Abstract
Background: Chemotherapy with capecitabine combined with leucovorin, oxaliplatin, and irinotecan plus bevacizumab (XELOXIRI-Bev) or fluorouracil, leucovorin, oxaliplatin, and irinotecan plus bevacizumab (FOLFOXIRI-Bev), is recently introduced as first-line treatment for metastatic colorectal cancer (mCRC). The comparison between the two strategies above in clinical efficacy has not been assessed. Methods: We retrospectively reviewed 138 patients with untreated metastatic colorectal cancer to receive either FOLFOXIR-Bev (group 1) or XELOXIRI-Bev (group 2). Up to 12 cycles of treatment were administered, followed by fluorouracil plus bevacizumab until disease progression. The primary end point was progression-free survival. Results: The mean progression-free survival was 13.5 months in the group 1, as compared with 10.4 months in the group 2 (hazard ratio for progression, 0.3; 95% confidence interval [CI], 0.12 to 0.83; P = 0.032). The objective response rate was 71% in the group 1 and 52.2% in the group 2 (P = 0.006). Overall survival was not found significant difference between the two groups (group 1 vs. 2; 31.3 vs. 24.6 months; hazard ratio for death, 0.6; 95% CI, 0.29 to 1.15; P = 0.115). The incidences of grade 3 or 4 neurotoxicity, stomatitis, diarrhea, and neutropenia were significantly higher in the group 1. Conclusion: FOLFOXIR-Bev, as compared with XELOXIRI-Bev, improved the outcomes in patients with mCRC, but increased the incidence of some adverse events.
Keywords: XELOXIRI-Bev, FOLFOXIRI-Bev, metastatic colorectal cancer (mCRC), outcomes
Introduction
Colorectal carcinoma (CRC) is one of the leading causes of cancer-related death in both men and women [1,2]. Every year, more than 1 million patients are newly diagnosed with CRC and most of them eventually develop into metastatic colorectal cancer (mCRC) [2-4], which is presented with synchronous or metachronous metastatic disease after the resection of the primary tumor [5]. In the past years, a number of studies have consistently demonstrated the poor mCRC patients’ prognosis with median overall survival (OS) ranging from 9 to 14 months [6-9]. In an attempt to better improve the life-quality of mCRC patients, several different strategies were developed with effective outcomes [10-13].
In the past few years, some different combinations of the newer cytotoxic agents, such as irinotecan and oxaliplatin, with fluorouracil and targeted agents including bevacizumab, cetuximab, and panitumumab, have evidently increased the tumor response and improved the survival of terminal colorectal cancer patients without resection [14]. In order to enhance therapeutic effects and to expand the proportion of patients responded to all active agents, more potential, active first-line chemo triplet regimens have been developed, including the combination of 5-FU with irinotecan and oxaliplatin. Especially, a phase III study carried out by the GONO [15] demonstrated that a combination of 5-FU with LV, irinotecan and oxaliplatin (FOLFOXIRI) might improve survival in mCRCas first-line treatment as a result of manageable toxicities and an increased tumor response rate and a higher rate of radical resection of metastases, and therefore seemed to be superior to 5-FU/LV and irinotecan (FOLFIRI). However, this regimen presented more grade 3/4 diarrhea, stomatitis, and neutropenia. Therefore, an advanced combination-FOLFOXIRI plus bevacizumab (FOLFOXIRI-Bev) was developed to be thought to be one of the most active and favorable induction chemotherapy regimens. However, a main defect to the FOLFOXIRI-Bev regimen is that continuous infusion of 5-FU with a biweekly schedule is hard to change.
The use of capecitabine instead of 5-FU, either with irinotecan or oxaliplatin, was proved to be more effect [16,17]. With the substitution of capecitabine for the infusion of 5-FU, the XELOXIRI regimen can simplify the treatment delivery of the FOLFOXIRI regimen and decrease the complications associated with the central venous catheters which are applied in the FOLFOXIRI regimen. A report by Yasushi et al. [11] with XELOXIRI plus bevacizumab (XELOXIRI-Bev) showed promising response rate with manageable toxicities, suggesting a feasible regimen for patients with mCRC and concluding it as a potential alternative to FOLFOXIRI-Bev. However, an investigation by GONO with XELOXIRI combination [18] revealed a high incidence of diarrhea, and therefore concluded that the combination is not preferable to FOLFOXIRI.
Up to now, few studies have conducted a comparison between FOLFOXIRI-Bev and XELOXIRI-Bev regimens. On the basis of the promising results of both two regimens, we performed this study to compare XELOXIRI-Bev with FOLFOXIRI-Bevin patients with mCRC.
Materials and methods
The protocol was approved by the Ethics Committee of Tianjin Third Central Hospital. All patients provided their written informed consent.
From January 2009 to May 2013, a total of 69 patients with previously untreated mCRC received the XELOXIRI-Bev regimen. As a matched-pair control group with a ratio of 1:1, 69 patients were selected from those who underwent FOLFOXIRI-Bev regimen for the treatment of mCRC at the same period. All patients were required to meet the following eligibility criteria: 1) colorectal cancer confirmed by histopathology, 2) unresectable mCRC, 3) age 18 to 75 years, 4) an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or lower, 5) presence of a measurable lesion according to WHO criteria, that is, leukocyte count ≥3,500 mm3, neutrophil count ≥1,500 mm3, platelet count ≥100,000 mm3, serum creatinine ≤1.3 mg/dL, serum bilirubin ≤1.5 mg/dL and serum aspartate aminotransferase and alanine aminotransferase 2.5* normal values or less (≤5 if liver metastases). The patients who had previous palliative chemotherapy, total colectomy or symptomatic chronic diseases for metastatic disease were excluded. Previous chemotherapy included irinotecan or oxaliplatin. Moreover, patients with myocardial infarction in the last 24 months or uncontrolled arrhythmia, active infections, inflammatory bowel disease were not included either.
Treatment was administered every 2 weeks for a maximum of 12 cycles until evidence of disease progression, unacceptable toxicity, and patient refusal. Patients in group 1 received up to 12 cycles of FOLFOXIRI-Bev. The regimen, described by Loupakis et al. [13], consisted of a 30-minute infusion of bevacizumab at a dose of 5 mg per kilogram, a 60-minute infusion of irinotecan at a dose of 165 mg per square meter, and a 120-minute infusion of oxaliplatin at a dose of 85 mg per square meter and a concomitant 120-minute infusion of leucovorin at a dose of 200 mg per square meter, followed by a 48-hour continuous infusion of fluorouracil to a total dose of 3200 mg per square meter. Individuals in group 2 underwent up to 12 cycles of XELOXIRI-Bev. The regimen was modified according to the regimen introduced by Yasushi et al. [11], consisting of an infusion of bevacizumab at a dose of 7.5 mg/kg on day 1 (the first infusion was delivered over 90 min, the second infusion over 1 h, and subsequent infusions over 30 min), a 60-minute infusion of irinotecan at a dose of 120 mg/m2 for dose levels 1, 2, and 3) in 250 ml of normal saline over 1 h on day 1, and an infusion of oxaliplatin 100 mg/m2 in 250 ml dextrose 5%, followed by an oral of capecitabine (1,700 mg/m2 per day) from day 2 to 14.
Evaluation criteria
Pre-treatment assessments were measured according to a detailed medical history and physical examination, blood chemistry, serum levels of carcinoembryonic antigen (CEA) and computed tomography scans (CT) of the chest and abdomen. WHO criteria was used to evaluate tumor response, the duration of which was determined from the first documentation of response to disease. The determination of progression free survival (PFS) was the interval between the initiation of treatment and the date when disease progression was first documented or the date of death from any cause. OS was measured from the date of treatment initiation to the date of death. The follow up time was measured from the day of first treatment administration to the time of the present analysis (for patients still alive) or death for deceased patients.
Statistical analysis
All statistical analyses were carried out using the SPSS software version 19.0. Data were expressed as mean ± SD. Paired Student’s t-test was conducted to analyze the differences between two groups. Survival curves were plotted by using the Kaplan-Meier method and compared by using the log-rank test. Cox proportional-hazards modeling was also performed as supportive analyses. Subgroup analyses of PFS were performed by means of an interaction test to determine the consistency of the treatment effect according to key baseline characteristics. The objective response rate, the resection rate for metastases, and the incidence of adverse events in the two groups were compared with the use of the chi-square test for heterogeneity or with Fisher’s exact test when appropriate. A P-value of <0.05 considered statistically significant.
Results
A total of 138 patients were enrolled in this study, and their baseline characteristics are summarized in Table 1. The mean age was 64 years in group 1, and 62 in group 2. A total of 55 patients had liver metastases, 26 out of whom were included in group 1 and remaining were in group 2. No patients had received previous adjuvant therapy. The follow-up period ranged from 3-54 months (median, 27 months; mean, 26.3 months). Among the 138 patients, a total of 85 patients died during the follow-up.
Table 1.
Patient characteristics
| Characteristics | FOLFOXIR-Bev (group 1) | XELOXIRI-Bev (group 2) | P value |
|---|---|---|---|
| Sample size | 69 | 69 | |
| Age | 64 ± 14.5 | 62 ± 13.9 | 0.749 |
| Gender | 39/30 | 36/33 | 0.608 |
| BMI | 25.3 ± 2.43 | 25.8 ± 2.52 | 0.903 |
| ECOG PS | 0.861 | ||
| 0 | 43 | 42 | |
| 1 | 26 | 27 | |
| Primary tumor | 0.564 | ||
| Colon | 52 | 49 | |
| Rectum | 17 | 20 | |
| Previous adjuvant chemotherapy | 0.830 | ||
| Yes | 13 | 14 | |
| No | 56 | 55 | |
| Time to metastases | 0.796 | ||
| Metachronous | 8 | 9 | |
| Synchronous | 61 | 60 | |
| No. of metastatic sites | 0.732 | ||
| 1 | 37 | 39 | |
| >1 | 32 | 30 | |
| Liver-only metastases | 0.602 | ||
| Yes | 26 | 29 | |
| No | 43 | 40 | |
| CEA | 0.607 | ||
| <100 | 42 | 43 | |
| ≥100 | 27 | 23 | |
| Köhne score | 0.493 | ||
| High-risk | 7 | 9 | |
| Intermediate-risk | 35 | 31 | |
| Low-risk | 27 | 29 |
Abbreviations: XELOX-Bev, capecitabine combined with oxaliplatin plus bevacizumab; FOLFOXIRI, fluorouracil, leucovorin, oxaliplatin, and irinotecan plus bevacizumab; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
All patients received at least one cycle of treatment and were evaluated for safety. Both regimens were relatively well tolerated and associated with manageable toxicities. As showed in Table 2, the median number of administered cycles was 11 in the FOLFOXIRI-Bev group and 11 in the XELOXIRI-Bev group. The relative dose-intensity of administered fluorouracil, oxaliplatin, irinotecan and bevacizumab ranged between 81% and 83% of planned for all agents in FOLFOXIRI-Bev group. The relative dose-intensity of administered capecitabine, oxaliplatin, irinotecan and bevacizumab ranged between 81% and 84% of planned for all agents in XELOXIRI-Bev group. Treatment interruptions because of toxicity were 4% for FOLFIRI and 9% for FOLFOXIRI (P = 0.19). Treatment-related grade 3 or 4 adverse events occurring in patients of both groups are summarized in Table 3. Most commonly observed toxicities were neutropenia, diarrhea, nausea, stomatitis, vomiting, peripheral neurotoxicity, asthenia, and hypertension. The incidence of grade 3 or 4 neutropenia (P = 0.002) and peripheral neuropathy (P = 0.001) was significantly higher in the FOLFOXIRI-Bev group than in the XELOXIRI-Bev group. No significant differences in bevacizumab-related adverse events were observed between groups.
Table 2.
Number of cycles and relative dose intensities
| Variables | FOLFOXIR-Bev (group 1) | XELOXIRI-Bev (group 2) |
|---|---|---|
| No. of cycles | ||
| Total | 687 | 683 |
| Median | 11 | 10 |
| Range | 1-16 | 1-16 |
| Relative dose intensity with respect to planned, % | ||
| Oxaliplatin | 83 | 84 |
| Fluorouracil | 82 | - |
| Irinotecan | 82 | 81 |
| Capecitabine | - | 82 |
| Bevacizumab | 81 | 83 |
Table 3.
Maximum toxicity per patient with most common grade 3 or 4
| Event | FOLFOXIR-Bev | XELOXIRI-Bev | P value |
|---|---|---|---|
| Neutropenia | 32 (46.4%) | 15 (21.7%) | 0.002 |
| Febrile neutropenia | 8 (11.6%) | 5 (7.2%) | 0.382 |
| Diarrhea | 14 (20.3%) | 7 (10.1%) | 0.097 |
| Stomatitis | 9 (13.0%) | 4 (5.8%) | 0.145 |
| Nausea | 3 (4.3%) | 2 (2.9%) | 0.649 |
| Vomiting | 6 (8.7%) | 2 (2.9%) | 0.145 |
| Asthenia | 10 (14.5%) | 6 (8.7%) | 0.228 |
| Peripheral neuropathy | 11 (15.9%) | 0 | 0.001 |
| Hypertension | 4 (5.8%) | 1 (1.4%) | 0.172 |
| Venous thromboembolism | 5 (7.2%) | 4 (5.8%) | 0.730 |
| Serious adverse events | 14 (20.3%) | 12 (17.4%) | 0.663 |
All patients were evaluated for tumor response, displaying in Table 4. The response rate was 71.0% in the FOLFOXIRI-Bevgroup, as compared with 52.2% in the XELOXIRI-Bev group (odds ratio, 2.2; 95% CI, 1.11 to 4.53; P = 0.023). In the multivariate analysis, only treatment with FOLFOXIRI was an independent predictive factor for response (hazard ratio [HR], 2.4; 95% CI, 1.2 to 4.33; P = .014). The rate of R0 resection of metastases was not significantly different in treatment groups (16% in the FOLFOXIRI-Bev group vs. 11% in the XELOXIRI-Bev group, P = 0.462).
Table 4.
Efficacy in patients with mCRC
| Variable | FOLFOXIR-Bev (group 1) | XELOXIRI-Bev (group 2) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Response | ||||
| Complete | 4 | 2 | ||
| Partial | 45 | 34 | ||
| Stable disease | 13 | 22 | ||
| Progression | 7 | 11 | ||
| Not assessable | 0 | 0 | ||
| Overall response rate | 49 (71.0%) | 36 (52.2%) | 2.2 (1.11-4.53) | 0.023 |
| PFS | ||||
| Progression event | 51 (73.9%) | 62 (89.9%) | 0.3 (0.12-0.83) | 0.032 |
| Months of PFS | 13.5 | 10.4 | ||
| OS | ||||
| Deaths | 38 (68.1%) | 47 (55.1%) | 0.6 (0.29-1.15) | 0.115 |
| Months of OS | 31.3 | 24.6 |
Abbreviations: PFS, progression-free survival; OS, overall survival.
As shown in Figures 1 and 2, patient survival analysis showed no difference in the OS time between the two groups, but and longer PFS time in (OS, P = 0.115, log-rank = 1.158; and PFS, P = 0.032, log-rank = 15.926) (Table 4). Subsequently, the Cox’s multivariate analysis demonstrates that the only in dependent prognostic factors for reduction of the death risk was liver metastases (HR, 0.59; 95% CI, 0.28 to 0.87; P = 0.004). Moreover, the Cox’s multivariate analysis demonstrates that treatment arm was only independent prognostic factor for reduction of the progression risk (HR, 0.62; 95% CI, 0.41 to 0.81; P = 0.001).
Figure 1.
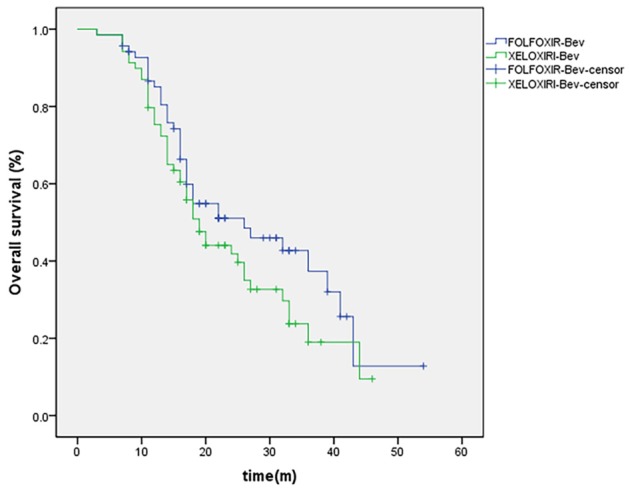
Kaplan-Meier Estimates of Overall Survival, According to Treatment Group.
Figure 2.
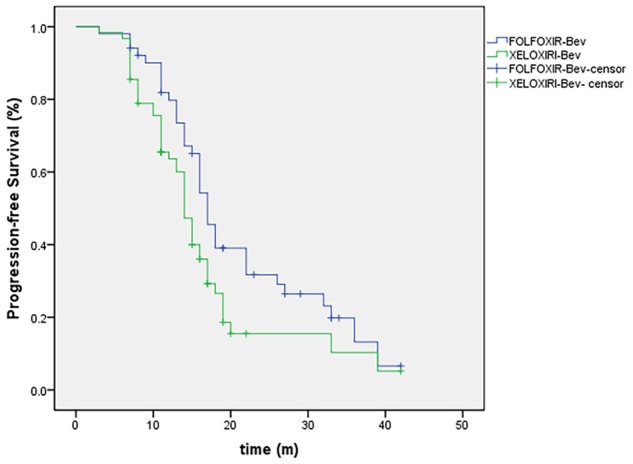
Kaplan-Meier Estimates of Progression-free, According to Treatment Group.
Discussion
To the best of our knowledge, this is the first study that evaluates the benefits and limitations of two highly active four-drug regimens-XELOXIRI-Bev and FOLFOXIR-Bev chemotherapy for the first-line treatment of mCRC. The results in this study showed improved progression-free survival among patients with mCRC after treatment with the combination of FOLFOXIRI-Bev, as compared with XELOXIRI-Bev. Moreover, an absolute increase of 18.8% in response rate was reported. However, the incidence of grade 3 or 4 neutropenia (P = 0.002) and peripheral neuropathy (P = 0.001) was significantly higher in the FOLFOXIRI-Bev group than in the XELOXIRI-Bev group.
In the late several years, considerable progress in the management of mCRC has been achieved, such as better efficacy of chemotherapy, increased use of surgery on metastases [19], and, more recently, the use of targeted agents [20,21]. Previous studies have fully explored the efficacy and safety of combination chemotherapy in mCRC patients [22-24]. Although published literature have demonstrated that the application of all the three main active cytotoxic agents can yield good outcome in unresectable patients [25], only 50% to 80% of patients can be tolerated to all three drugs in a successive strategy with doublets. Moreover, there is consistent evidence that a greater proportion of patients who received increased the activity of chemotherapy can undergo a secondary surgery on metastases, and can obtain longer survival time [26]. Many previous reports have showed that the addition of bevacizumab to chemotherapy in patients could acquire effective outcomes [27-30].
Generally, FOLFOXIRI-Bev regimens are even more active than FOLFOXIRI and have achieved high response rates of 80% and a considerably high R0 resection rate of 40% in patients with liver-only metastases [13,31]. A phase 2 study has investigated the efficacy and safety of FOLFOXIRI-Bev, and the results has revealed a response rate of 77% with median PFS time of 13.1 months and median OS time of 30.9 months [32]. However, in several investigations of FOLFOXIRI-Bev regimens, the main grade 3 or 4 adverse events were neutropenia in 49%, diarrhea in 14% and hypertension in 11%, which might be caused by infusional 5-FU [13].
With infusional 5-FU substituted by capecitabine, the XELOXIRI regimen can simplify the treatment delivery of the FOLFOXIRI regimen and reduce the complications as a result of the use of the central venous catheters in the FOLFOXIRI regimen. Yasushi et al. in their study showed the XELOXIRI-Bev regimen feasible with manageable toxicities [11]. However, the most concern in the application of the XELOXIRI combination is gastrointestinal toxicity and worse grade 3/4 diarrhea associated with substitution of capecitabine, compared with 5-FU regimens [14]. Vasile et al. reported the major concern with the GONO- XELOXIRI regimen, which is gastrointestinal toxicity, in particular, grade 3/4 diarrhea found in 30% of patients [18]. Furthermore, for the COI-XELOXIRI regimen, the main symptom of toxicity was grade 3/4 diarrhea that is experienced by 24% of the patients during treatment [33].
The current study compared XELOXIRI-Bev with FOLFOXIRI-Bev in patients with mCRC. Our results show that the combination of FOLFOXIRI-Bev is more effective in mCRC patients with higher response rate, and longer PFS time, compared to XELOXIRI-Bev regimen. However, XELOXIRI-Bev regimen is safer than FOLFOXIRI-Bev regimen as a result of lower incidence of grade 3 or 4 neutropenia and peripheral neuropathy. Several limitations of the current study should be considered. This is a retrospective study in which bias was inevitable, and sample size was relatively small.
Conclusion
In summary, FOLFOXIRI-Bev revealed to be superior to XELOXIRI-Bev in efficacy that longer PFS time and higher tumor response rate were achieved in patients with mCRC. However, XELOXIRI-Bev could decrease incidence of grade 3 or 4 neutropenia and peripheral neuropathy, compared to FOLFOXIRI-Bev, and, therefore, XELOXIRI-Bev seemed to be safer. In view of several limitations of this study, more studies involving well-designed randomized controlled trials are needed to be investigate whether XELOXIRI-Bev regimen used as an alternative to FOLFOXIRI-Bev.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Ciombor KK, Berlin J. Targeting metastatic colorectal cancer-present and emerging treatment options. Pharmgenomics Pers Med. 2014;7:137–144. doi: 10.2147/PGPM.S47582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim R, Schell MJ, Teer JK, Greenawalt DM, Yang M, Yeatman TJ. Co-evolution of somatic variation in primary and metastatic colorectal cancer may expand biopsy indications in the molecular era. PLoS One. 2015;10:e0126670. doi: 10.1371/journal.pone.0126670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 7.Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, Silver J, Ogino S, Hooshmand S, Kwak E, Freed E, Meyerhardt JA, Saridaki Z, Georgoulias V, Finkelstein D, Fuchs CS, Kulke MH, Shivdasani RA. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. 2009;101:465–472. doi: 10.1038/sj.bjc.6605164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 9.Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, Hurwitz HI, Kabbinavar F, Novotny WF, Hillan KJ, Koeppen H. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 10.Stein A, Glockzin G, Wienke A, Arnold D, Edelmann T, Hildebrandt B, Hollerbach S, Illerhaus G, Konigsrainer A, Richter M, Schlitt HJ, Schmoll HJ. Treatment with bevacizumab and FOLFOXIRI in patients with advanced colorectal cancer: presentation of two novel trials (CHARTA and PERIMAX) and review of the literature. BMC Cancer. 2012;12:356. doi: 10.1186/1471-2407-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato Y, Ohnuma H, Hirakawa M, Takahashi M, Osuga T, Okagawa Y, Murase K, Takada K, Kawano Y, Iyama S, Hayashi T, Sato T, Miyanishi K, Takimoto R, Kobune M, Okita K, Mizuguchi T, Furuhata T, Hirata K, Kato J. A dose-escalation study of oxaliplatin/capecitabine/irinotecan (XELOXIRI) and bevacizumab as a first-line therapy for patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2015;75:587–594. doi: 10.1007/s00280-014-2672-9. [DOI] [PubMed] [Google Scholar]
- 12.Vamvakas L, Matikas A, Karampeazis A, Hatzidaki D, Kakolyris S, Christophylakis C, Boukovinas I, Polyzos A, Georgoulias V, Souglakos J. Capecitabine in combination with oxaliplatin and bevacizumab (AXELOX) as 1st line treatment for fit and vulnerable elderly patients (aged >70 years) with metastatic colorectal cancer (mCRC): a multicenter phase II study of the Hellenic Oncology Research Group (HORG) BMC Cancer. 2014;14:277. doi: 10.1186/1471-2407-14-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 14.Bekaii-Saab T, Wu C. Seeing the forest through the trees: a systematic review of the safety and efficacy of combination chemotherapies used in the treatment of metastatic colorectal cancer. Crit Rev Oncol Hematol. 2014;91:9–34. doi: 10.1016/j.critrevonc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crino L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, Schoffski P, Sobrero A, Van Cutsem E, Diaz-Rubio E. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J. Clin. Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, van Bochove A, Sinnige HA, Creemers GJ, Tesselaar ME, Slee PH, Werter MJ, Mol L, Dalesio O, Punt CJ. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): A phase III randomised controlled trial. Lancet. 2007;370:135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 18.Vasile E, Masi G, Fornaro L, Cupini S, Loupakis F, Bursi S, Petrini I, Di Donato S, Brunetti IM, Ricci S, Antonuzzo A, Chiara S, Amoroso D, Andreuccetti M, Falcone A. A multicenter phase II study of the combination of oxaliplatin, irinotecan and capecitabine in the first-line treatment of metastatic colorectal cancer. Br J Cancer. 2009;100:1720–1724. doi: 10.1038/sj.bjc.6605075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill S, Goldberg RM. First-line treatment strategies to improve survival in patients with advanced colorectal cancer. Drugs. 2004;64:27–44. doi: 10.2165/00003495-200464010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J. Clin. Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 23.Mattioli R, Massacesi C, Recchia F, Marcucci F, Cappelletti C, Imperatori L, Pilone A, Rocchi M, Cesta A, Laici G, Bonsignori M, Lippe P. High activity and reduced neurotoxicity of bi-fractionated oxaliplatin plus 5-fluorouracil/leucovorin for elderly patients with advanced colorectal cancer. Ann Oncol. 2005;16:1147–1151. doi: 10.1093/annonc/mdi222. [DOI] [PubMed] [Google Scholar]
- 24.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, O’Mahony MS, Maughan TS, Parmar M, Langley RE FOCUS2 Investigators; National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol. 2004;22:1209–1214. doi: 10.1200/JCO.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 27.Kozloff MF, Berlin J, Flynn PJ, Kabbinavar F, Ashby M, Dong W, Sing AP, Grothey A. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology. 2010;78:329–339. doi: 10.1159/000320222. [DOI] [PubMed] [Google Scholar]
- 28.Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J. Clin. Oncol. 2009;27:199–205. doi: 10.1200/JCO.2008.17.7931. [DOI] [PubMed] [Google Scholar]
- 29.Price TJ, Zannino D, Wilson K, Simes RJ, Cassidy J, Van Hazel GA, Robinson BA, Broad A, Ganju V, Ackland SP, Tebbutt NC. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: A subgroup analysis from the AGITG MAX trial: An international randomised controlled trial of Capecitabine, Bevacizumab and Mitomycin C. Ann Oncol. 2012;23:1531–1536. doi: 10.1093/annonc/mdr488. [DOI] [PubMed] [Google Scholar]
- 30.Venderbosch S, Doornebal J, Teerenstra S, Lemmens W, Punt CJ, Koopman M. Outcome of first line systemic treatment in elderly compared to younger patients with metastatic colorectal cancer: a retrospective analysis of the CAIRO and CAIRO2 studies of the Dutch Colorectal Cancer Group (DCCG) Acta Oncol. 2012;51:831–839. doi: 10.3109/0284186X.2012.699193. [DOI] [PubMed] [Google Scholar]
- 31.Bruera G, Ricevuto E. Intensive chemotherapy of metastatic colorectal cancer: Weighing between safety and clinical efficacy: Evaluation of Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: A phase 2 trial. Lancet Oncol 2010;11:845-52. Expert Opin Biol Ther. 2011;11:821–824. doi: 10.1517/14712598.2011.582462. [DOI] [PubMed] [Google Scholar]
- 32.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, Ciarlo A, Del Monte F, Cortesi E, Amoroso D, Granetto C, Fontanini G, Sensi E, Lupi C, Andreuccetti M, Falcone A. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 33.Bajetta E, Celio L, Ferrario E, Di Bartolomeo M, Denaro A, Dotti K, Mancin M, Bajetta R, Colombo A, Pusceddu S. Capecitabine plus oxaliplatin and irinotecan regimen every other week: a phase I/II study in first-line treatment of metastatic colorectal cancer. Ann Oncol. 2007;18:1810–1816. doi: 10.1093/annonc/mdm347. [DOI] [PubMed] [Google Scholar]


