Abstract
Objective: The aim of this study is to compare the short-term results of intra-articular platelet-rich plasma (PRP) and hyaluronic acid (HA) administrations in early knee osteoarthritis. Materials and methods: One hundred and eighteen patients (mean age: 59.3±8.55) who were clinically and radiologically documented with a knee osteoarthritis diagnosis between May and December 2013 were evaluated. For the radiological evaluation, the Kellgren-Lawrence radiological classification scale was employed. The data of stage 1 and 2 patients with osteoarthritis were gathered retrospectively according to the Kellgren-Lawrence classification. The patients were given intra-articular PRP or HA treatments a total of three times, one week apart. 61 patients (102 knees) were involved in the PRP group, and 57 patients (97 knees) were involved in the HA group. The patients were evaluated using the Knee Society’s Knee Scoring System (KSS) and the Visual Analog Scale (VAS) scoring system before the treatment and at three and six months after the treatment. Results: In the PRP and HA groups, when pre-treatment KSS and VAS scores were compared with post-treatment three and six-month scores, a statistically significant difference was seen. When the groups were compared with each other, there was no significant difference between pre-treatment KSS and VAS pain scores; however, a significant difference was found between post-treatment three and six-month scores. Conclusion: In this study, the intra-articular PRP administration was more efficient than the HA administration in early knee osteoarthritis.
Keywords: Platelet-rich plasma, hyaluronic acid, knee osteoarthritis, intra-articular treatment
Introduction
Osteoarthritis is a progressive joint disease associated with cartilage degeneration that disturbs quality of life and daily activities by leading to a loss of movement [1]. Although it may affect all joints, knee osteoarthritis is the most common type among adults, with a prevalence of 6% and with its frequency reaching up to 40% with advancing age [2].
The biochemical and biomechanical changes of osteoarthritis in joints is the cause an inadequate repair processes of damaged cartilage. The first pathological changes observed are the loss and fibrillation of the joint cartilage together with reshaping and thickening of the subchondral bone. Finally, full layer cartilage loss is seen. Starting from the load bearing area of the joint cartilage, the muscles surrounding the joint, the joint capsule, synovium, ligaments and subchondral bone are affected [3]. Afterward, movement at joints is clinically restricted, and pain appears. Today, in the pharmacological treatment of osteoarthritis, analgesics, non-steroid anti-inflammatory drugs, corticosteroids, glucosamine, chondroitin sulfate and viscosupplementation are used. These drugs have a symptomatic effect on osteoarthritis. The treatment is focused on relieving the findings and symptoms and slowing the progress of the disease [2]. Intra-articular injection is also an option for pharmacological treatment and has been shown to be effective particularly in knee osteoarthritis [4]. Besides commonly used steroids and HA preparations, growth factors, radioisotopes, botulinum toxin type A, tanezumab and platelet-rich plasma (PRP) are also intra-articular administered agents [4-8]. PRP is a treatment that stimulates the natural healing process through the growth factors contained in platelets. PRP accelerates the physiological recovery process at the administration site, provides support for cell connection, relieves pain and shows anti-inflammatory and anti-bacterial activity [9]. PRP is an inexpensive and simple way of obtaining growth factors and cytokines [10]. It is a new treatment method that can be easily prepared autogenously [11]. In the literature, there are studies on the use of PRP in degenerative joint diseases [12-20]. In our study, the aim is to determine the effect on pain and functions of PRP, which is used in the treatment of knee osteoarthritis, and to compare its short-term results with HA preparations frequently used in clinical practice.
Materials and methods
In this study, the data from the patients who have been administered intra-articular PRP or HA because of knee osteoarthritis at stage 1 or stage 2 based on the Kellgren-Lawrence classification between May and December 2013 were gathered retrospectively. Those with diabetes mellitus, rheumatic diseases, coagulation disorders, infection, immunosuppressive diseases, receiving anticoagulant treatment, non-steroidal anti-inflammatory drugs within five days prior to the treatment, those with an abnormal blood table and those at stages 3 and 4, according to the Kellgren-Lawrence classification, were not included in the study. One hundred and eighteen patients meeting the criteria were included in the study. The anterior-posterior and lateral graphics of all patients in a standing position were evaluated. Staging was made based on the Kellgren-Lawrence classification. The patients were divided into two groups as the PRP administered group and the HA administered group. Sixty-one patients (102 knees) were involved in the PRP group and 57 patients (97 knees) were involved in the HA group. The patients were given intra-articular PRP or HA treatments a total of three times, one week apart. The patients in both groups were evaluated using the Knee Society’s Knee Scoring System (KSS) and the Visual Analog Scale (VAS) scoring system before the treatment and at three and six months after the treatment. In preparing the PRP, 1.5 mL of the 15 mL blood sample taken from the upper extremities was used for a platelet count before centrifuge. The remaining 13.5 mL venous blood was put into a 15 mL centrifuge tube containing 1.5 mL 3.2% sodium citrate and centrifuged at room temperature for eight minutes at 3,600 rpm. After the centrifuge, 2.5 ml of PRP was obtained from between the bottom erythrocyte and upper plasma layers. Then, a 0.5 mL PRP sample was reserved for the platelet count. 2.0 ml PRP was used for the intra-articular injection. The average platelet count before centrifuge was 215.000/μL (133.000/μL-297.000/μL) and was 825.000/μL (575.000/μL-1.215.000/μL) after centrifuge. The average change in the platelet count was calculated as 3.8 times. In HA administration, 20 mg/2 mL preparations were used. The knee to be injected was prepared using a sterile cover, and PRP or HA injections were made via the anterolateral portal. After injection, the patient was sent home and advised to take one day of rest and apply ice to the area. After the first day, the patients were permitted to carry out tolerable daily activities. Paracetamol was recommended as a pain reliever.
Statistical analysis
Data analysis was performed using the Statistical Package the Social Sciences (SPSS, Inc., Chicago, II, version 15.0) for Windows program. Descriptive were expressed as number and percentage for categorical variables and as mean, standard deviation, minimum and maximum for numerical variables. Chi-square test was used for paired group comparison of categorical variables. For the comparison of KSS scores and VAS scores, kruskal wallis test was used for ordinal variables. A p value smaller than 0.05 was considered statistically significant.
Results
This study involved 118 patients with an average age of 59.3±8.55. In 61 patients (102 knees), PRP was employed and in 57 patients (97 knees) HA was employed. The demographic properties of the patient groups are shown in Table 1. Between the patients of both groups, no statistically significant difference was determined with respect to gender, age, osteoarthritic stage or involvement (unilateral/bilateral). No infection, deep venous thrombosis or similar serious complications were seen during or after the treatment. Only in the PRP administered group, swelling occurred at three knees which was recovered after applying ice. In the PRP group, the average KSS score was 60.89±4.21 before treatment, 75.75±3.65 at the third month and 84.36±7.2 at the sixth month (Figure 1).
Table 1.
Demographic properties of the treated patients
| PRP N (%) | HA N (%) | P value | |
|---|---|---|---|
| Gender | 0.746 | ||
| Female | 50 (82) | 48 (84.2) | |
| Male | 11 (18) | 9 (15.8) | |
| Age | 58.9±10.4 | 59.7±6.7 | 0.607 |
| Stage | 0.318 | ||
| Stage 1 | 28 (45.9) | 21 (36.8) | |
| Stage 2 | 33 (54.1) | 36 (63.2) | |
| Knee involvement | 0.729 | ||
| Unilateral | 20 (32.8) | 17 (29.8) | |
| Bilateral | 41 (67.2) | 40 (70.2) |
Figure 1.
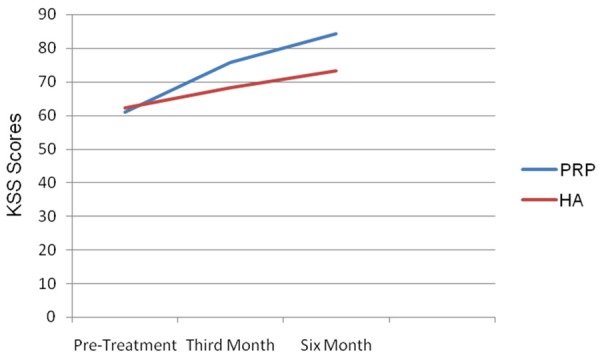
Pre-treatment, 3rd and 6th month KSS scores of Prp and ha groups.
The VAS score of this group was determined 7.2±1.07 before treatment, 3.59±0.76 at the third month and 2.13±0.67 at the sixth month (Figure 2). In the PRP group, when pre-treatment KSS and VAS scores were compared with the post-treatment third and sixth month scores, a statistically significant difference was seen. In the HA group, the KSS score was 62.33±3.81 before treatment, 68.26±3.74 at the third month and 73.25±4.46 at the sixth month (Figure 1). In this group, the VAS score was 7.11±0.90 before treatment, 5.23±0.80 at the third month and 4.00±0.90 at the sixth month (Figure 2).
Figure 2.
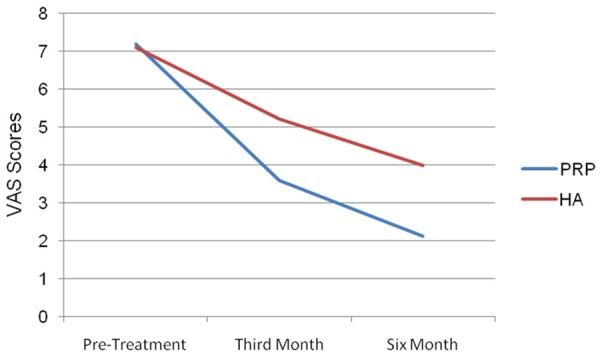
Pre-treatment, 3rd and 6th month VAS scores of Prp and ha groups.
Also in the HA group, when pre-treatment KSS and VAS scores were compared with the post-treatment scores at the third and sixth months, a statistically significant difference was seen. No statistically significant difference was found for the KSS scores between the two groups (P=0.086). However, the KSS scores were noticeably different between the two groups at the third and sixth months (P<0.001, P<0.001, respectively). VAS scores were not different between the two groups before treatment (P=0.069). VAS scores were noticeably different between the two groups at three and six months (P<0.001, P<0.001, respectively) (Table 2).
Table 2.
Comparison of Pre-treatment, 3rd and 6th month KSS and VAS scores of the groups
| PRP Group n:102 | HA Group n:97 | P value | |
|---|---|---|---|
| KSS Score | Mean ± SD | Mean ± SD | |
| Pre-treatment | 60.89±4.21 | 62.33±3.81 | 0.086 |
| Post-treatment third month | 75.75±3.65 | 68.26±3.74 | <0.001* |
| Post-treatment sixth month | 84.36±7.2 | 73.25±4.46 | <0.001* |
| P value | <0.001* | <0.001* | |
| VAS Score | Mean ± SD | Mean ± SD | |
| Pre-treatment | 7.2±1.07 | 7.11±0.90 | 0.069 |
| Post-treatment third month | 3.59±0.76 | 5.23±0.80 | <0.001* |
| Post-treatment sixth month | 2.13±0.67 | 4.00±0.90 | <0.001* |
| P value | <0.001* | <0.001* |
KSS: Knee Society’s Knee Scoring System, VAS: Visual Analog Scale, HA: Hyaluronic acid, PRP: Platelet-rich plasma, SD: Standard deviation;
Statistically significant difference.
Discussion
Both pharmacologic and non-pharmacologic treatment alternatives are available for osteoarthritis. Combined use of these two alternatives is recommended for the treatment of osteoarthritis [21,22]. However, still there is not a treatment that can both eradicate osteoarthritis and change the course of the disease. Intra-articular injection is a preferred alternative in the treatment of symptomatic osteoarthritis. Different treatment protocols exist such as HA, corticosteroids, non-steroid anti-inflammatory drugs and PRP [23]. HA is a natural component of the connective tissue and cartilage. Besides contributing to the viscoelastic capacity of the synovial fluid, it also functions as a physiological nutrient factor, and has been used in osteoarthritis patients when the HA concentration was determined to decrease and chain length was shortened. The increased weight on the damaged cartilage results in decreased lubrication and causes degeneration on the cartilage surface and collagen network in the long-term [24,25].
The mechanism of HA in the cartilage after administration is not known. However, among the randomized placebo controlled multi-center studies, there are studies demonstrating that HA is useful in knee osteoarthritis [26,27]. Additionally, HA treatment was found to be more efficient in early osteoarthritis patients than late osteoarthritis patients [28]. For intra-articular administrations, there are HA preparations with different formulations and molecular weights. However, in the randomized controlled studies, no difference was found between HA products with respect to efficiency [29].
PRP was first used in 1987 in cardiac surgery in order to prevent excessive blood transfusion [30]. There are several studies reporting the use of PRP in joint pathologies [10,12-20]. There are more than 30 bioactive proteins in the alpha granules of the platelets [31]. The platelet origin growth factor, transforming growth factor, venous endothelial growth factor, insulin-like growth factor (etc.) and the proteins, such as fibrin, fibronectin, vitronectin and thrombospondin contained in PRP play a role in many stages of tissue recovery. The growth factors activate the cells that function in tissue recovery [10]. PRP’s mechanism of action on the degenerative knee joint can be stated as recurring inflammation and angiogenesis through its proteins and growth factors, anabolic and cartilage protecting activity, cell differentiation and synovial cell modulation. However, PRP’s mechanisms of action on joint pathologies are not yet clear and are more complex than previously thought [32]. PRP is derived from the patient’s blood and the platelet ratio it contains is much higher than the full blood. PRP contains a high concentration of platelets, growth factors, proteins and cytokines [33]. Anitua has reported that PRP is effective at platelet counts over 300.000/μl [34]. In another study, it was reported that the efficacy of PRP is maximized when the platelet concentration is 2.5 times the basal platelet count [35]: it was determined as 3.8 times in our study. PRP can be easily prepared in laboratory centrifuges, outpatient clinics and similar units. Because of the fact that the study protocols are not standard and different methods are used for PRP preparation, comparing studies is difficult [36]. There are various factors affecting the properties of the PRP products produced such as volume of the blood sample, volume of the plasma obtained, existence of the red and/or white blood cells, addition of thrombin or calcium chloride for fibrin formation and the addition of PH modifying components [37]. In a limited number of studies, it was demonstrated that, considering the intra-articular PRP or HA administrations in the treatment of osteoarthritis, the results of PRP were better [38-40]. In our study, when knee function scores at the three and six months were compared with pre-treatment function scores, a statistically significant difference was demonstrated. In addition, a significant reduction was determined in pain scores. Again, in our study, in the inter-group comparison, the variations in the pain and function scores of the PRP group are significantly better than the HA group. In the series of 115 patients who were administered PRP by Kon et al., it was found that the functional scores were different at the sixth month, but the efficiency decreased after the ninth month [16]. In Filardo et al., it was discovered that the clinical recovery of the patients who were administered PRP and followed for two years was best at about the ninth month [13]. In our study too, although six-month follow-up results are good, long-term results are not known. We think that the long term studies to be conducted will be more enlightening. In another study, patients who were administered PRP or HA were followed-up at the second, sixth and twelfth months and although it was reported that the patients up to stage 2, according to the Kellgren-Lawrence classification exhibited a better clinical recovery, no apparent intergroup difference was determined [39].
The manual method employed in obtaining PRP in our study is inexpensive and efficient. Because PRP is obtained from patients’ own blood, immune reaction or blood-borne diseases are not at stake. In any of the reviewed studies, no serious side effects or complications were demonstrated regarding PRP [33]. No complication was seen in our study either. This study’s limitations include its short follow-up periods and retrospective nature. Nevertheless, it was demonstrated that intra-articular PRP was more efficient than HA in the treatment of early knee osteoarthritis.
Disclosure of conflict of interest
None.
References
- 1.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RE. The prevalence of knee osteoarthritis in the elderly, the framingham osteoarthritis study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 2.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil. 2006;85(Suppl 11):S2–11. doi: 10.1097/01.phm.0000245568.69434.1a. quiz S12-4. [DOI] [PubMed] [Google Scholar]
- 4.Sinusas K. Osteoarthritis: diagnosis and treatment. Am Fam Physician. 2012;85:49–56. [PubMed] [Google Scholar]
- 5.Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intraarticular Injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26:910–913. [PubMed] [Google Scholar]
- 6.Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-Based knee injections for the management of arthritis. Pain Med. 2012;13:740–753. doi: 10.1111/j.1526-4637.2012.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frizziero A, Giannotti E, Ferraro C, Masiero S. Platelet rich plasma intra-articular injections: a new therapeutic strategy for the treatment of knee osteoarthritis in sport rehabilitation. A systematic review. Sport Sci Health. 2012;8:15–22. [Google Scholar]
- 8.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez M, Anitua E, Orive G, Mujika I, Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39:345–354. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 10.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269–278. doi: 10.1016/j.arthro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Cerza E, Carni S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, De Biasi G, Ciuffreda M. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 13.Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:528–535. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 14.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: Study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobbi A, Karnatzikos G, Mahajan V, Malchira S. Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health. 2012;4:162–172. doi: 10.1177/1941738111431801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18:472–479. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 17.Kon E, Mandelbaum B, Buda R, Filardo G, Delcogliano M, Timoncini A, Fornasari PM, Giannini S, Marcacci M. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy. 2011;27:1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez M, Guadilla J, Fiz N, Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford) 2012;51:144–150. doi: 10.1093/rheumatology/ker303. [DOI] [PubMed] [Google Scholar]
- 20.Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 21.Altman RD. Practical considerations for the pharmacologic management of osteoarthritis. Am J Manag Care. 2009;15(Suppl):236–243. [PubMed] [Google Scholar]
- 22.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 23.Hameed F. Ihm j Injectable medication for osteoarthritis. PM R. 2012;4(Suppl):s75–s81. doi: 10.1016/j.pmrj.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Brockmeier SE, Shaffer BS. Viscosupplementation therapy for osteoarthritis. Sports Med Arthrosc. 2006;14:155–162. doi: 10.1097/00132585-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Fajardo M, Di Cesare PE. Disease-modifying therapies for osteoarthritis: current status. Drugs Aging. 2005;22:141–161. doi: 10.2165/00002512-200522020-00005. [DOI] [PubMed] [Google Scholar]
- 26.Curran MP. Hyaluronic acid (supartz): a review of its use In osteoarthritis of the knee. Drugs Aging. 2010;27:925–941. doi: 10.2165/11205920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A metaanalysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86-a:538–545. doi: 10.2106/00004623-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Migliore A, Granata M. Intra-articular use of hyaluronic acid in the treatment of osteoarthritis. Clin Interv Aging. 2008;3:365–369. doi: 10.2147/cia.s778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das A, Neher JO, Safranek S. Clinical inquiries. Do hyaluronic acid injections relieve OA knee pain. J Fam Pract. 2009;58:281c–281e. [PubMed] [Google Scholar]
- 30.Ferrari M, Zia S, Valbonesi M, Henriquet E, Venere G, Spagnolo S, Grasso MA, Panzani I. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif organs. 1987;10:47–50. [PubMed] [Google Scholar]
- 31.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 32.Andia I, Sanchez M, Maffulli N. Joint pathology and platelet-rich plasma therapies. Expert Opin Biol Ther. 2012;12:7–22. doi: 10.1517/14712598.2012.632765. [DOI] [PubMed] [Google Scholar]
- 33.Saucedo JM, Yaffe MA, Berschback JC, Hsu WK, Kalainov DM. Platelet-rich plasma. J Hand Surg Am. 2012;37:587–589. doi: 10.1016/j.jhsa.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 35.Graziani E, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–219. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 36.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, Ayeni OR, Bhandari M. Efficacy of autologous platelet rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 37.Arnoczky SP, Delos D, Rodeo SA. What is platelet-rich plasma? Oper Tech Sports Med. 2011;19:142–148. [Google Scholar]
- 38.Cerza F, Carnì S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, De Biasi G, Ciuffreda M. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 39.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;23:229. doi: 10.1186/1471-2474-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]


