Abstract
Objectives: Changes of chest CT images in Mycobacterium and non-Mycobacterium abscesses in patients with lung disease were with a view to making an early diagnosis. Methods: 124 primary patients diagnosed with non-tuberculosis Mycobacterium infections with a positive sputum acid-fast smear were enrolled in this retrospective study. CT images and clinical data of these patients were analyzed. Results: The 52 Mycobacterium abscess lung disease cases included bronchiectasis 82.7% (43/52), which was more easily detected bilaterally than unilaterally (29/52 vs. 14/52), lung consolidation 44.2% (23/52), nodules 44.2% (22/52), cavities 32.7% (17/52), tree-in-bud pattern 42.3% (22/52) and patchy shadow 63.5% (33/52) in CT images. Tree-in-bud pattern was more common in Mycobacterium abscess compared with non-Mycobacterium abscess lung disease (42.3% vs. 18.1%, P = 0.004). A significant difference of the lung area involved by tree-in-bud in CT was found between non-Mycobacteria abscess and Mycobacterium abscess lung disease (17.0% vs. 7.2%, P < 0.001), and tree-in-bud occurred more readily unilaterally (21.2% vs. 6.9%, P = 0.029), and in the inferior lobe of the right lung (3.2% vs. 0.2%, P = 0.029) in Mycobacterium abscess lung disease. Patchy shadow was more common in non-Mycobacterium abscess lung disease (63.5% vs. 80.1%, P = 0.041). Further multi-factor analysis confirmed that tree-in-bud was an independent predictor of Mycobacterium abscess lung disease. Conclusions: Different CT results existed between non-Mycobacterium abscess and Mycobacterium abscess lung diseases. The tree-in-bud pattern might be helpful to choose a suitable therapy in patients, with an acid-fast bacilli smear-positive diagnosis of lung disease.
Keywords: Mycobacterium abscess, lung disease, acid-fast bacilli, smear positive lung disease, chest CT performance
Introduction
Non-tuberculosis Mycobacterium diseases are those caused by non-tuberculous Mycobacterium (NTM) except for Mycobacterium tuberculosis and Mycobacterium leprae, with the lungs being the most commonly involved organ. In recent years, the incidence of infection caused by NTM has been gradually rising, thus making it an important pathogenic bacterium in the clinic [1-3]. Mycobacterium abscess is a fast-growing NTM that produces lung infection. Research in the USA has shown that more than 80% of fast-growing NTM lung diseases are caused by Mycobacterium-induced abscesses [1]. 12% of Mycobacterium abscesses were isolated from 17,915 NTM strains in South Korea, which was only secondary to Mycobacterium avium-intracellulare composite group [4]. In addition, different therapy regimes were given due to different sensitivities of the bacteria to drugs, particularly Mycobacterium and non-Mycobacterium abscess; the Mycobacterium abscess was independently separated according to the American Thoracic Society guidelines. At present, more and more iatrogenic infections and outbreaks caused by Mycobacterium abscess have been reported, which have become one of the most common bacteria for iatrogenic infection, thus attracting the close attention of Chinese and other scholars [5-7]. However, effective treatment of Mycobacterium abscess pulmonary disease is still lacking, with broad-spectrum antibiotics being administered intravenously as the main treatment in hospitals. Unfortunately, these bacteria are resistant to most antibiotics and are naturally resistant to anti-tuberculosis drugs. Their low efficacy, high resistance rate, gastrointestinal toxicity are well known and found even for presently well-established effective antibiotics, including amikacin, cefoxitin or imipenem and others, thereby resulting in a worse prognosis for Mycobacterium abscesses infection compared to non-Mycobacterium infection [6,8,9].
Because of the relatively higher incidence of Mycobacterium abscess in NTM infections, the risk of outbreak, spread of infection, drug resistance, and poor prognosis by these bacteria was increased, making them potentially more harmful to public health. Therefore, early diagnosis of Mycobacterium abscess infection, especially early differential diagnosis of non-Mycobacterium abscess, would be of important significance for the clinical diagnosis and treatment of Mycobacterium abscess infection.
Sputum acid-fast bacilli (AFB) smear microscopy is one of the most commonly used and effective methods for early clinical diagnosis of Mycobacterium lung disease [10]. Samples from patients who are AFB positive are cultured so that the infecting bacterial strain(s) can be identified, but clinicians often need other tests to provide a differential diagnosis of NTM (mainly due to the length of time required for strain identification). Imaging can provide important information to help identification of the disease and understand its potential severity e.g. lesion size. Thus, CT imaging before confirmed bacterial culture results is of great clinical interest for the early differential diagnosis and treatment of NTM related diseases.
Therefore, this study set out to compare chest CT imaging differences between Mycobacterium abscesses and non-Mycobacterium abscesses in patients with lung disease. The aims were to determine the characteristics of Mycobacterium abscess lung disease using imaging techniques and to assess the possibility of using imaging as a predictor of Mycobacterium abscess lung disease.
Patients and methods
Patients
AFB smear-positive patients (4,167 of 13,509 cases of sputum specimens analyzed) were diagnosed and treated in Shanghai Pulmonary Hospital, which is affiliated to Tongji University, from January 2011 to January 2014. Samples were collected at least twice from AFB smear-positive patients. To varying degrees, sputum specimens, bacterial culture and strain identification were performed for all AFB smear-positive patients. In total, 4,043 patients were diagnosed with tuberculosis by strain identification, 124 patients with a non-tuberculosis Mycobacterium infection (of which 52 were Mycobacterium abscesses) and 72 cases were non-Mycobacterium (NM) abscesses. All patients underwent CT imaging and the general clinical data from the primary diagnosis plus the chest CT examination results were compared between Mycobacterium abscesses and NM abscesses-lung disease (LD) infections. The Tongji University and the ethics committee of Shanghai Pulmonary Hospital, which is affiliated to Tongji University, approved the study. All of the patients enrolled in the study signed consent forms.
Diagnosis of pulmonary Mycobacterium abscesses-LD and NM abscesses-LD
An AFB smear was performed on all the sputum specimens. Polymerase chain reaction (PCR) was performed for Mycobacterium tuberculosis using the IS6110 probe (Life Technologies, Carlsbad, USA). Modified Roche medium (BaiHui, Wuxi, China) was employed for Mycobacterium cultivation. Confirmed diagnosis of Mycobacterium abscesses-LD and NM abscesses-LD was based on the results of Mycobacterium cultures and American Thoracic Society guidelines [1].
CT scanning methods
Computer tomography (CT) imaging was performed in the radiology department of the Shanghai Pulmonary Hospital within 3 months of AFB smear analysis. A conventional CT scan was performed using multi slice spiral CT (Brilliance CT 64-channel, Philips, Eindhoven, Netherlands), with a scanning layer thickness of 5 mm and an interval of 5 mm. HRCT scan was performed with a slice thickness of 1 mm and an interval of 10 mm. The scan range was from the apex pulmonis to the costophrenic angle position under maximal inspiratory amplitude. CT performance of Mycobacterium abscesses-LD and NM abscesses-LD was recorded according to the following description: tree-in-bud (centrilobular nodules with a linear branching pattern), nodules (d ≥ 1 cm), bronchiectasis, thin walled cavity (d < 3 cm), thin walled cavity (d ≥ 3 cm), thick wall cavity (d < 3 cm), thick wall cavity (d ≥ 3 cm), lung consolidation, patchy shadow, nodules (d < 1 cm), atelectasis, ground glass nodules, reticular opacity, decrease of lung volume, mediastinal lymphadenectasis, hilar lymphadenopathy, calcified lymph nodes, bronchial cystic expansion, pleural effusion, interstitial fibrosis, pneumothorax and pleural thickening. All the CT images were analyzed and confirmed by two radiological and lung disease experts, who were blinded to the microbiology results. The sensitivity and specificity for the identification of Mycobacterium abscess lung disease by CT imaging were calculated as: sensitivity = true positive/cases group and specificity = true negative/cases group
Statistical analysis
All statistical analysis was performed using SPSS for Windows (Version 16.0, Chicago, SPSS Inc). The chi-square test or Fisher’s exact test were employed for comparison of classification variables and an independent sample t-test was employed for comparison of continuous variables. Differences of CT performance characteristics were evaluated using single factor analysis. Multi-factor analysis using a logistic regression model was performed to determine the independent predictor of Mycobacterium abscesses-LD and NM abscesses-LD. P < 0.05 was considered to be statistically significance.
Results
Among the NTM strains detected in our study, 52 (41.9%) were Mycobacterium abscesses in 21 males and 31 women, who had a median age of 58 years (range 23~80 years). Intracellular Mycobacteria (42/72, 58%) was the most common strain in the 72 non-Mycobacteria abscesses patients (Table 1), among who were 39 males and 33 women, with a median age of 59 years (range 18-78). No significant differences of gender, age, smoking history, malignant tumor or other basic diseases were found between the two groups (Table 2).
Table 1.
Species identification result of NTM
| Species | Quantity |
|---|---|
| Mycobacterium abscesses | 52 (41.9) |
| Mycobacterium intracellulare | 42 (34.7) |
| Mycobacterium avium | 8 (6.5) |
| Mycobacterium kansasii | 7 (5.6) |
| Mycobacterium fortuitum | 5 (4.0) |
| Mycobacterium Gordon | 6 (4.8) |
| Mycobacterium chelonae | 2 (1.6) |
| Mycobacterium paraffinum | 1 (0.8) |
Table 2.
Clinical characteristics of patients with Mycobacterium abscesses-LD and NM abscesses-LD
| Mycobacterium abscesses (n = 52) | NM abscesses (n = 72) | P | |
|---|---|---|---|
| Age | 58 | 59 | 0.994 |
| Male | 31 | 39 | 0.585 |
| Ever smoker | 20 | 24 | 0.574 |
| Diabetes mellitus | 10 | 13 | 1.0 |
| Malignancy | 1 | 0 | 0.419 |
| Autoimmune disease | 1 | 0 | 0.419 |
| COPD | 11 | 12 | 0.641 |
| Pneumoconiosis | 0 | 1 | 1.0 |
Based on the CT findings on laterality and the distribution of Mycobacterium abscesses and NM abscesses-LD (Supplementary Table 1), the most common CT manifestations in the 124 cases of NTM lung disease was bronchiectasis (85.5%, 106/124) and patchy shadow (73.4%, 91/124), followed by nodules (47.6%, 59/124), lung consolidation (41.1%, 51/124), cavity (27.4%, 34/124) and tree-in-bud (28.2%, 35/124).
The most common CT manifestations in the 52 cases of Mycobacterium abscesses-LD was also bronchiectasis (82.7%, 43/52), which was easier to detect bilaterally rather than unilaterally (29/52 vs. 14/52), and tended to be present more in the middle lobe of right lung and left lingual (35/146 vs. 26/146) (Supplementary Table 1; Figure 1A), followed by patchy shadow (63.5%, 33/52), lung consolidation (44.2%, 23/52) and nodules (44.2%, 23/52). No obvious laterality and distribution differences were found for lung consolidation and nodules. Cavities were found in 17 patients (32.7%, 17/52), which were mainly located in the upper lobe (9/17) (Supplementary Table 1; Figure 1B), without obvious laterality. However, a tree-in-bud pattern was detected in 42.3% (22/52) of Mycobacterium abscesses-LD patients, which was 2.3 times (42.3% vs. 18.1%, P = 0.004) higher than that found in the NM abscesses-LD patients (Table 3 and Figure 1C).
Figure 1.
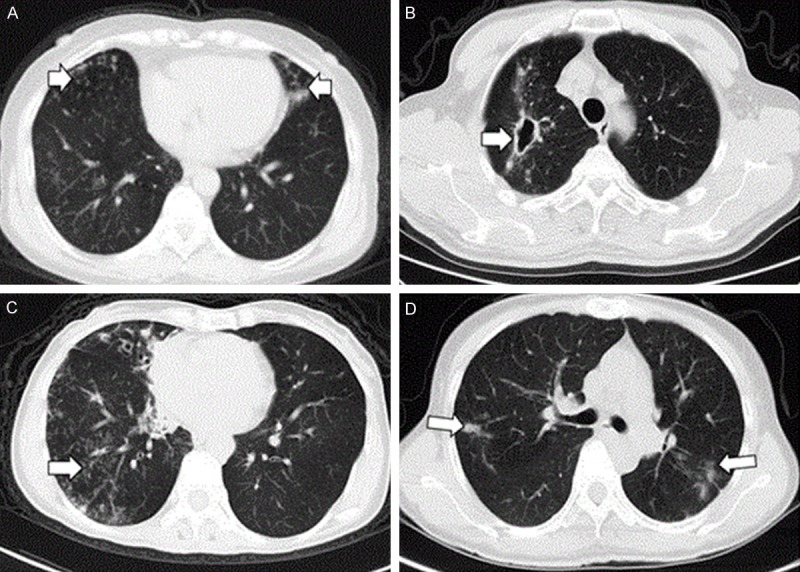
Different CT results in different patients with Mycobacterium abscesses and intracellular mycobacteria. A. Chest thin layer CT in a 59-year-old female with mycobacterial abscess lung disease indicated bronchiectasis in the middle lobe of the right lung and left lingula. B. Chest thin layer CT in a 49-year-old male with mycobacterial abscess lung disease revealed a cavity in the upper lobe of the right lung. C. Chest thin layer CT in a 61 year-old female with mycobacterial abscess lung disease showed small centrilobular nodules in the right lower lung and bronchiectasis in the middle lobe of the right lung. D. Chest CT in a 49 year-old male with non-myobacterial abscess lung disease shows a patchy shadow in the bilateral upper lobe.
Table 3.
Comparative chest CT findings of Mycobacterium abscesses and NM abscesses
| CT findings | Number of patients involved | Number of involved lobes | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mycobacterium abscesses (n = 52) | NM abscesses (n = 72) | P | Mycobacterium abscesses (n = 312) | NM abscesses (n = 432) | P | |
| Tree-in-bud pattern | 22 | 13 | 0.004 | 53 | 31 | < 0.001 |
| Nodules (d ≥ 1 cm) | 23 | 27 | 0.465 | 47 | 46 | 0.092 |
| Bronchiectasis | 43 | 63 | 0.606 | 146 | 176 | 0.115 |
| Thin-walled cavity (d < 3 cm) | 11 | 14 | 0.824 | 14 | 16 | 0.706 |
| Thin-walled cavity (d ≥ 3 cm) | 12 | 9 | 0.148 | 17 | 10 | 0.029 |
| Thick-walled cavity (d < 3 cm) | 21 | 18 | 0.080 | 38 | 28 | 0.009 |
| Thick-walled cavity (d ≥ 3 cm) | 25 | 25 | 0.143 | 43 | 40 | 0.059 |
| Consolidation | 23 | 29 | 0.714 | 43 | 56 | 0.744 |
| Patches | 33 | 58 | 0.041 | 94 | 147 | 0.268 |
| Nodules (d < 1 cm) | 22 | 37 | 0.365 | 63 | 91 | 0.784 |
| Atelectasis | 1 | 0 | 0.419 | 1 | 0 | 0.419 |
| Ground-glass opacity | 2 | 8 | 0.190 | 2 | 15 | 0.011 |
| Reticular opacities | 1 | 0 | 0.419 | 1 | 0 | 0.419 |
| Volume reduction | 6 | 6 | 0.556 | 16 | 18 | 0.595 |
| Mediastinal lymphadenopathy | 4 | 10 | 0.391 | 0 | 0 | |
| Hilar lymphadenopathy | 3 | 8 | 0.356 | 0 | 0 | |
| Calcified lymph nodes | 0 | 5 | 0.074 | 0 | 0 | |
| Cystic changes | 3 | 1 | 0.308 | 3 | 2 | 0.655 |
| Pleural effusion | 8 | 9 | 0.792 | 12 | 18 | 0.853 |
| Interstitial fibrosis | 1 | 4 | 0.398 | 5 | 17 | 0.079 |
| Pneumothorax | 0 | 1 | 1.0 | 3 | 3 | 0.699 |
| Pleural thickening | 0 | 2 | 0.509 | 0 | 9 | 0.012 |
A significant difference was found by CT in amount of lung area lesions between non-Mycobacterium abscess and Mycobacterium abscess lung disease patients (17.0% (53/312) vs. 7.2% (31/432), P < 0.001). The lesions were more prominent unilaterally (21.2% vs. 6.9%, P = 0.029) (Supplementary Table 1; Figure 2A) and in the inferior lobe of the right lung (3.2% vs. 0.2%, P = 0.029) (Figure 2B), while patchy shadow was more common in NM abscess lung disease (63.5% vs. 80.1%, P = 0.041) (Figure 1D). The sensitivity and specificity of tree-in-bud and patchy shadows for the diagnosis of Mycobacterium abscess lung disease was further analyzed, and we found that the sensitivity and specificity of patchy shadows was 63.5% (33/52) and 18.1% (13/72) respectively, and was 42.3% (22/52) and 81.9% (59/72) for small centrilobular tree-in-bud development. A further multi-factor analysis confirmed that tree-in-bud was an independent predictor for Mycobacterium-LD diagnosis (P = 0.004, OR = 3.662, 95% CI = 1.524-8.798) (Table 4).
Figure 2.
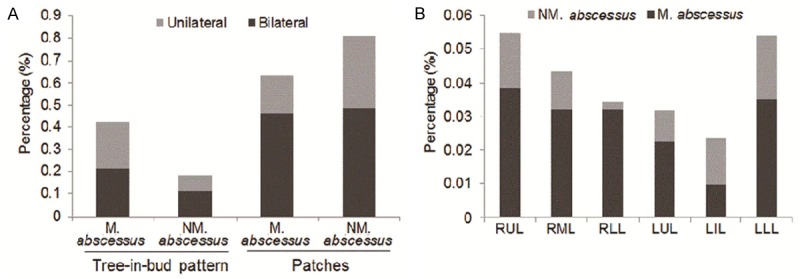
Percentage of tree-in-bud and patchy shadow in bilaterally and unilaterally locations, and the proportion of involved lobes in Mycobacterium abscesses-LD and NM abscesses LD. A. Higher percentage of tree-in-bud was found unilaterally in Mycobacterium abscesses LD (P = 0.029), but no significant difference for patchy shadows. B. A Higher proportion of small centrilobular nodules in the right lower lung (RRL) were found in Mycobacterium abscesses-LD than in NM abscesses-LD.
Table 4.
Multivariate analyses of predictors for Mycobacterium and NM abscesses
| CT findings | P | Odds ratio | 95% CI | |
|---|---|---|---|---|
| Tree-in-bud pattern | 0.004 | 3.662 | 1.524-8.798 | Mycobacterium abscesses |
| Thick-walled cavity (d < 3 cm) | 0.126 | 1.930 | 0.831-4.482 | |
| Patches | 0.014 | 0.326 | 0.133-0.799 | NM abscesses |
| Calcified lymph nodes | 0.223 | 0.343 | 0.062-1.916 |
Discussion
The present study analyzed the CT imaging characteristics of Mycobacterium abscesses-LD patients with positive sputum AFB in China. It represents the largest sample size of Mycobacterium abscesses of any similar reports on NTM. Since all the collected samples were characterized by bacteria identification, the finding that tree-in-bud is an independent predictor of Mycobacterium abscesses-LD is potentially of great interest.
Most research has considered nodules, tree-in-bud, patchy shadow (consolidation, ground glass), cavities, bronchiectasis and fibrous stripes to be the major CT manifestations of NTM lung diseases [11,12], and indeed similar results were found in the present study. In research on the CT characteristics of Mycobacterium abscesses-LD carried out by Han et al. and Jeong et al. the main findings were that tree-in-bud, bronchiectasis, fibrous cavities and nodules, were present, mainly in the upper lung, findings partly consistent with our research, since bronchiectasis was the most common CT result in our study (43/52), with cavity being detected relatively less frequently (17/52). Other CT imaging characteristics (e.g., patchy shadows, interstitial fibrosis, lymph nodes) also appeared, and patchy shadows as well as lung consolidations were common detected in patients with Mycobacterium abscesses-LD.
Although the CT manifestations of Mycobacterium abscesses-LD were comparatively recognized, to our knowledge, no reports on a comparison of the CT characteristics between Mycobacterium abscesses and NM abscesses-LD have been published. Chung et al. compared CT characteristics between Mycobacterium abscesses and Mycobacterium avium intracellular-LD, and found no differences between nodules and bronchiectasis between the two groups [13], findings which were certainly similar to our results; however, it is noteworthy that tree-in-bud was more common in Mycobacterium abscesses-LD. Tree-in-bud refers to small airway (at the bronchiole level) involvement of lesions, resulting in expansion of the airway and infiltration of pathological substances into the tube cavities, which manifests as nodular shadows of diameter of 2~4 mm and branch line shadows connected with these nodules in thin layer CT, which look like tree-in-buds. In a report on 50 cases of NTM lung diseases by Jun et al. the occurrence rate of tree-in-bud was 50% (25/50), which was similar to our results (42.3%).
Based on the studies of Chung et al. and Han et al. the CT characteristics of Mycobacterium abscesses-LD mainly occurred bilaterally and without a preferred lung lobe distribution, which was different from our findings. In the present study we also found that patchy shadows were more common in NM abscesses-LD, but the proportion of the lung lobe affected by patchy shadow, laterality and preferred location showed no significant differences compared to Mycobacterium abscesses-LD, which has not previously been reported. The reason might be that patchy shadow was not included in the CT imaging analysis of previous reports. Complications such as emphysema or pulmonary bullae, pleural effusion or pleural thickening adhesions, atelectasis or destroyed lung and mediastinal lymphadenectasis may exist and affect the multiple lung lobe CT results. For the differential diagnosis of NTM-LD, both microbiological analysis and clinical imaging data are considered to be indispensable [14]. Microbial analysis is highly specific but necessarily takes a longer time to complete, and therefore early differential diagnosis of Mycobacterium abscesses-LD by CT appearances before confirmed microbiological diagnosis is important clinically so that appropriate treatment can be initiated.
Above all, common characteristics of NTM-LD can be found in both Mycobacterium abscesses and NM abscesses-LD, such as bronchiectasis, tree-in-bud, cavity, lung consolidation and nodule shadows. However, bronchiectasis, a higher proportion of lung lobes involved by tree-in-bud, preferred unilateral, and inferior lobe lesions of the right lung was more common in Mycobacterium abscesses-LD, but patchy shadow was more common in NM abscesses-LD.
Due to some similar imaging findings between the two types of NTM-LD and certain imaging similarity with secondary pulmonary tuberculosis in some patients, the differential diagnosis of the two types of NTM lung disease should be considered closely with the clinical manifestations. NTM lung disease should be considered for those patients with positive clinical respiratory secretions AFB but ineffective anti-tuberculosis treatment.
In summary, if lesions were mainly bronchiectasis and accompanied by cavity, lung consolidation, tree-in-bud and nodule shadows, NTM-LD infection should be strongly suspected. If a higher proportion of the lung lobe is involved by tree-in-bud, and with preferred unilateral occurrence in the inferior lobe of the right lung, Mycobacterium abscesses-LD is likely indicated. For those with a higher percentage of patchy shadows, but without preferred unilateral tree-in-bud occurrence in the inferior lobe of the right lung, NM abscesses-LD may be indicated. CT imaging results may facilitate the early diagnosis and differentiation of NTM-LD before confirmed microbiological diagnosis reports are available.
Acknowledgements
The project was supported by grant No. 12ZR1426200 from the Natural Science Foundation of Shanghai science and technology committee; grant No. 14411962900 from the Medical Guide Program of Shanghai science and technology committee; grant No. 81101231 from the National Natural Science Foundation of China and grant No. 1511219019 from Young Talent Training Program of Tongji University.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002;23:553–567. doi: 10.1016/s0272-5231(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 3.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 4.Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, Park YK, Kang S. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal. 2008;22:415–420. doi: 10.1002/jcla.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tettelin H, Davidson RM, Agrawal S, Aitken ML, Shallom S, Hasan NA, Strong M, de Moura VC, De Groote MA, Duarte RS, Hine E, Parankush S, Su Q, Daugherty SC, Fraser CM, Brown-Elliott BA, Wallace RJ Jr, Holland SM, Sampaio EP, Olivier KN, Jackson M, Zelazny AM. High-level relatedness among Mycobacterium abscessus subsp. massiliense strains from widely separated outbreaks. Emerg Infect Dis. 2014;20:364–371. doi: 10.3201/eid2003.131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benwill JL, Wallace RJ Jr. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis. 2014;27:506–510. doi: 10.1097/QCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 7.Orme IM, Ordway DJ. Host response to nontuberculous mycobacterial infections of current clinical importance. Infect Immun. 2014;82:3516–3522. doi: 10.1128/IAI.01606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catherinot E, Roux AL, Vibet MA, Bellis G, Lemonnier L, Le Roux E, Bernède-Bauduin C, Le Bourgeois M, Herrmann JL, Guillemot D, Gaillard JL OMA group. Inhaled therapies, azithromycin and Mycobacterium abscessus in cystic fibrosis patients. Eur Respir J. 2013;41:1101–1106. doi: 10.1183/09031936.00065612. [DOI] [PubMed] [Google Scholar]
- 9.Wallace RJ Jr, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother. 2014;69:1945–1953. doi: 10.1093/jac/dku062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O’Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 11.Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231:880–886. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y, Song JW, Chae EJ, Lee HJ, Lee CW, Do KH, Seo JB, Kim MY, Lee JS, Song KS, Shim TS. CT findings of pulmonary non-tuberculous mycobacterial infection in non-AIDS immunocompromised patients: a case-controlled comparison with immunocompetent patients. Br J Radiol. 2013;86:20120209. doi: 10.1259/bjr.20120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, Kim S. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. J Korean Med Sci. 2005;20:777–783. doi: 10.3346/jkms.2005.20.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somoskovi A, Salfinger M. Nontuberculous mycobacteria in respiratory infections: advances in diagnosis and identification. Clin Lab Med. 2014;34:271–295. doi: 10.1016/j.cll.2014.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


