Abstract
Objective: Previous studies which investigated the relationship between reduced E-cadherin and prognosis of endometrial cancer were ambiguous and conflicting. Therefore, the aim of the present study was to evaluate the relationship between reduced expression of E-cadherin and endometrial cancer by meta-analysis approach. Method: AfterPubmed and Embasewere deliberately searched via the internet, 8 pieces of literaturewere totally included in final meta-analysis. After the data had been abstracted, the pulled odds ratio (OR) and hazard ratio (HR) were calculated by STATA with random or fixed effect model depending on their heterogeneity. The publication bias of included literature were tested by Begg’s funnel plot and Egger’s test. Results: The pulled data showed that the reduced expression of E-cadherin was significantly associated with overall survival (OS), HR=2.42, 95% CI: 1.50-3.89. The clinical parameters such as lymph node metastasis (LNM), myometrial invasion (MI), International Federation of Gynecology and Obstetrics (FIGO) stage, histological type and pathological type were also significantly associated with reduced expression of E-cadherin. The results of publication biasshowed there were no significant publication bias. Conclusion: Endometrial cancer patients with reduced expression of E-cadherin may have a poorer prognosis than those with normal or higher expression of E-cadherin.
Keywords: E-cadherin, endometrial cancer, prognostic factor, survival
Introduction
Endometrial cancer is one of the most common malignant diseases in female in developed countries and 5-year survival rate of the patients is comparatively higher if patientsare conformed early stage [1,2]. Optimally, patients suffered from early stage endometrial cancer can be treated with the staging operation including hysterectomy and bilateral salpingo-oophorectomy, followed with appropriate adjuvant therapy considering their postoperative pathology [3]. The most common clinicopathologic parameters which contained International Federation of Gynecology and Obstetrics (FIGO) stage, histological type, histological grade, depth of myometrial invasion (MI), lymph node metastasis (LNM) always faced with criticism because of their poor reproducibility [4]. Recently, scientists began to concentrate on several markers such as HABP1 (Hyaluronic acid binding protein 1), LRG1 (leucine-rich-alpha-2-glycoprotein1), cyclin A and cyclin B [5-7] which involved in the prognosis of patients with endometrial cancer, however, the value of these biological factors as prognostic indicators was controversial and heterogeneous. So it is urgent and essential for clinicians and scientists to find novel precise and accurate marker with the purpose of predicting the outcome of endometrial cancer patients.
E-cadherin is one of the most important cell adhesion molecule members which played vital role in cytoskeleton toregulate cellular differentiation and keep the structural integrity and polarity of cells [8]. In recent years, evidence revealed that E-cadherin associated with enhancing invasiveness in vitro, facilitating metastasis in vivo and unfavorable clinicopathologic parameters in several human cancers such as breast, stomach and lung [9-11]. What’s more, there were a number of studies [12-19] investigating the relationship between the expression of E-cadher in and its prognostic significance in endometrial cancer. E-cadherinhas been at the forefront in implicating in the outcome of endometrial patients and it may be considered as a potential predict factor in pathology.
Unfortunately, previous researches argued that the relationship between reduced or absent E-cadher in expression and overall survival (OS) might be disputable. Therefore, for better guiding clinical practice, we conducted this meta-analysis to clarify this unsettled and conflicting issue.
Material and methods
Publication search
Pubmed and Embase database had been searched via the internet with a combination of the following keywords: “endometrial cancer”, “endometrial tumor”, “endometrial carcinoma”, “endometrial neoplasm” and “E-cadherin”. Summary were scanned based on the searched results. The reference lists of acquired articles and relevant reviews were also searched to identify other eligible studies. The overlapping articles were affirmed through data included the period, hospital and treatment information and only the most informative and latest articles were included in the present study.
Eligibility criteria for meta-analysis
Considering the purpose of the present study is the prognostic significance of reduced E-cadherin expression, the criteria were set to identify eligible studies, which including: (1) evaluate the expression of E-cadherin in endometrial cancer; (2) the correlation between OS or clinicopathologic features and expression of E-cadherin was recorded in articles; (3) hazard ratio (HR) and 95% confidence intervals (CIs) was reported in articles or there was sufficient data to calculate the approximately the HR and 95% CIs. The excluding criteria were: (1) studies were published other than in English; (2) studies published in reviews or conference ab-stracts; (3) articles involved in overlapping population.
Data extraction
Data was elaborately extracted independently by two investigators through predefined form which included the following topic such as first author, year of publication, country, total number of included patients, cut-off scores, clinicopathologic parameters and treatment. The controversy was solved by discussion in accordance with the criteria mentioned above.
Statistical analysis
The data were analyzed using STATA 11.0 software (Stata Corporation, College Station, TX, USA). The pulled ORs on clinicopathological parameters including LNM, histological type, histological grade, MI and FIGO stage were calculated with odds ratio (OR) with its 95% CI. HRs and their 95% CIs were aggregated to estimate the impact of E-cadherin aberrant expression on OS. Subgroup analyses were performed by survival analysis (multivariate analysis or the univariate analysis). In summary, HR could be obtained directly when the articles recorded. If HR was not given specific in the publications, they were calculated with the following parameters introduced by Tierney et al [20]. If the total number of events, the number of patients at risk in each group and the log-rank statistic or its p-value were recorded in articles, then the value of HR on OS were approximately estimated; If the data mentioned above were unavailable, HRs were calculated with data read from Kaplan-Meier survival curves with the software of Engauge Digitizer 2.11 version (Mark Mitchell, Boston, USA); If a HR of an event on preserved E-cadherin arm versus the reduced E-cadherin arm wasrecorded rather than vice versa, then a HR of the reduced E-cadherin arm versus preserved E-cadherin arm wasgot by taking the reciprocal of the HR i.e. 1/HR and associated CI. By convention, the polled HR>1 means a worse survival for patients with the reduced expression of E-cadherin.
Heterogeneity assumption was tested by the chi-square-based Q-test with the definition that a P value more than 0.1 indicated absent heterogeneity among studies. The fixed-effects model was used to calculate the pooled OR or HR if the study lack of heterogeneity. Otherwise, the model of random-effects was employed. Begg’s funnel plot and Egger’s test were carried out to evaluate the bias of publication and the p-value less than 0.05 was recognized as statistically significant.
Results
Study characteristic
There were 436 articles were detected in Pubmed and Embase database totally. After removing duplicate papers, 303 pieces of literature remained. After abstracts of remained articles were read, there were 23 full text articles were reviewed carefully and eventually only eight studies was eligible for included with criteria. A total of 1120 patients were enrolled in the present meta-analysis. The characteristics of research were illustrated in Table 1. The expression of E-cadherin was measured by immunohistochemistry (IHC) in all researches. However, the articles showed a variety of the cut-off value for E-cadherin expression and reduced expression of E-cadherin ranged from 25% to 44%.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Number of patients | Cases (Preserved/Reduced) | Cut-off scores | Clinicopathologic parameters | Treatment |
|---|---|---|---|---|---|---|---|
| González-RodillaI | 2013 | Spain | 126 | NA | Score ≥5 | OS | Surgery |
| Tanaka Y | 2013 | Japan | 354 | 213/141 | Score ≥3 | HT, HG, LNM, MI, FIGO stage, OS | Surgery |
| Stefansson IM | 2004 | Norway | 286 | 159/127 | Scores ≥3 | HT, HG, FIGO stage, MI, OS | Surgery ± radiotherapy |
| Mell LK | 2004 | USA | 102 | 76/26 | Scores ≥3 | HT, HG, FIGO stage, MI, OS | surgery |
| Singh M | 2001 | USA | 42 | 28/14 | Scores ≥2 | HT, HG, OS | Tamoxifen + medroxyprogesterone acetate |
| Koyuncuoglu M | 2012 | Turkey | 95 | NA | ≥4% of tumor cells | HT, HG, OS | Surgery ± chemo radiotherapy |
| Yi ZT | 2011 | China | 82 | 42/40 | ≥10% of tumor cells | HG, stage, LNM, MI, OS | Surgery + chemotherapy |
| Kim YT | 2002 | Korea | 33 | 22/11 | Scores ≥3 | HG, HT, stage, MI, LNM, OS | Surgery |
MI: myometrial invasion; LNM: lymph node metastasis; HG: histological grade; HT: histological type; FIGO: International Federation of Gynecology and Obstetrics; OS: overall survival; NA: not known.
The correlation of E-cadherin expression and OS
All of 8 researches were included to aggregate HR involving OS and the expression of E-cadherin. Because there is significant heterogeneity (P<0.0001) in the included studied, the random effect model was chose to conduct the present meta-analysis. The pulled HR was 2.42 (95% CI: 1.50-3.89, P=0.004). The subgroup analyzes in multivariate analysis group and univariate analysis group showed that the reduced expression of E-cadherin were significantly associated with poor prognosis (the results showed in Figure 1).
Figure 1.
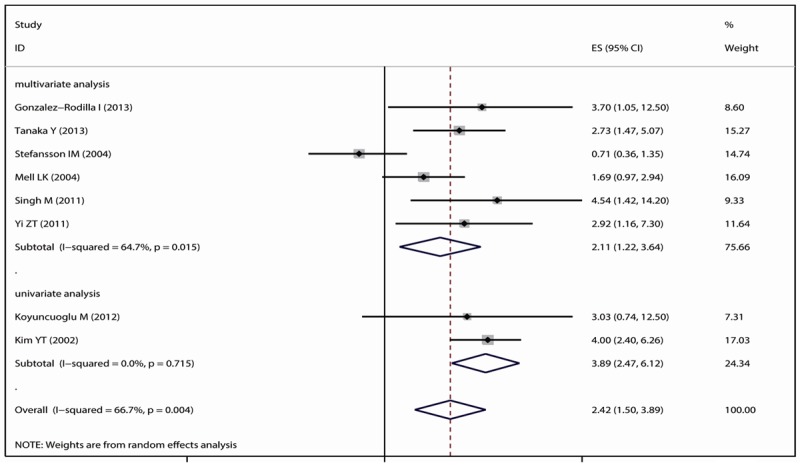
Meta-analysis with a random-effects model for the association between the reduced expression of E-cadherin and OS. Subgroup analyses were performed by survival analysis (multivariate analysis or the univariate analysis).
The association between the reduced expression of E-cadherin and clinicopathological parameters in endometrial cancer
When data was pulled from clinicopathological parameters, the heterogeneity was calculated using the corresponding effect model. Results showed that there was a significant relationship between reduced E-cadherin expression and clinicopathological figures such as LNM, MI, FIGO stage, histological type and pathological type. Specifically, the aggregated ORs were as follows: 3.94 (2.088-7.436) for LNM (with LNM vs. without LNM), 1.785 (1.089-2.925) for MI (with MI ≥1/2 vs. with MI <1/2), 3.008 (2.224-4.068) for tumor grade (grade3 vs. grade 1 and grade 2), 3.769 (1.812-7.844) for FIGO stage (stage III/IV vs. stage IB/II), 0.266 (0.181-0.391) for histological type (non-endometrioid tumor vs. endometrioid tumor). The detailed information was listed in Table 2.
Table 2.
The correlation between the reduced E-cadherin expression and clinicopathologic parameters
| Clinicopathologic parameter | Literature number | Heterogeneity | Effect model | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| LNM (+ vs. -) | 3 | No | Fixed model | 3.940 | 2.088-7.436 | 0.00 |
| P=0.493 | ||||||
| MI (≥1/2 vs. <1/2) | 6 | Yes | Random model | 1.785 | 1.089-2.925 | 0.021 |
| P=0.041 | ||||||
| FIGO stage (III+IV vs. I+II) | 6 | Yes | Random model | 3.769 | 1.812-7.844 | 0.00 |
| P=0.015 | ||||||
| Histological grade (G3 vs. G1+G2) | 7 | Yes | Random model | 3.44 | 1.827-6.476 | 0.00 |
| P=0.03 | ||||||
| Pathological type (Non-endometrioid vs. Endometrioid) | 6 | No | Fixed model | 3.75 | 2.557-5.52 | 0.00 |
| P=0.070 |
MI: myometrial invasion; LNM: lymph node metastasis; FIGO: International Federation of Gynecology and Obstetrics.
Publication bias analysis
Publication bias were analyzed with Egger’s and Begg’stest. The results didn’t show any publication bias between clinicopathological parameters and E-cadherin expression (P>0.05) with Egger’s test (Table 3). There was no publication bias in both Begg’s funnel plot test (P=0.902) (Figure 2) and Egger’s test (P=0.684) in 8 studies demonstrating OS.
Table 3.
Publication bias between E-cadherin expression and the clinicopathologic parameters analyzed by Egger’s test
| Clinicopathologic parameters | t | 95% CI | P | |
|---|---|---|---|---|
| LNM | 0.46 | -19.47493 | 20.9336 | 0.726 |
| MI | -0.63 | -6.090773 | 3.841344 | 0.564 |
| FIGO stage | 0 | -4.371616 | 4.363077 | 0.998 |
| Histological grade | 0.84 | -2.101621 | 4.152923 | 0.438 |
| Pathological type | 0.66 | -2.951051 | 4.795335 | 0.545 |
| OS | 0.43 | -3.915626 | 5.575062 | 0.684 |
Figure 2.
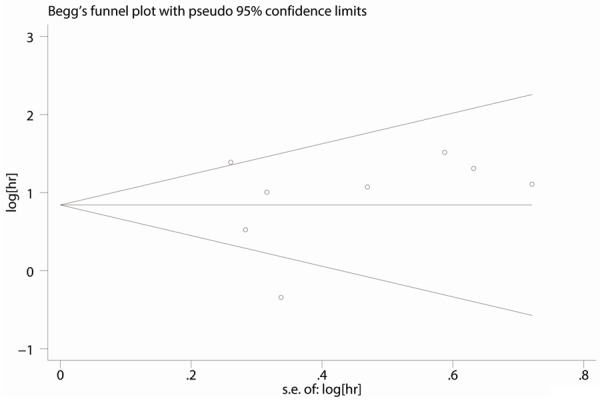
Begg’s funnel plot of studies examining the association between the reduced expression of E-cadherin and OS.
Discussion
Recently, tons of researches have deeply investigated the molecular mechanism of reduced E-cadherin expression in neoplasm. Zhou Y [21] revealed that in the cell lines of breast cancer, the expression of E-cadherin was enhanced via ERβ1 and resulted in inhibiting migration and invasion of cells. What’s more, So WK [22] and his colleagues reported that the down-regulated E-cadherin stimulated the invasive ability of ovarian cancer cells via lig and amphiregulin (AREG) which enhanced the expression of transcriptional repressors of E-cadherin such as SNAIL, SLUG and ZEB1. Considering endometrial cancer, Carico E [23] successfully provided a mouse model for the deficiency of E-cadherin expression via knockdown Msh2 enzyme and hemizygous for E-cadherin. These mouse developed endometrioid-like tumor in uterus in the end, which can provide the robust evidence to certify the relationship between reduced expression of E-cadherin and endometrial cancer.
In the present study, 8 studies including a total of 1120 patients were enrolled in meta-analysis, and our results showed that the reduced E-cadherin was significantly associated with higher risk of unfavorable clinicopathological parameters such as histological grade, histological type, MI, LNM and FIGO stage. At the same time, this meta-analysis also revealed that patients with reduced expression E-cadherin had worse OS, which was consistent with other carcinomas such as lung cancer, esophageal cancer and oral cancer involved with E-cadherin [24-26].
Combined our results with previous studies, there are several implications for clinical practice. To begin with, the reduced expression of E-cadherin can be used to predict clinicopathologic parameters such as FIGO stage, LNM, MI and histological grade in endometrial cancer. Koyuncuoglu M [27] also reported that negative expression of E-cadherin was significantly associated with advanced stage (P=0.001) and poor differentiation (P=0.024) respectively. Secondly, E-cadherin can be regarded as a potential marker for endometrialcancer diagnosis in clinical practice. Carico E [28] conformed that the expression of E-cadherin down-regulated in neoplastic endometrium than in normal and hyperplastic endometrium with the method of immunohistochemistry. What’s more, the study conducted by Montserrat Nand his colleague [29] proved that the expression of E-cadherin repressors such as HMGA2 and TWIST1 exceeded the expression in normal endometrium, at the same time, CDH1, the gene of E-cadherin decreased correspondingly in endometrial cancer. Considering the evidence mentioned above, E-cadherin can be used as an efficient biomarker for discriminating benign and malignant tumors.
As we know, heterogeneity is the major problem influence the explanation of ultimate results of meta-analysis. Considering the present study, subgroup analysis was performed on the basis of survival analyze in original paper. However, the heterogeneity in the subgroup of multivariate analysis was not decreased. This can derive from other variations. For example, the studies involved in the meta-analysis commonly used immunohistochemistry for the reason thatit was a low-cost way to measure the expression of E-cadherin in the specimen and also easy to be applied. However, because of various primary antibodies, different dilutions and cut-off values, a wide range of decreased protein expression was observed in previous studies, which can lead to observation heterogeneity in the end. On the other hand, Clinical heterogeneity orientated from different patients and various treatments can also causeheterogeneity.
When the original publication bias were calculated by Begg’s funnel plot, the results revealed that the funnel plot was symmetric (P>0.05) without substantial impact on final outcome, which further increase the credibility of conclusion for this meta-analysis. At the same time, a few limitations should be admitted in the present study. Firstly, we only searched the published literature written in English, omitting the unpublished papers reported negative results and conference abstract. Secondly, the evaluated HR may be less exact compared with the data directed from published articles. All of these may exert subtle influence on the finalresults.
Conclusively, the pulled data on HR suggested that E-cadherin expression status is an important factor in the prognosis of endometrial cancer patients. It may be applied as an effective predictive biomarker for the patients suffered from endometrial cancer. For the best of our knowledge, this is the first meta-analysis investigated the association between reduced expression of E-cadherin and overall survival in endometrial cancer. However, considering the limitations existed in the meta-analysis, further studies and larger well-designed prospective researches should be conducted in the future to preciselyevaluate the correlations between E-cadherin and endometrial cancer.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 30901713) and a grant from Shandong Provincial Natural Science Foundation (No. ZR2009CQ019).
Disclosure of conflict of interest
None.
References
- 1.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004;35:649–62. doi: 10.1016/j.humpath.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom B, Strang P, Lindgren A, Bergstrom R, Tribukait B. Carcinoma of the endometrium: do the nuclear grade and DNAploidy provide more prognostic information than do the FIGO and WHO classifications? Int J Gynecol Pathol. 1996;15:191–201. doi: 10.1097/00004347-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Santala S, Talvensaari-Mattila A, Soini Y, Honkavuori-Toivola M, Santala M. High expression of cyclin A is associated with poor prognosis in endometrial endometrioid adenocarcinoma. Tumour Biol. 2014;35:5395–9. doi: 10.1007/s13277-014-1703-9. [DOI] [PubMed] [Google Scholar]
- 6.Wen SY, Zhang LN, Yang XM, Zhang YL, Ma L, Ge QL, Jiang SH, Zhu XL, Xu W, Ding WJ, Yang BQ, Zhang ZG, Teng YC. LRG1 is an independent prognostic factor for endometrial carcinoma. Tumour Biol. 2014;35:7125–33. doi: 10.1007/s13277-014-1953-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Liu T, Yu G, Wang J. Overexpression of HABP1 correlated with clinicopathological characteristics and unfavorable prognosis in endometrial cancer. Tumour Biol. 2015;36:1299–306. doi: 10.1007/s13277-014-2761-8. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa H, Hikiba Y, Hirata Y, Font-Burgada J, Sakamoto K, Hayakawa Y, Taniguchi K, Umemura A, Kinoshita H, Sakitani K, Nishikawa Y, Hirano K, Ikenoue T, Ijichi H, Dhar D, Shibata W, Akanuma M, Koike K, Karin M, Maeda S. Loss of liver E-cadherin induces sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad Sci U S A. 2014;111:1090–5. doi: 10.1073/pnas.1322731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, Van Roy F, Cornelisse CJ, Cleton-Jansen AM. Simultaneous loss of E-cadherin andcatenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–411. doi: 10.1002/(SICI)1096-9896(199712)183:4<404::AID-PATH1148>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 11.Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996;69:184–189. doi: 10.1002/(SICI)1097-0215(19960621)69:3<184::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Rodilla I, Aller L, Llorca J, Munoz AB, Verna V, Estevez J, Schneider J. The e-cadherin expression vs. tumor cell proliferation paradox in endometrial cancer. Anticancer Res. 2013;33:5091–5. [PubMed] [Google Scholar]
- 13.Tanaka Y, Terai Y, Kawaguchi H, Fujiwara S, Yoo S, Tsunetoh S, Takai M, Kanemura M, Tanabe A, Ohmichi M. Prognostic impact of EMT (epithelial-mesenchymal-transition) related protein expression in endometrial cancer. Cancer Biol Ther. 2013;14:13–9. doi: 10.4161/cbt.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in p-cadherin expression and related cell adhesion markers in endometrial cancer. J. Clin. Oncol. 2004;22:1242–52. doi: 10.1200/JCO.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Mell LK, Meyer JJ, Tretiakova M, Khramtsov A, Gong C, Yamada SD, Montag AG, Mundt AJ. Prognostic significance of E-cadherin protein expression in pathological stage i-iii endometrial cancer. Clin Cancer Res. 2004;10:5546–53. doi: 10.1158/1078-0432.CCR-0943-03. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Darcy KM, Brady WE. Cadherins, catenins and cell cycle regulators: impact on survival in a gynecologic oncology group phase ii endometrial cancer trial. Gynecol Oncol. 2011;123:320–8. doi: 10.1016/j.ygyno.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyuncuoglu M, Okyay E, Saatli B, Olgan S, Akin M, Saygili U. Tumor budding and e-cadherin expression in endometrial carcinoma: are they prognostic factors in endometrial cancer? Gynecol Oncol. 2012;125:208–13. doi: 10.1016/j.ygyno.2011.12.433. [DOI] [PubMed] [Google Scholar]
- 18.Yi TZ, Guo J, Zhou L, Chen X, Mi RR, Qu QX, Zheng JH, Zhai L. Prognostic Value of E-Cadherin Expression and CDH1 Promoter Methylation in Patients With Endometrial Carcinoma. Cancer Invest. 2011;29:86–92. doi: 10.3109/07357907.2010.512603. [DOI] [PubMed] [Google Scholar]
- 19.Kim YT, Choi EK, Kim JW, Kim DK, Kim SH, Yang WI. Expression of e-cadherin and alpha-, beta-, gamma-catenin proteins in endometrial carcinoma. Yonsei Med J. 2002;43:701–11. doi: 10.3349/ymj.2002.43.6.701. [DOI] [PubMed] [Google Scholar]
- 20.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Ming J, Xu Y, Zhang Y, Jiang J. ERβ1 inhibits the migration and invasion of breast cancer cells through upregulation of E-cadherin in a Id1-dependent manner. Biochem Biophys Res Commun. 2015;457:141–7. doi: 10.1016/j.bbrc.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 22.So Wk, Fan Q, Lau MT, Cheng JC, LLeung PC. Amphiregulin induces human ovarian cancer cell invasion by down-regulating E-cadherin expression. FEBS Lett. 2014;588:3998–4007. doi: 10.1016/j.febslet.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Carico E, Atlante M, Giarnieri E, Raffa S, Bucci B, Giovagnoli MR, Vecchione A. Increased incidence of endometrioid tumors caused by aberrations in E-cadherin promoter of mismatch repair-deficient mice. Anticancer Res. 2010;30:4993–7. [Google Scholar]
- 24.Wu Y, Liu HB, Ding M, Liu JN, Zhan P, Fu X, Lu G. The impact of e-cadherin expression on non-small cell lung cancer survival: A meta-analysis. Mol Bio Rep. 2012;39:9621–8. doi: 10.1007/s11033-012-1827-1. [DOI] [PubMed] [Google Scholar]
- 25.Xu XL, Ling ZQ, Chen SZ, Li B, Ji WH, Mao WM. The impact of E-cadherin expression of the prognosis of esophageal cancer: A meta-analysis. Dis Esophagus. 2014;27:79–86. doi: 10.1111/dote.12024. [DOI] [PubMed] [Google Scholar]
- 26.Luo SL, Xie YG, Li Z, Ma JH, Xu X. E-cadherin expression and prognosis of oral cancer: a meta-analysis. Tumour Biol. 2014;35:5533–7. doi: 10.1007/s13277-014-1728-0. [DOI] [PubMed] [Google Scholar]
- 27.Koyuncuoglu M, Okyay E, Saatli B, Olgan S, Akin M, Saygili U. Tumor budding and E-Cadherin expression in endometrial carcinoma: are they prognostic factors in endometrial cancer? Gynecol Oncol. 2012;125:208–13. doi: 10.1016/j.ygyno.2011.12.433. [DOI] [PubMed] [Google Scholar]
- 28.Carico E, Atlante M, Giarnieri E, Raffa S, Bucci B, Giovagnoli MR, Vecchione A. E-cadherin and alpha-catenin expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res. 2010;30:4993–7. [PubMed] [Google Scholar]
- 29.Montserrat N, Mozos A, Llobet D, Dolcet X, Pons C, De Herreros AG, Matias-Guiu X, Prat J. Epithelial to mesenchymal transition in early stageendometrioid endometrial carcinoma. Hum Pathol. 2012;43:632–43. doi: 10.1016/j.humpath.2011.06.021. [DOI] [PubMed] [Google Scholar]


