Abstract
Background: The purpose of this study is to evaluate the intima-media thickness (IMT) and arterial elasticity of the common carotid artery (CCA) in patients with cerebral small vessel disease (SVD) by applying radiofrequency (RF) ultrasound technology. Methods: Fifty SVD subjects (SVD group) and fifty-three matched controls (Control group) were enrolled in the study. Structural and functional changes in the common carotid arterial wall were investigated by quality intima-media thickness (QIMT) and quanlity arterial stiffness (QAS) with a Mylab Twice ultrasound instrument. The vessel related variables between these two groups were analyzed. Results: There was a significant higher value of CCA-IMT in SVD group than that in control group (P<0.01). Pulse wave velocity (PWV), stiffness coefficient (α) and stiffness index (β) were remarkably greater (P<0.01) while compliance coefficient (CC) decreased significantly (P<0.01) in the SVD group than control group. Furthermore, significant difference was found on IMT between left and right CCA in SVD (P<0.01) and control group (P<0.01) while no significant difference was found on CC, α, β and PWV between left and right CCA in SVD (P>0.05) and control group (P>0.05). Conclusions: Decreased arterial elasticity of the CCA in patients with SVD compared with normal subjects. Ultrasound RF technology can be used to non-invasively and quantitatively detect the change in the structure and function of the CCA in SVD subjects for evaluating preclinical atherosclerosis.
Keywords: Ultrasound, cerebral small vessel disease, common carotid artery, carotid distensibility
Introduction
Cerebral small vessel disease (SVD) refers to a group of pathological processes with various aetiologies that affect the small arteries, arterioles, venules, and capillaries of the brain [1]. The consequences of small vessel disease on the brain parenchyma are mainly lesions located in the subcortical structures such as lacunar infarcts, white matter lesions, large haemorrhages, and microbleeds [2]. SVD is an important clinico-pathological condition as it is the cause of 20% of strokes worldwide, and the most common cause of vascular and mixed dementia [3]. Rapid and accurate diagnosis of SVD is urgently needed in clinical practice.
Sub-clinical atherosclerotic changes manifesting on arterial walls involve thickening of the intima media and decreased vascular elasticity. Common carotid arterial intima-media thickness (CCA-IMT) has demonstrated as a usefulness marker for cardiovascular risk and as a method for detection of the early development of arterial atherosclerotic disease [4]. It also well established that the assessment of local, regional or systemic indices of arterial stiffness and compliance (pulse wave velocity [PWV], carotid distensibility [CD]) are useful for predicting the future risk of cardiovascular events such as coronary heart disease (CHD) and stroke [5,6].
Recent advances in cardiovascular imaging provide the opportunity on early detection of atherosclerotic vascular disease and quantify its progression. Innovations in ultrasound (US) technology have enabled the automatic and accurate measurement of CCA-IMT and arterial elasticity by means of US radiofrequency (RF)-data technology. This technology combines the advantages of B-mode imaging (visual morphology) with integrated RF-data technology for quantitative assessment of the properties of the walls of blood vessels. It also provides feedback on the quality of the measurement.
The aim of this investigation was to detect the changes in the intima-media thickness (IMT) and arterial elasticity of the common carotid artery (CCA) in patients with cerebral small vessel disease (SVD) by applying radiofrequency (RF) ultrasound technology.
Patients and methods
Subjects
The study was carried out on 103 subjects from December 2011 to June 2012. Fifty patients with SVD (mean age: 56.56±8.46 years; range: 30-80 years) and fifty-three aged-matched control subjects (mean age: 53.64±8.36 years; range: 39-70 years). All the patients were performed with cranial Magnetic Resonance Imaging (MRI). Inclusion criteria of SVD patients were consistent with neuroimaging diagnosis standards [7]. Patients with cerebral hemorrhage, cerebral tumor, history of carotid endarterectomy,carotid artery stenosis and occlusion, severe heart, liver or kidney disease were excluded. Individuals in the control group were free from established heart disease, diabetes mellitus and hypertension.
The whole study protocol was approved by the Institutional Ethics Committee. Written informed consent was obtained from all subjects.
Clinical characteristics
Height, weight, and blood pressure (BP) were measured in all participants. The BMI was calculated as weight (kg) divided by the square of height (m). Clinical BP was determined by performing three systolic blood pressure tests (SBP), and diastolic blood pressure (DBP) measurements. Pulse pressure (PP) was calculated by SBP minus DBP. For office BP measurement, the patient was seated for at least 10 min in a quiet room during the run-in period assessment. Blood samples for measurement of the level of glucose, triglycerides (TG), total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein (LDL)-cholesterol were obtained after 12 h of fasting.Glucose levels were measured using the glucose oxidase method.
US assessment
US examinations were performed with a Mylab 90 Platform (Esaote Medical Systmes, Firenze, Italy) using 4-13 Hz vascular probe LA 523 with built in RF quality intima-media thickness (QIMT) and RF quality arterial stiffness (QAS) software. This software used a complex algorithm that could process all data coming from the lesion as RF signals. It can be used for quantitative evaluation of the properties of the vessel wall.
The subject was placed in supine position with head elevation ≤45 and a side tilt of 30° to fully expose the neck. Longitudinal images of the bilateral CCA were obtained by placing linear probe on the neck. A clear definition of anterior or posterior carotid walls was achieved by small movements of the probe. The distal 1-1.5 cm of the CCA just proximal to the bulb was measured by a computer analysis system (RFQIMT technology). IMT measurements were automatically taken over six cardiac cycles and the mean value of IMT was obtained. Standard deviation (SD) value was controlled under a cutoff value 15. RF-QAS was then used to measure the elasticity of the CCA. Measurement of carotid distensibility (CD) was automatically taken over six cardiac cycles. Then the mean and SD values automatically calculated and the SD value was controlled under a cutoff value 15. QAS data analysis software also calculated the pulse wave velocity (PWV), compliance coefficient (CC), and stiffness index (α and β) according to the following formulae: CC = π (Ds × Ds-Dd × Dd)/[4(Ps-Pd)]; α = ln (Ps/Pd)/[(As-Ad)/Ad]; PWVβ = √(β × DBP/2ρ) where β = ln (Ps/Pd)/[(Ds-Dd)/Dd], where As = systolic area, Ad = diastolic area, Ds = systolic diameter, Dd = diastolic diameter, Ps = systolic blood pressure, Pd = diastolic blood pressure, Ρ = blood density.
Intra-observer and inter-observer variability
Ten patients were randomly picked among the SVD and control subjects. The imaging data was acquired twice, in two different sessions separated by 30 min, by the same operator. Then the data collection was performed by a different operator. Intra-observer and inter-observer variability were assessed based on the acquired data.
Statistical analysis
All the data were presented with mean ± SD and Student’s t test was performed after passing the normality test. Bland-Altman plots were employed to assess the intra- and inter-observer variability. P<0.05 was considered as significant difference. Statistical analyses were performed with SPSS software version 18.0 (SPSS Inc. Chicago, IL, US).
Results
Patient characteristics
The main clinical and pathological data for patients at the beginning of the study are shown in Table 1. SBP, DBP, PP and BMI were significantly higher in SVD group than control group (P<0.05). No significant difference was found on age, fasting blood glucose (FBG), TG, TC, HDL-C and LDL-C between 2 groups (P<0.05).
Table 1.
Comparison of the clinical characteristic between SVD Group and Control
| SVD Group (n = 50) | Control Group (n = 53) | t value | p value | |
|---|---|---|---|---|
| Age (Year) | 56.56±8.46 | 53.64±8.36 | 1.716 | 0.081 |
| BMI (kg/m2) | 24.31±2.26 | 23.37±2.09 | 2.195 | 0.031 |
| SBP (mmHg) | 140.36±15.70 | 116.53±13.46 | 8.248 | 0.000 |
| DBP (mmHg) | 87.94±10.69 | 75.81±9.94 | 5.967 | 0.000 |
| PP (mmHg) | 52.42±12.87 | 40.72±8.74 | 5.426 | 0.000 |
| FBG (mmol/L) | 5.12±0.65 | 5.06±0.49 | 1.764 | 0.081 |
| TG (mmol/L) | 1.74±0.75 | 1.69±0.86 | 1.838 | 0.071 |
| TC (mmol/L) | 4.95±0.76 | 4.88±0.82 | 1.712 | 0.090 |
| HDL-C (mmol/L) | 1.30±0.43 | 1.31±0.56 | 1.717 | 0.089 |
| LDL-C (mmol/L) | 3.46±0.58 | 3.38±0.66 | 1.139 | 0.266 |
Note: BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: pulse pressure; FBG: fasting blood glucose; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein; LDL-C: low-density lipoprotein.
Arterial structure and stiffness comparison
As shown in Table 2, IMT (P<0.01), α (P<0.01), Β (P<0.01) and PWV (P<0.01) were significantly higher in SVD group than control group while CC (P<0.01) was significantly lower in SVD than that in control group.
Table 2.
Comparison of the diameter, IMT and elastic parameters of the common carotid artery (CCA) between the SVD group and control group
| SVD Group (n = 50) | Control Group (n = 53) | p value | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SVD/Control | left/right | |||||||
|
| ||||||||
| Left CCA | Right CCA | Left CCA | Right CCA | Left CCA | Right CCA | SVD group | Control group | |
| IMT (μm) | 694.88±77.63 | 637.42±93.30 | 586.87±62.12 | 545.13±62.28 | <0.001 | <0.001 | 0.001 | 0.001 |
| CC (mm2/KPa) | 0.89±0.13 | 0.91±0.09 | 0.96±0.08 | 0.99±0.08 | = 0.002 | <0.001 | 0.487 | 0.088 |
| α | 5.68±1.23 | 5.46±1.19 | 4.77±0.62 | 4.74±0.96 | <0.001 | = 0.002 | 0.360 | 0.876 |
| β | 11.25±1.01 | 11.14±1.02 | 9.24±1.24 | 9.13±1.20 | <0.001 | <0.001 | 0.585 | 0.668 |
| PWV (m/s) | 9.49±1.09 | 9.29±1.05 | 7.22±1.11 | 7.07±1.22 | <0.001 | <0.001 | 0.348 | 0.513 |
Note: IMT: intima-media thickness; CC: compliance coefficient; α: stiffness indicator; β: stiffness parameter; PWV: pulse wave velocity.
Furthermore, we also compare these variables between right and left CCA. The left CCA IMT was significantly higher than that on right CCA in both SVD group (P<0.01) and control group (P<0.01). No significant difference was found on CC, α, β and PWV between right and left CCA.
Repeatability comparison
Intragroup and intergroup IMT and PWV values were comparable between different acquisitions (intragroup: a mean bias of 5.6±11.49 μm; intergroup: 1.3±43.50 μm for IMT; intragroup: a mean bias of 0.001±0.329 cm/s; intergroup: 0.028±0.326 cm/s for PWV). Bland-Altman analysis showed a consistent trend in the difference and mean values of IMT and PWV by repeated measurement (Figures 1 and 2).
Figure 1.
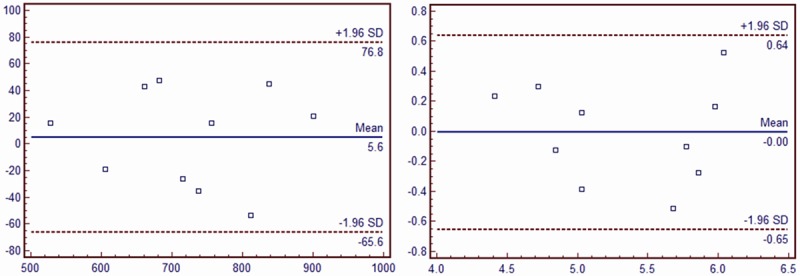
Intra-observer repeatability of PWV and IMT measurements of the same patient at different time point. A. PWV; B. IMT.
Figure 2.
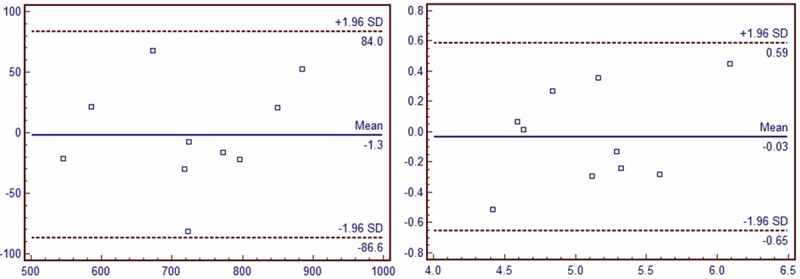
Inter-observer repeatability of PWV and IMT measurements of the same patient at the same time point by two different observer. A. PWV; B. IMT.
Discussion
The present study found decreased arterial elasticity of the CCA in patients with SVD compared with normal subjects and demonstrated US RF technology can be used to non-invasively and quantitatively detect the change in the structure and function of the CCA in SVD subjects for evaluating preclinical atherosclerosis.
SVD is a group of pathological processes with various aetiologies that affect the small arteries, arterioles, venules, and capillaries of the brain. It is characterized by loss of smooth muscle cells from the tunica media, deposits of fibro-hyaline material, narrowing of the lumen, and thickening of the vessel wall [8]. In addition, ischemic stroke can be caused by SVD. Until now, no consensus has reached on the etiology of the SVD and it is proposed that SVD is manifestation of multiple brain vessel risk factors and hereditary factors, including lacunar infarcts and white matter lesion. SVD has an important role in cerebrovascular disease and is a leading cause of cognitive decline, functional loss and even vascular dementia. Currently, MRI is usually employed for SVD diagnosis. However, high cost, unsuitable for large population screen and contraindication caused by ferromagnetic materials hinder its use [9]. As a window of systemic atherosclerosis, carotid atherosclerosis has showed close relationship with cerebral small vessel on pathology and morphology. Since vessel US has been widely used in the examination of carotid artery lesion, it can also be employed for SVD detection.
RF ultrasound could quantitatively detect the change in CCA-IMT and arterial elasticity at an early stage and IMT is associated with the degree of atherosclerosis [10]. Increased thickness of IMT was found in the patient with hypertension [11]. Sojkovaet et al [12] suggested that IMT is related to regional cerebral blood flow. We also found a significant increasing of IMT in SVD subjects than control subjects. Previous studies also showed that side differences of carotid intima-media thickness were found in predicting cardiovascular events among patients with coronary artery disease [13]. Furthermore, Luo et al suggested right CA-IMT correlated better with haemodynamic parameters while left CA-IMT showed better correlation with biochemical indices [14]. In present study, we found the bilateral IMT were significantly higher in SVD group than control group and left CA-IMT was significantly higher than the right CA-IMT. These results were consistent with previous studies.
Since atherosclerosis is a long-term pathological process, more and more attention have been paid to detect the structural changes before the onset of the disease. Recent studies have been shown that endothelial cell dysfunction serve as the early marker of atherosclerosis. It is well established that measurement of carotid arterial IMT is a valid method to define early atherosclerosis and to predict eventual cardiovascular events in the general population. However, IMT can only reflect the structure feature of arterial wall but not the endothelial cell dysfunction. Many researches showed to us that arterial stiffness is correlated with the presence and severity of arterial atherosclerosis [15]. By using QAS techniques, high resolution ultrasound acquisitions based on RF signals allow us to assess local IMT and stiffness in a rapid and specific manner. And these features make it possible in early prevention and diagnosis of SVD. In this study, the stiffness index (α, β) and PWV were significantly higher in SVD group than that in control group while compliance coefficient (CC) was significant lower in SVD group.
Recent studies have shown a further association between excessive pressure pulsatility and a number of afflictions of aging that share a predominant microvascular etiology, including many forms of kidney disease and cognitive impairment. In these disorders, microvascular remodeling and impaired regulation of local blood flow, which are related to large artery stiffness and pressure pulsatility, are associated with evidence of diffuse microscopic tissue damage [16]. Mitchell et al showed that aortic dilatation, wall stiffening and increased pulse wave velocity result from elastin fragmentation, leading to a premature reflected pressure wave that contributes to elevated PP [17]. Boss et al showed that stiffness index and 5-year prediction of cardiovascular death were significantly higher in hypertension groups than control group [18]. These results support the early intervention of hypertension can slow down the progress of atherosclerosis. We found here that SBP and PP were significantly higher in SVD group than control group. These results suggested increased SBP and PP might be involved in the process of atherosclerosis and have impact on the elasticity of the artery.
Our study has several limitations. Firstly, the low patient number in SVD group might result in some degree of statistical error. Secondly, selection bias could be presented due to the higher admission rate of lacunar infarcts and severe clinical symptoms were found in the patients with SVD. Lastly, most of enrolled patients were presented with simple hypertension and the conclusion conducted here might only be suitable for those patients but not the patients with other diseases (such as diabetes, hyperlipidaemia and so on). Further studies are needed to confirm these conclusions.
In conclusion, we conducted that US RF technology can be used to non-invasively and quantitatively detect the change in the structure and function of the CCA in SVD subjects for evaluating preclinical atherosclerosis. However, further studies are needed to confirm the conclusion before its application in clinical practice.
Disclosure of conflict of interest
None.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F, Wright CB. Current pathophysiological concepts in cerebral small vessel disease. Front Aging Neurosci. 2014;6:24. doi: 10.3389/fnagi.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper MK, Raza K, Nuttall SL, Stevens R, Toescu V, Heaton S, Gardner-Medwin J, Hiller L, Martin U, Townend J, Bacon PA, Gordon C. Impaired endothelial function in systemic lupus erythematosus. Lupus. 2007;16:84–8. doi: 10.1177/0961203306074842. [DOI] [PubMed] [Google Scholar]
- 5.Wykretowicz A, Gerstenberger P, Guzik P, Milewska A, Krauze T, Adamska K, Rutkowska A, Wysocki H. Arterial stiffness in relation to subclinical atherosclerosis. Eur J Clin Invest. 2009;39:11–6. doi: 10.1111/j.1365-2362.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 6.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–86. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien J, Barber B. Neuroimaging in dementia and depression. Advances in Psychiatric Treatment. 2000;6:109–19. [Google Scholar]
- 10.Eigenbrodt ML, Bursac Z, Tracy RE, Mehta JL, Rose KM, Couper DJ. B-mode ultrasound common carotid artery intima-media thickness and external diameter: cross-sectional and longitudinal associations with carotid atherosclerosis in a large population sample. Cardiovasc Ultrasound. 2008;6:10. doi: 10.1186/1476-7120-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu FH, Lin HJ, Ye XH, Zhang XL. [Evaluation of carotid artery elasticity in patients with obstructive sleep apnea syndrome using quantitative arterial stiffness technique] . Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:691–4. [PubMed] [Google Scholar]
- 12.Sojkova J, Najjar SS, Beason-Held LL, Metter EJ, Davatzikos C, Kraut MA, Zonderman AB, Resnick SM. Intima-media thickness and regional cerebral blood flow in older adults. Stroke. 2010;41:273–9. doi: 10.1161/STROKEAHA.109.566810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SW, Hai JJ, Kong SL, Lam YM, Lam S, Chan PH, Chan KW, Wong KL, Tam CC, Chan RH. Side differences of carotid intima-media thickness in predicting cardiovascular events among patients with coronary artery disease. Angiology. 2011;62:231–6. doi: 10.1177/0003319710379109. [DOI] [PubMed] [Google Scholar]
- 14.Luo X, Yang Y, Cao T, Li Z. Differences in left and right carotid intima-media thickness and the associated risk factors. Clin Radiol. 2011;66:393–8. doi: 10.1016/j.crad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V. Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2012;43:103–8. doi: 10.1161/STROKEAHA.111.626200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–11. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 18.Boos CJ, Lane DA, Karpha M, Beevers DG, Haynes R, Lip GY. Circulating endothelial cells, arterial stiffness, and cardiovascular risk stratification in hypertension. Chest. 2007;132:1540–7. doi: 10.1378/chest.07-0428. [DOI] [PubMed] [Google Scholar]


