | 15 (29.4) | 21 (41.2) | 12.987 | 0.011 |
| Weak positive | 113 | 19 (16.8) | 63 (55.8) | 31 (27.4) | ||
| Negative | 62 | 18 (29.0) | 22 (35.5) | 22 (35.5) | ||
| Her-2 | ||||||
| Strong positive | 119 | 24 (20.2) | 49 (41.2) | 46 (38.7) | 12.286 | 0.015 |
| Weak positive | 42 | 15 (35.7) | 13 (31.0) | 14 (33.3) | ||
| Negative | 65 | 13 (20.0) | 38 (58.5) | 14 (21.5) | ||
| Luminal A type | 72 | 9 (25.7) | 30 (41.7) | 33 (48.5) | 13.437 | 0.037 |
| Luminal B type | 54 | 15 (42.9) | 25 (34.7) | 14 (20.6) | ||
| Her-2 overexpression type | 27 | 6 (17.1) | 13 (18.1) | 8 (11.8) | ||
| Basal like type | 22 | 13 (19.1) | 4 (5.6) | 5 (14.3) |
Note: Lumina A type: ERα+, PR+, and Her-2-; Lumina B type: ERα+, PR+, and Her-2+; Her-2 overexpression type: ERα-, PR-, and Her-2+; basal like type: ERα-, PR-, and Her-2-.
Cyclin D1 expression is correlated with ER and Her-2 expression
To analyze the correlation of cyclin D1 expression with clinical indicators of breast cancer, Spearman rank correlation was performed. The data demonstrated that the expression of cyclin D1 was not correlated with histological grades, clinical stages, age of menarche, or PR (P > 0.05), but was positively correlated with ER positive expression (rs = 0.139, P < 0.05) and negatively correlated with Her-2 positive expression (rs = -0.131, P < 0.05) (Table 3). These results suggest that cyclin D1 expression is correlated with ER and Her-2 expression.
Table 3.
Correlation analysis of clinical indicators of breast cancer
| Menstruation | Histological grades | Staging | Cyclin D1 | Her-2 | ER-a | PR | |
|---|---|---|---|---|---|---|---|
| Menstruation | 1 | 0.012 | 0.017 | 0.013 | 0.086 | 0.175# | 0.035 |
| Histological grades | 0.012 | 1 | 0.296# | -0.005 | 0.132* | 0.051 | 0.057 |
| Staging | 0.017 | 0.296# | 1 | 0.016 | 0.181# | 0.013 | 0.084 |
| Cyclin D1 | 0.013 | -0.005 | 0.016 | 1 | -0.131* | 0.139* | -0.027 |
| Her-2 | 0.086 | 0.132* | 0.181# | -0.131* | 1 | 0.037 | 0.112 |
| ER-a | 0.175# | 0.051* | 0.013* | 0.139* | 0.037 | 1 | 0.373# |
| PR | 0.035 | 0.057 | 0.084 | -0.027 | 0.112 | 0.373# | 1 |
P < 0.05;
P < 0.01.
Overexpression of cyclin D1 and ER prolongs the survival time of breast cancer patients after surgery, but overexpression of Her-2 reduces the survival time
To examine the relationship between cyclin D1 expression and the prognosis of breast cancer patients, Kaplan-Meier method was used to perform survival analysis. Tumor-free survival time in cyclin D1 negative group was 10.242 years, and that in cyclin D1 weak positive group was 9.997 years. Of note, tumor-free survival time in cyclin D1 strong positive group was 10.680 years, being significantly longer than those in the other two groups (χ2 = 6.307, P < 0.05) (Figure 2A). Tumor-free survival time in ER negative group was 9.446 years, and that in ER weak positive group was 10.682 years. Of note, tumor-free survival time in ER strong positive group was 11.710 years, being significantly longer than those in the other two groups (χ2 = 6.413, P < 0.05) (Figure 2B). Tumor-free survival time in Her-2 negative group was 10.278 years, and that in Her-2 weak positive group was 10.705 years. Of note, tumor-free survival time in Her-2 strong positive group was 9.793 years, being significantly longer than those in the other two groups (χ2 = 8.280, P < 0.05) (Figure 2C). These results indicate that overexpression of cyclin D1 and ER prolongs the survival time of breast cancer patients after surgery, but overexpression of Her-2 reduces the survival time.
Figure 2.
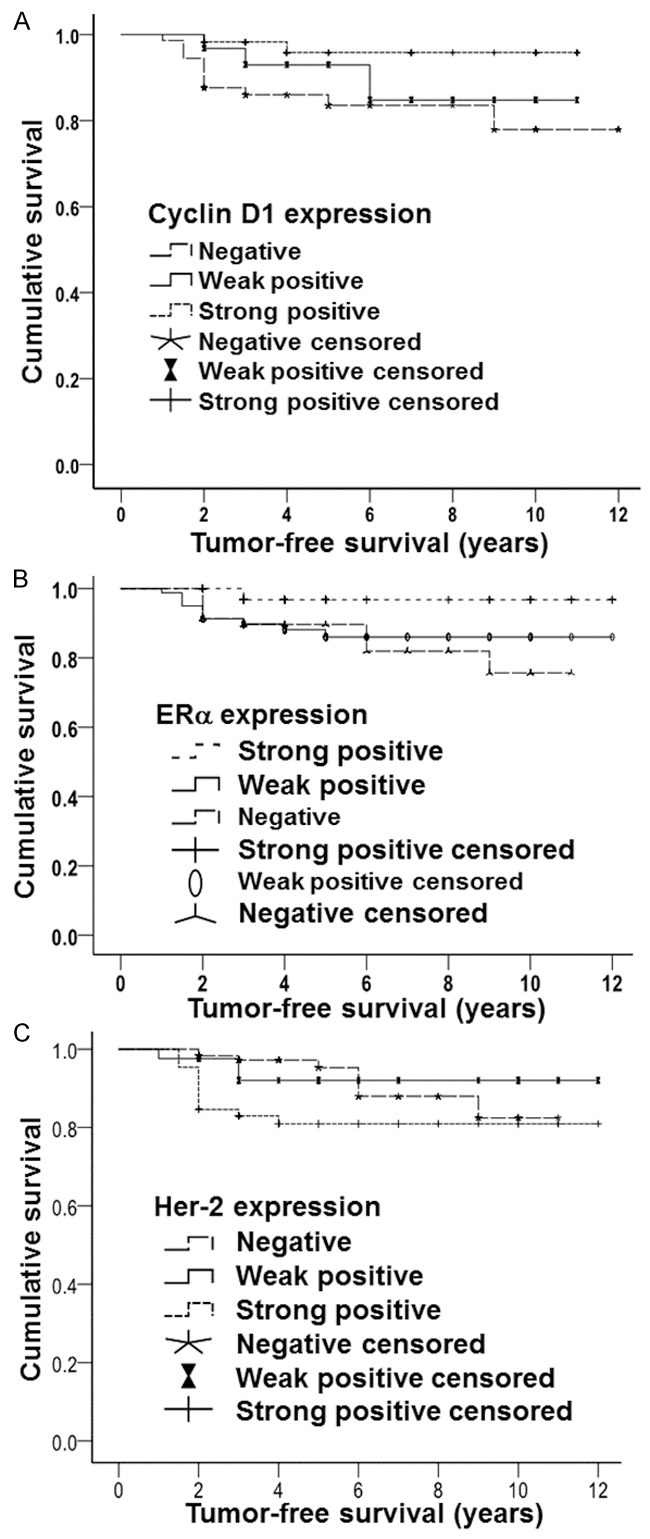
Survival curves of breast cancer patients in correlation with (A) cyclin D1, (B) estrogen receptor α (ERα), and (C) human epidermal growth factor receptor-2 (Her-2). Tumor-free survival time and survival rate of patients with negative, weak positive and strong positive expression of cyclin D1, ERα and Her-2 were recorded.
Discussion
Cyclin D1 is identified as an independent activator of ER [13]. After the activation of ERα-mediated ER pathway, Wnt pathway is activated by the activation of β-catenin, leading to the elevation of cyclin D1 expression [13]. Another study shows that ER in breast cancer induces the overexpression of cyclin D1, and breast cancer with cyclin D1 overexpression tends to be ER-positive, with higher degrees of tissue differentiation [14]. Hwang et al. find that overexpression of cyclin D1 and ER in the same breast cancer case usually results in longer survival time of the patient [15]. Gillett et al. report that higher cyclin D1 expression in breast cancer patients indicates longer survival time and lower recurrence rate [16]. In addition, elevated cyclin D1 expression is commonly observed in breast cancer tissues with good differentiation and positive expression of ER [16]. Therefore, detection of cyclin D1 and ER is valuable for the prediction of recurrence and prognosis for breast cancer patients. By now, only a few reports suggest that cyclin D1 overexpression indicates poor prognosis in breast cancer patients with positive expression of ER. For example, Kenny et al. report that overexpression of cyclin D1 and positive expression of ER are related to poor prognosis [17]. Sutherland et al. find that cutoff of estrogen supply enhances the expression of a kind of tumor-suppressor gene, and that the expression of cyclin D1 and cyclin-dependent kinases is reduced, leading to the arrestment of cell cycle at G1 phase. However, continued estrogen supply elevates cyclin D1 mRNA and protein expression, and results in smoothly completed cell cycle [18]. More evidence demonstrates that cyclin D1 is induced by estrogen in breast cancer. Paola et al. show that the binding of cyclin D1 with estrogen response element may be induced by stimulating ER [19]. Tumors with high expression of cyclin D1 are likely to have positive expression of ER, or even functional ER. In the present study, analysis of the correlation of cyclin D1 expression with ER or PR suggests that cyclin D1 expression is not correlated to PR expression (P > 0.05), but is positively correlated to the positive expression of ER in breast cancer tissues (P < 0.05). The results indicate that the activity of cyclin D1 may be mediated by ER expression, and cyclin D1 can act as the independent activator of ER.
By now, the relationship between Her-2 expression and cyclin D1 expression is not clear yet. A study indicates that overexpression of Her-2 in lymph node-positive tumors induces the aggregation of β-catenin in cytoplasm and activates Wnt signaling pathway [20]. However, another study shows that β-catenin aggregates in cytoplasm under negative expression of ER and silenced Her-2 gene amplification, leading to the activation of Wnt signaling pathway [21]. Jukkola et al. report that Her-2 overexpression is closely correlated to the negative expression of ER [22]. In the present study, we analyzed the correlation of cyclin D1 expression with Her-2 expression, and showed that cyclin D1 expression is negatively correlated to Her-2 expression (P < 0.05), and positively correlated to ER expression. These may be related to decreased cyclin D1 expression or the activation of Wnt signaling pathway.
In recent clinical researches, breast cancer is classified into four subtypes, including Luminal A type (ERα+, PR+, and Her-2-), Luminal B type (ERα+, PR+, and Her-2+), Her-2 overexpression type (ERα-, PR-, and Her-2+), and basal like type (ERα-, PR-, and Her-2-). In the present study, comparison of the expression of cyclin D1 among all subtypes shows that the percentage of Luminal A type is the highest in cyclin D1 positive group, and the percentage of Luminal B type is the highest in cyclin D1 negative group. It is reported that ER expression in Luminal A type is high [23]. Although positive expression of ER is observed in Luminal B type, its expression is relatively low, and Her-2 is overexpressed. In addition, the percentage of cyclin D1 negative expression is high in basal like type, leading to poor prognosis in patients with basal like type breast cancer. Negative expression of cyclin D1 may represent poor prognosis, and the percentage of Her-2 overexpression type is not high in breast cancer. Therefore, no significant difference is observed. These observations further demonstrate that the activity of cyclin D1 may be mediated by ER, and the expression of cyclin D1 may be used as a predictor for the prognosis of breast cancer patients.
Hwang et al. suggest that breast cancer with cyclin D1 overexpression tends to have good prognosis [21]. Gillett et al. report that the conditions of breast cancer patients turn to be better in the presence of cyclin D1 overexpression [16]. The cumulative tumor-free survival rates calculated in the present study showed that the median tumor-free survival time in cyclin D1 negative group was 10.242 years, that in cyclin D1 (+ to ++) group was 9.997 years, and that in cyclin D1 strong positive group was 10.680 years. The results indicate that patients with overexpression of cyclin D1 have higher tumor-free survival rate, and its cumulative tumor-free survival time is longer than patients in cyclin D1 negative and cyclin D1 strong positive groups (P < 0.05). These results indicate that breast cancer patients with overexpression of cyclin D1 have longer survival time and better prognosis. The survival curves calculated according to ER expression show that the median tumor-free survival time in ER negative group was 9.446 years, that in ER weak positive group was 10.682 years, and that in ER strong positive group was 11.710 years. As the increase of ER positive expression, the tumor-free survival time is prolonged, suggesting that breast cancer patients with ER overexpression have longer survival time and better prognosis. The facts that cyclin D1 positive expression is positively correlated to ER positive expression, and that patients with ER overexpression have longer tumor-free survival time, further demonstrate that patients with cyclin D1 overexpression have good prognosis.
Many researches show that breast cancer patients with Her-2 overexpression or gene amplification have poor differentiation of tumor tissues and higher levels of malignancy. According to the survival curves calculated from Her-2 expression in the present study, the median tumor-free survival time in Her-2 negative group was 10.278 years, that in Her-2 weak positive group was 10.705, and that in Her-2 strong positive group was 9.793. Patients with overexpression of Her-2 have reduced tumor-free survival rate, and their cumulative tumor-free survival time was shorter than that in Her-2 weak positive group or Her-2 negative group (P < 0.05), suggesting that patients with Her-2 overexpression have shorter survival time and poorer prognosis. The present study shows that positive expression of cyclin D1 is negatively correlated to positive expression of Her-2, and that patients with Her-2 overexpression have shorter tumor-free survival time. These results demonstrate that patients with cyclin D1 overexpression have longer survival time and better prognosis. In addition, the fact that positive expression of cyclin D1 is positively correlated to the positive expression of ER suggests that cyclin D1 positive breast cancer may be more sensitive to estrogen, having lower malignancy. In conclusion, high expression of cyclin D1 in breast cancer tissues is closely related to the progression of breast cancer. This early event suggests that the expression of cyclin D1 may be connected to the occurrence and proliferation of estrogen-sensitive breast cancer, as well as good prognosis. The detection of cyclin D1 expression can provide necessary evidence for the evaluation and prediction of prognosis of breast cancer.
Acknowledgements
This work was supported by the General Surgery Specialty Construction Project of China.
Disclosure of conflict of interest
None.
References
- 1.Wang Y, Xu XM, Deng JJ, et al. The Expression of p16 and Cyclin D1 in breast cancer and its clinical significance. Medical Recapitulate. 2014;20:4358–4360. [Google Scholar]
- 2.Ji A, Tang J. Expression of Cyclin A1, A2, D1, and E1 after breast cancer surgery and its clinical significance. Acta Universitatis Medicinalis Nanjing (Natural Science) 2010;30:1290–129. [Google Scholar]
- 3.Zhang RJ, Zang JX, Fu DY, et al. The research of expression and clinical significance of SOX7, β-catenin and cyclinD1 protein in invasive breast cancer. International Journal of Surgery. 2013;40:599–604. [Google Scholar]
- 4.Zhao X, Zhao YY, Wang J, et al. Expression and significance of CyclinD1, p16 in esophageal squamous carcinoma. Guangdong Medical Journal. 2015;36:273–276. [Google Scholar]
- 5.Li X, Huo X, Li W, Yang Q, Wang Y, Kang X. Genetic association between cyclin D1 polymorphism and breast cancer susceptibility. Tumour Biol. 2014;35:11959–11965. doi: 10.1007/s13277-014-2489-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SY, Caamano J, Cooper F, Guo X, Klein-Szanto AJ. Immunohistochemistry of cyclin D1 in human breast cancer. Am J Clin Pathol. 1994;102:695–698. doi: 10.1093/ajcp/102.5.695. [DOI] [PubMed] [Google Scholar]
- 7.Donnellan R, Chetty R. Cyclin D1 and human neoplasia. Clin Pathol: Mol Part. 1998;51:1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Liang Z, Liu T. Expression of CyclinD1 in invasive lobular breast cancer and its significance. Medical Journal of Peking Union Medical College Hospital. 2012;3:8–12. [Google Scholar]
- 10.Fan Y, Zhu Y, Meng J. Expression of survivin, p53 protein and cyclin D1 in breast cancer and their correlation. Clinical Misdiagnosis & Mistherapy. 2010;23:101–104. [Google Scholar]
- 11.Cai B, Zhu Y. Expression of ck/34BEl2, actin, P63, S-100, C-erbB-2, PCNA, and cyclin Dl in cyclomastopathy and breast cancer. China Practical Medicine. 2008;3:33–35. [Google Scholar]
- 12.Kohlberger PD, Breitenecker F, Kaider A, Lösch A, Gitsch G, Breitenecker G, Kieback DG. Modified truecolor computer-assisted image analysis versus subjective scoring of estrogen receptor expression in breast cancer: A comparison. Anticancer Res. 1999;19:2189–2193. [PubMed] [Google Scholar]
- 13.Luo J, Chen Y, Xu H. Expression of B-catenin, cyclinD1 and Erα in breast cancer tissues. Practical Preventive Medicine. 2010;7:1502–1504. [Google Scholar]
- 14.Umekita Y, Ohi Y, Sagara Y, Yoshida H. Overexpression of cyclin D1 predicts for poor prognosis in estrogen receptor negative breast cancer patients. Int J Cancer. 2002;98:415–418. doi: 10.1002/ijc.10151. [DOI] [PubMed] [Google Scholar]
- 15.Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS. Prognostic value of combined analysis of cyclinD1 and estrogen receptor status in breast cancer patients. Pathol Int. 2003;53:74–80. doi: 10.1046/j.1440-1827.2003.01441.x. [DOI] [PubMed] [Google Scholar]
- 16.Gillet C, Smith P, Gregory W, Richards M, Millis R, Peters G, Barnes D. Cyclin D1 and prognosis in human breast cancer. Int J Cancer. 1996;69:92–99. doi: 10.1002/(SICI)1097-0215(19960422)69:2<92::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, Sutherland RL, Robertson JF. Overexpression of CyclinD1 messenger RNA predicts for poor prognosis in estrogen receptor positive breast cancer. Clin Cancer Res. 1999;5:2069–2076. [PubMed] [Google Scholar]
- 18.Sutherland R, Rrall O, Alle K. Cyclin-dependent kinases as dowmstream targets of orxtrogen action:potential prognostic in dicators and therapeutic targets. Endorine Related Cancer. 1997;4:357. [Google Scholar]
- 19.Collecchi P, Passoni A, Rocchetta M, Gnesi E, Baldini E, Bevilacqua G. CyclinDl expression in node-positive(N+) and node-negative(N-) infiltrating human mammary carcinomas. Int J Cancer. 1999;84:139. doi: 10.1002/(sici)1097-0215(19990420)84:2<139::aid-ijc8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Khalil S, Tan GA, Giri DD, Zhou XK, Howe LR. Activation Status of Wnt/ß-Catenin Signaling in Normal and Neoplastic Breast Tissues: Relationship to HER2/neu expression in Human and Mouse. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0033421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. β-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 22.Raka EA, El-rehin DA, Paish C, Green AR, Lee AH, Robertson JF, Blamey RW, Macmillan D, Ellis IO. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006;42:3149–3156. doi: 10.1016/j.ejca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]


