Abstract
To explore the protective effect of dexmedetomidine (Dex) on rats with renal ischemia-reperfusion injury and the influence of Dex on the expression of tight junction protein in kidney. Grouped 40 SPF male rats into 4 groups, sham operation group (group S), ischemia-reperfusion group (group I/R), pretreatment with Dex group (group Pre/Dex), post-treatment with Dex group (group Post/Dex), randomly, 10 rats each group. Rats in group S were anaesthetized and set up with removal of right kidney; rats in group I/R were set up with removal of right kidney and left renal artery clamping for 45 min followed by 60 min reperfusion; rats in group Pre/Dex were intravenous injected with Dex (1 μg/kg) for 30 min after indwelling catheter via femoral vein puncture; rats in group Post/Dex were intravenous injected with Dex (1 μg/kg) for 30 min after left renal reperfusion. The kidneys in each group were made out pathologic slices after 6 h I/R, stained with HE; blood samples were taken with separation plasma, creatinine (Scr) and urea nitrogen (BUN) were detected by automatic biochemical analyzer; IL-1β and TNF-α were detected by Enzyme-linked Immunosorbent Assay (ELISA); the expression level of tight junction protein ZO-1 and protein occludin in kidney were detected by Western-blot. The results of HE staining showed that, comparing to group S, the tissue of kidney in group I/R were damaged heavily with tubules dilatation and inflammation obviously, while lightened in group Pre/Dex and group Post/Dex. The results of detection of renal function and inflammatory factors showed that, comparing to group S, Scr, BUN, IL-1β and TNF-α were all enhanced in group I/R, group Pre/Dex and group Post/Dex, significantly (P < 0.05), while the inflammatory factors in group Pre/Dex and group Post/Dex were lower than in group I/R, significantly (P < 0.05). The results of Western-blot showed that the expression of protein ZO-1 and occludin in group Pre/Dex and group Post/Dex were higher than in group I/R, significantly (P < 0.05). Dex could reduce renal dysfunction induced by I/R, inhibit inflammatory response, up-regulate the expression of protein ZO-1 and occludin and protect renal.
Keywords: Dexmedetomidine (Dex), renal, ischemia-reperfusion, renal function, tight junction protein
Introduction
Kidney transplantation is one of the most ideal to treating with terminal renal diseases. Since the first successful case of renal transplantation 50 years ago, there had more than 50 million of patients with renal failure receipted renal transplantation and lived their lives [1,2]. However, during the perioperation of renal transplantation, ischemia-reperfusion injury was still an important problem. Because of the structure characteristics, renal became one of the most sensitive organs in the ischemia-reperfusion injury [3]. With renal ischemia, renal blood flow reduced and tubular endothelial cells were injured, which causing renal tubular blockage, cell metabolism dysfunction after reperfusion, inflammation medium releasing, inflammation reaction aggravation, and leading to abnormal renal function and organs damage, even the damage of brain, lung, heart, liver and so on [4,5]. Recently, the studies on prevention and treatment aiming at IRI renal injury mainly focused on regulating one point of IRI, such as using anti-apoptotic chemicals, antioxidants, nuclear selective antagonists, alone [6,7]. All of the agents could not exert therapeutic effects integrally and were limited in clinical application, without reaching clinical effect.
Researches showed that α2 receptor agonist could sustain renal medulla blood flow to prevent from contrast induced nephropathy, and active the adrenergic receptors on presynaptic membrane of central and peripheral sympathetic nerve, thus reducing the stress reaction induced by surgery and plasma catecholamine concentration [8,9]. Dex was an α2 adrenoceptor agonists with high selectivity, not only initiating and sustaining natural sleeping state by activating central nervous system and leading to sedation and hypnosis, but also preventing from adverse reaction induced by α1 adrenergic receptor [10,11]. Bollucuoglu et al. [12] found that ischemia-reperfusion was one of the reasons leading to acute renal failure. By constructing the ischemia reperfusion model in mice, they found the renal tubular cells swollen, pyknosis and necrosis in renal tissues, and hyperemia in renal medulla; however, by intraperitoneal injection with Dex, renal injury decreased significantly, which indicating Dex could increase tolerance of renal injury. By Meta analysis, Tan et al. [13] found that Dex could induce sympathetic inhibitory effect, useful for long-term oxygen supply and demand in myocardium and exerting protective effect. But there were barely reported about the protective effect on organs and the relationship between the mechanism and anti-inflammatory response of Dex in renal ischemia reperfusion injury. We studied on the protective effect of Dex on renal ischemia reperfusion injury and investigated the mechanism for further researches on prevention and treatment of renal ischemia reperfusion injury in renal transplantation.
Materials and methods
Animals and grouping
40 male SD rats (SPF), weighting 250 to 300 g, obtained from the experimental animal center of GENERAL HOSPITAL OF SHENYANG MILITARY, grouped into 4 groups randomly, sham operation group (group S), ischemia-reperfusion group (group I/R), pretreatment with Dex group (group Pre/Dex), post-treatment with Dex group (group Post/Dex), 10 in each group. Rats in group S were anaesthetized and set up with removal of right kidney; rats in group I/R were set up with removal of right kidney and left renal artery clamping for 45 min followed by 60 min reperfusion; rats in group Pre/Dex were intravenous injected with Dex (1 μg/kg) for 30 min after indwelling catheter via femoral vein puncture; rats in group Post/Dex were intravenous injected with Dex (1 μg/kg) for 30 min after left renal reperfusion.
Construction of renal ischemia reperfusion models
According to the reference [10], rats were in eating and drinking’s abstention for 6 h before operation. Injected intraperitoneally with 10% chloral hydrate (3 mg/kg) to anesthesia. Details were followed: opened along the ventral median line (4.0 to 5.0 cm) and abdomen was exposed; moved the ileum, colon and spleen to the left, separated the right renal, ligated renal pedicle and removed; moved the ileum, colon and spleen to the right, exposed the left renal and pedicle, and occluded with non-injured vascular clamp; loosened the clamp after 45 min and followed by reperfusion, if renal turned from dark purple into red, it was successful to construct the model; closed the peritoneal.
Collecting samples
After successfully constructing renal ischemia-reperfusion models for 6 h, rats in each group were anesthetized with 10% chloral hydrate, collected blood from eyes, centrifuged at 3,000 r/min for 5 min, separated the supernatant into 1.5 mL EP tubes, stored at -80°C; took renal tissues (stored at -80°C) and fixed in neutral buffered formalin.
Detection
Renal pathological changes
Fixed the renal tissues in neutral buffered formalin for one or two days; washed with flowing water for 4 to 5 h, dried the tissues with filter paper, placed in 70% ethanol, overnight; placed in 80% ethanol with the same method, overnight; rehydrated with gradient ethanol, placed in xylene for 20 min; placed the tissues in wax for 3 h; embedded the tissues with metal embedding box; sectioned with thickness 3 to 5 μm; placed the sections in oven at 37°C, overnight; placed in xylene for dewaxing; rehydrated with gradient ethanol, placed the sections in distilled water for 3 min; stained in hematoxylin for 5 min; placed in clean water to recovered into blue, stained eosin for 30 s; placed in gradient ethanol to dehydrate, placed in xylene for 10 min; sealed with neutral gum and observed the renal pathological changes with microscope.
Renal function detecting
Collected the blood samples, placed in anticoagulative tube and sent to inspection department, detected Scr and BUN with automatic biochemical detector.
Detecting IL-1β and TNF-α by ELISA
Detected the serum samples by ELISA kit after centrifuging. The details were followed the specification: added 50 μL samples in the first and second well of the first row on enzyme label plate, diluted in multiple proportions; added 40 μL diluted samples each well, added 10 μL samples; incubated at 37°C for 30 min; removed the sealing membrane, discarded the liquid, added washing buffer with standing for 30 s, discarded the liquid, repeated 5 times, then dried; added 50 μL enzyme sign the second antibody, incubated at 37°C for 30 min; washed with washing buffer; added chromogenic reagent A and B (50 μL of each) in order, colored at 37°C in dark for 10 min; added 50 μL terminal liquid; detected the 450 nm absorbance value with enzyme mark instrument, drew calibration curve and analyzed the results.
Detecting the expression of protein ZO-1 and Occludin by western-blot
Added RIPA buffer in the tissue (stored at -80°C), centrifuged the tissue homogenate at 4°C with 12,000 r/min for 10 min, absorbed the supernatant, determined the total protein content by BCA method; added two times (1:1) of Loading-buffer, boiled for 5 min; the samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 5% stacking gel and 10% separating gel; placed the gel in buffer solution on the horizontal rotator for 30 min; transferred to polyvinylidene fluoride (PVDF) membrane for 1 h; washed with TBST, blocked in 5% dried milk at 4°C on the rotator, overnight; washed with TBST and added rabbit anti ZO-1 and rabbit anti occludin (both 1:800), separately, incubated for 1 h; washed 3 times and added HRP-goat anti rabbit IgG (1:3000), placed on the rotator for 1 h; washed with clean water, developed with ECL system, analyzed the gray value of western blot.
Statistic analysis
The data were showed as mean ± standard (x̅ ± s). With IBM-SPSS 19.0, analysis of variance was conducted for comparison between groups, there was a significant difference with a value of P < 0.05.
Results
Renal histology changes
Renal injury in group I/R was more serious than group S, with obvious inflammation, dilatation of tubules, hyperemia, edema, glomerular nephritis; renal injury in group Pre/Dex and group Post/Dex were lighten, inflammation reaction reduced, cells with abnormal morphology were less (Figure 1).
Figure 1.
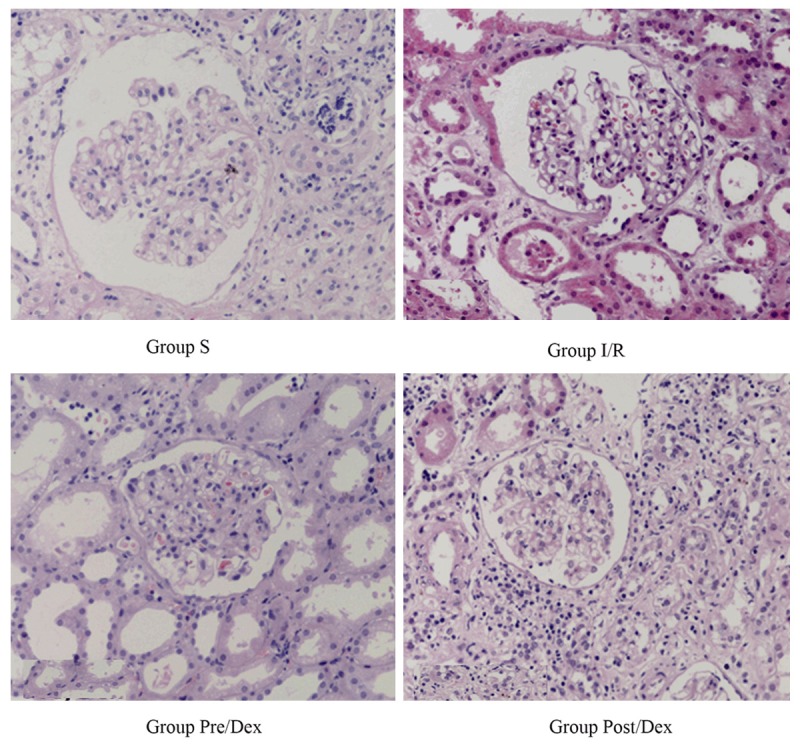
Pathological slices of renal ischemia reperfusion injury × 200.
Detection of renal function
All the samples were detected by automatic biochemical detector. The results were followed (Table 1). Comparing to group S, Scr and BUN in other groups all increased significantly (P < 0.05). There was no significant difference of Scr and BUN between group Pre/Dex and group Post/Dex (P > 0.05), while both lower than in group I/R (P < 0.05).
Table 1.
Comparison of Scr and BUN
| Groups | Group S | Group I/R | Group Pre/Dex | Group Post/Dex |
|---|---|---|---|---|
| Scr (μmol/L) | 41.21 ± 5.31 | 134.34 ± 12.79* | 81.29 ± 11.42*,Δ | 78.63 ± 12.57*,Δ |
| BUN (mmol/L) | 5.31 ± 0.51 | 29.76 ± 5.34* | 18.34 ± 4.28*,Δ | 17.98 ± 3.47*,Δ |
Note: comparing to S;
P < 0.05.
Comparing to I/R;
P < 0.05.
Detection of inflammatory factor
Detecting IL-1β and TNF-α concentration with ELISA kit, the results were followed (Table 2). Comparing group S, IL-1β and TNF-α in other groups were increased significantly (P < 0.05); there were no significant differences of IL-1β and TNF-α between group Pre/Dex and group Post/Dex (P > 0.05), while significantly comparing to group R (P < 0.05).
Table 2.
Comparison of inflammatory factors
| Groups | Group S | Group I/R | Group Pre/Dex | Group Post/Dex |
|---|---|---|---|---|
| IL-1β (ng/L) | 85.34 ± 16.13 | 172.16 ± 23.04* | 108.97 ± 23.08*,Δ | 117.29 ± 18.48*,Δ |
| TNF-α (ng/L) | 58.637 ± 13.22 | 141.06 ± 19.21* | 98.78 ± 18.19*,Δ | 97.69 ± 16.28*,Δ |
Note: comparing to S;
P < 0.05.
Comparing to I/R;
P < 0.05.
Expression of protein ZO-1 and occludin
Comparing to group S, expression level of protein ZO-1 and occludin all decreased in other groups (P < 0.05). Comparing to group I/R, ZO-1 and occludin increased significantly (P < 0.05) (Figure 2 and Table 3).
Figure 2.
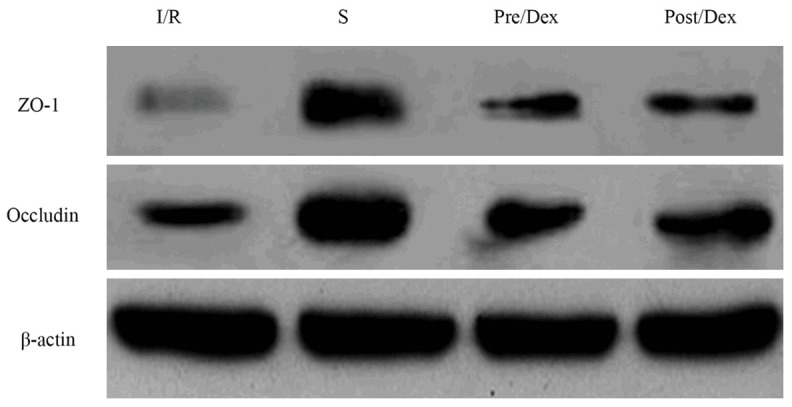
Expression of protein ZO-1 and occludin in renal tissues of rats.
Table 3.
Expression changes of ZO-1 and occludin in each group (x̅ ± s)
| Groups | Group S | Group R | Group Pre/Dex | Group Post/Dex |
|---|---|---|---|---|
| ZO-1 | 0.66 ± 0.10 | 0.21 ± 0.04* | 0.48 ± 0.06*,Δ | 0.51 ± 0.04*,Δ |
| occludin | 0.85 ± 0.11 | 0.36 ± 0.05* | 0.64 ± 0.06*,Δ | 0.59 ± 0.05*,Δ |
Note: comparing to S;
P < 0.05.
Comparing to I/R;
P < 0.05.
Discussion
Recently, IRI was a commonly pathological phenomenon in clinical. For the structural and functional particularity of renal, ischemia-reperfusion injury in kidney transplant operation was typical and serious, absorbing attentions. The development mechanism mainly included periods of ischemia and reperfusion. Blood flow decreased in renal ischemia and renal was in anoxic condition. Although ischemic tissue recovered after reperfusion, there were problems about energy metabolism, changing of cells permeability, activating inflammation reaction and aggravating tissue injury [1-5]. By constructing IRI models in rats, we simulated clinical renal I/R process and found that renal injury was serious after 6 h of reperfusion. With staining in HE, dilatation of tubules and inflammation were obvious, and there was glomerulonephritis. Scr and BUN were both increased significantly by detection of blood biochemistry, which indicated the successful construction of IRI models in rats, consistent with human medicine clinic.
Inflammatory process started from vascular endothelial injury, renal tubular cell dysfunction, and immuno-regulatory factors, including IL-1β, IL-6, IL-8, TNF-α and monocyte chemoattractant protein 1 (MCP-1), were released into renal tissue and blood circulation [14]. During the period of IRI, renal tubular epithelial cells producing cytokines, including IL-1β, IL-6, IL-8, TNF-α and so on, which could urge vascular adhesion molecules to express increasingly, thus mediated adhesive action between leukocytes and endothelial cells [15]. We found that after 45 min renal ischemia and 6h reperfusion, IL-1β and TNF-α increased significantly, which indicated that inflammatory reaction played an important role in IRE process.
It is very important to investigate the endogenous protective mechanisms of ischemic preconditioning to the kidney at present. In order to protect renal, activating or inhibiting some factors in the body played an important role in renal transplantation and the functional recovery of grafts [16,17]. Dederer [18] found that α2 receptor agonist could sustain renal medulla blood flow, prevent from contrast nephropathy and active the adrenergic receptors at the presynaptic membrane of central and peripheral sympathetic nerve, thus reduced stress response induced by surgery, with the effect of sedation and analgesia. Dex was a α2 adrenoceptor agonists with high selectivity, presenting diversity according to distributing in different cells, tissues and organs [19]. Dex, with strong selectivity to central α2 adrenoceptor agonists, inhibited the release of norepinephrine by activating α2 receptors at presynaptic membrane, and terminated the conduction of pain signals. By activating postsynaptic receptors, Dex inhibited sympathetic activity, thus induced decreasing of blood pressure and heart rate, with the effect of sedation and analgesia [20,21]. Otherwise, Dex, with locally vascular effect, directly activated α2 adrenoceptor agonists of renal tissues, thus reduced the release of presynaptic noradrenaline, locally, influencing renal vessels [22]. Due to the effect of Dex to reduce the concentration of norepinephrine in blood circulation and renal tissue, it was effective to avoid the adverse effect of vasoconstriction reduced by norepinephrine. Halaszynski [23] found that Dex, with the effect of maintaining hemodynamic stability, could reassign cardiac output when circulating blood volume decreasing, thus ensured the perfusion of organs, maintained renal blood flow and glomerular filtration. With previous studies, we had investigated that Dex had the protective effect on brain in cardiopulmonary bypass. In our researches, we pretreated and post-treated with Dex in ischemia-reperfusion models in rats, and observed the renal pathological slices. The results showed that renal injury reduced in groups with treatment with Dex, indicating the protective effect of Dex on renal in ischemia-reperfusion.
Tight junctions were the key structure to maintain the functions of the epithelial cells, playing an important role in renal developing process and nephron formation [24]. With the cell culture models, tight junction protein in different tissues fractured and the expression level changed, with the regulation on renal tubular paracellular permeability, under the condition of ischemic injury, free radical injury and lacking of ATP [25]. Ischemia-reperfusion injury was the most common reason leading to acute renal injury, changing the structure of tight junction, increasing apoptosis and separation of renal tubular cell [26]. Tight junction protein ZO, occludin and claudin were found in many tissues. ZO-1 was one of the most significant protein in tight junction. As the organizer of tight junction, ZO-1 mediated signal transduction and interaction between proteins [24]. Phosphorylation state of occludin was related to regulating membrane, with its expression of truncated status at C-terminal increasing paracellular permeability of molecules with low weight [26]. Our studies showed that the expression of tight junction protein ZO-1 and occludin were down regulated in I/R models, while a certain dose of Dex could up regulate the expression level of ZO-1 and occludin. But the mechanisms about how Dex plays in protecting renal in I/R, and how ZO-1 and occludin regulate in I/R, still need further researches.
In conclusion, Dex could reduce inflammatory reaction induced by renal ischemia-reperfusion, improve renal function, and up-regulate the tight junction protein ZO-1 and occludin, which might be one of the protective mechanism.
Disclosure of conflict of interest
None.
References
- 1.Yoo CS, Shin YH, Ko JS, Gwak MS, Kim GS. Anesthetic management including extracorporeal membrane oxygenation therapy of liver transplant recipient with life-threatening hypoxemia -a case report- Korean J Anesthesiol. 2013;65:151–157. doi: 10.4097/kjae.2013.65.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duty BD, Conlin MJ, Fuchs EF, Barry JM. The current role of endourologic management of renal transplantation complications. Adv Urol. 2013;2013:246520. doi: 10.1155/2013/246520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya P, Pandey AK, Paul S, Patnaik R, Yavagal DR. Aquaporin-4 Inhibition Mediates Piroxicam-Induced Neuroprotection against Focal Cerebral Ischemia/Reperfusion Injury in Rodents. PLoS One. 2013;8:e73481. doi: 10.1371/journal.pone.0073481. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.de Vries DK, Khairoun M, Lindeman JH, Bajema IM, de Heer E, Roest M, van Zonneveld AJ, van Kooten C, Rabelink TJ, Schaapherder AF, Reinders ME. Renal Ischemia-reperfusion induces release of angiopoietin-2 from human grafts of living and deceased donors. Transplantion. 2013;96:282–289. doi: 10.1097/TP.0b013e31829854d5. [DOI] [PubMed] [Google Scholar]
- 5.Chang WT, Li J, Vanden Hoek MS, Zhu X, Li CQ, Huang HH, Hsu CW, Zhong Q, Li J, Chen SJ, Vanden Hoek TL, Shao ZH. Baicalein preconditioning protects cardiomyocytes from ischemia-reperfusion injury via mitochondrial oxidant signaling. Am J Chin Med. 2013;41:315–331. doi: 10.1142/S0192415X13500237. [DOI] [PubMed] [Google Scholar]
- 6.Pendergrass KD, Boopathy AV, Seshadri G, Maiellaro-Rafferty K, Che PL, Brown ME, Davis ME. Acute preconditioning of cardiac progenitor cells with hydrogen peroxide enhances angiogenic pathways following ischemia-reperfusion injury. Stem Cells Dev. 2013;22:2414–2424. doi: 10.1089/scd.2012.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghali A, Mahfouz AK, Ihanamäki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-Tenon’s anesthesia. Saudi J Anaesth. 2011;5:36–41. doi: 10.4103/1658-354X.76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu J, Sun P, Zhao H, Watts HR, Sanders RD, Terrando N, Xia P, Maze M, Ma D. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olutoye OA, Glover CD, Diefenderfer JW, McGilberry M, Wyatt MM, Larrier DR, Friedman EM, Watcha MF. The effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:490–495. doi: 10.1213/ANE.0b013e3181e33429. [DOI] [PubMed] [Google Scholar]
- 10.Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–133. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 11.Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag. 2010;6:111–121. doi: 10.2147/tcrm.s5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollucuoglu K, Hanci V, Yurtlu S, Okyay D, Ayoglu H, Turan IO. Comparison of propofol-dexmedetomidine, tiopental-dexmedeto- midine and etomidate-dexmedetomidine combinations’ effects on the tracheal intubation conditions without using muscle relaxants. Bratisl Lek Listy. 2013;114:514–518. doi: 10.4149/bll_2013_107. [DOI] [PubMed] [Google Scholar]
- 13.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–939. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 14.Olutoye OA, Glover CD, Diefenderfer JW, McGilberry M, Wyatt MM, Larrier DR, Friedman EM, Watcha MF. The effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:490–495. doi: 10.1213/ANE.0b013e3181e33429. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Burgstad C, Ching K, Bellon M, Holloway RH. The effects of sedation on gastric emptying and intra-gastric meal distribution in critical illness. Intensive Care Med. 2008;34:454–460. doi: 10.1007/s00134-007-0942-2. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Wang Y, Zhou LN, Zheng F. ARB treatment prevents the decrease in endothelial progenitor cells and the loss of renal microvasculature in remnant kidney. Am J Nephrol. 2011;33:550–557. doi: 10.1159/000328632. [DOI] [PubMed] [Google Scholar]
- 17.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dederer H, Berger M, Meyer T, Werr M, Ilg T. Structure-activity relationships of acetylcholine derivatives with Lucilia cuprina nicotinic acetylcholine receptor α1 and α2 subunits in chicken β2 subunit hybrid receptors in comparison with chicken nicotinic acetylcholine receptor α4/β2. Insect Mol Biol. 2013;22:183–198. doi: 10.1111/imb.12014. [DOI] [PubMed] [Google Scholar]
- 19.Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, Huang WQ, Liu KX. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116:1035–1046. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
- 21.Lili X, Jianjun S, Haiyan Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth. 2012;26:556–561. doi: 10.1007/s00540-012-1354-1. [DOI] [PubMed] [Google Scholar]
- 22.Wakita R, Kohase H, Fukayama H. A comparison of dexmedetomidine sedation with and without midazolam for dental implant surgery. Anesth Prog. 2012;59:62–68. doi: 10.2344/11-11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halaszynski TM. Dexmedetomidine: a look at a promising new avenue of use. Saudi J Anaesth. 2012;6:104–106. doi: 10.4103/1658-354X.97019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denker BM, Sabath E. The biology of epithelial cell tight junctions in the kidney. J Am Soc Nephrol. 2011;22:622–625. doi: 10.1681/ASN.2010090922. [DOI] [PubMed] [Google Scholar]
- 25.Eadon MT, Hack BK, Xu C, Ko B, Toback FG, Cunningham PN. Endotoxemia alters tight junction gene and protein expression in the kidney. Am J Physiol Renal Physiol. 2012;303:F821–F830. doi: 10.1152/ajprenal.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]


