Abstract
To observe the effect of progesterone (PROG) on blood-brain barrier (BBB) permeability, brain tissue water content and dynamic changes of aquaporin-4 (AQP-4) in neonatal rats with hypoxic-ischaemic brain damage (HIBD). 72 neonatal Wistar rats, aged 7 days old, were randomly divided into control, hypoxic-ischaemic (6, 24 and 72 h, and 7 d subgroups) and drug groups (6, 24 and 72 h, and 7 d subgroups). The HIBD animal model was established. BBB was detected via an Evans blue tracer. Brain water content was determined by the dry/wet method. The AQP-4 expression in the cerebral cortex was observed through immunohistochemistry and Western blot. BBB permeability in the cerebral cortex of the neonatal rats, brain water content and AQP-4 expression in the hypoxia-ischaemia group were significantly higher than those of the control group after hypoxia for 6 h (P < 0.05), continued to rise within 24 h and then reached the peak at 72 h. BBB permeability in the cerebral cortex of the neonatal rats, brain water content and AQP-4 expression in the drug group were significantly lower than those of the hypoxia-ischaemia group after hypoxia for 6, 24 and 72 h (P < 0.05). Moreover, BBB permeability and BBB expression were positively correlated with the AQP-4 expression. In conclusion, PROG protects the brain of HIBD neonatal rats by alleviating the damage of BBB and cerebral oedema. The protective effect of PROG may be related to the down-regulation of AQP-4 expression in the cerebral cortex of neonatal rats.
Keywords: Progesterone, hypoxia-ischaemia, aquaporin, blood-brain barrier, cerebral oedema
Introduction
Neonatal hypoxic-ischaemic brain damage (HIBD) is the main disease that causes neonatal death or subsequent neurological development disorders [1-3]. The consequences of hypoxic-ischaemic brain damage are devastating and permanent, but its pathogenesis is not entirely clear, and no specific treatment is available. Therefore, effective treatment strategies should be urgently identified and developed [4,5]. Cerebral oedema is the most prominent histological change during hypoxia-ischaemia. The occurrence, development and severity of cerebral oedema are closely related to the prognosis of the disease.
Aquaporins (AQPs) are groups of proteins related to the transmembrane transport of water, and their discovery provides the molecular basis for this transport. A total of 13 kinds of AQPs are cloned in mammals. The AQP family performs an important function in regulating water homeostasis in brain tissue, cerebrospinal fluid (CSF) formation, and cerebral oedema [6]. AQP-4 is an important member of the AQP family. Its main function is to participate in metabolic balance regulation in brain, and its expression is closely related to the occurrence and development of cerebral oedema [7,8].
A large number of previous studies showed that progesterone (PROG) functions in protecting brain tissue in HIBD [9,10]. However, the dynamic changes of AQP-4 in the brain tissue of neonatal HIBD rats and the mechanism of cerebral oedema alleviation by PROG have yet to be reported. This experiment aimed to further explore the mechanism of PROG in the treatment of cerebral ischaemic injury from AQP-4 expression encephaloedema and BBB permeability.
Materials and methods
Animals and grouping
Seventy-two 7-day-old Wistar rats, which include an unlimited number of males and females, were randomly divided into control, hypoxia-ischaemia (HI), and drug groups. The HI and drug groups were also divided into 6, 24 and 72 h, and 7 d subgroups. The rats in the control group were not treated with ischaemia and hypoxia. The rats in the HI group were treated according to the animal model method. Finally, the rats in the drug group were treated according to the animal model method and intraperitoneally injected with 0.5 g/L PROG solution at a dosage of 8 mg/kg 30 min before hypoxia. The rats in the control and HI group were injected with normal saline solution. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Xinxiang Medical University.
Model establishment
The neonatal rats were fixed on the test bed in a supine position after inhalation of anaesthesia with anhydrous ether. The skin in the middle of the neck was sliced open after alcohol disinfection. The left common carotid artery was separated from the deep layer in the medial sternocleidomastoid, ligated with silk thread and snipped from the middle. The wound was then sutured. The animals recovered at room temperature for 2 h to 3 h. Then, the animals were placed in a 37°C homothermal closed container ventilated with 8% O2 and 92% N2 at 1.5 L/min for 2.5 h to prepare the hypoxia-ischaemia animal models [11,12].
BBB permeability detection
BBB permeability was detected through the Evans blue (EB) content. The skin and eyes of the rats turned blue after their heart chambers were injected with 20 g/L EB saline solution (20 mg/kg). The rats were sacrificed and immediately decapitated after 1 h. The left brain tissue was weighed. Two volumes of formamide were added. The sample was incubated for 72 h in a water bath, and the supernatant was separated afterwards. Absorbance was detected by spectrophotometry. The EB content was calculated in the measurement sample. The results are shown with the EB content in wet brain tissue (μg/g).
Determination of water content
The neonatal rats were decapitated at different times after HIBD, and the brains were quickly removed. Approximately 80 mg cortical tissue was removed and placed in a glass bottle. Then, the wet weight was weighed. The brain tissue was placed in an electrothermal 110°C constant-temperature dry box to bake for 48 h. Dry weight was measured after a constant weight was achieved. The brain tissue water content by the dry-wet method was calculated according to the following formula: brain water content = [(wet weight-dry weight)/wet weight] × 100%.
Immunohistochemistry
The experimental animals were killed at different times after hypoxia-ischaemia treatment. The brain was rapidly removed, segmented behind the optic chiasma, fixed overnight in 10% formalin, routinely dehydrated, transparentised, embedded in paraffin, cut into 5 μm sections, dewaxed, baked and preserved in a 4°C refrigerator. AQP immunohistochemical staining was performed according to kit instructions (Beijing Zhongshan Bio Technology Co., Ltd., Beijing, China). Instead of a primary antibody, a phosphate buffer was added into the negative control group, and other steps were same. Positive cells were expressed in the AQP protein. The cytoplasm and membrane were brown-yellow. The positive cell count method involved observations under 400 × light microscope, analysis by an image analysis system and calculation of the average optical density.
Western blot
The experimental animals were killed at different times after HI treatment. The brain was removed and placed on ice. The partial cortex in the injured side was used to detect proteins. Protein lysate was added, and the protein was extracted by centrifugation. The supernatant was considered as the total protein. Protein concentration was detected by using bicinchoninic acid method. The target protein was separated through polyacrylamide gel electrophoresis. Proteins were transferred on a nitrocellulose membrane and closed. Rabbit anti-rat AQP-4 antibody and β-Actin primary antibody were added. The kit was provided by Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, China). The sample was incubated overnight at 4°C. Then, the secondary antibody was added and incubated. The film was scanned using a gel or film transient display system. The optical density (OD) value of the target band was analysed using an image software, and the relative content was shown with the grey-level ratio of the target and reference protein bands.
Statistical analysis
All experimental data were shown using SPSS13.0 statistical analysis. The measurement data were presented as mean ± standard deviation (x̅ ± s). The comparison between different groups was carried out using single-factor variance analysis. Pairwise comparison was presented using t-test. The comparison between different indexes was carried out using the correlation analysis. P < 0.05 was used to indicate statistical significance.
Results
Immunohistochemistry
Immunohistochemical results showed that AQP-4 positive cells were round or oval. The cytomembrane and cytoplasm were coloured. AQP-4 expression in the cerebral cortex of the HI group was significantly higher than that of the control group after hypoxia for 6, 24 and 72 h (P < 0.01). AQP-4 expression in the cerebral cortex of the drug group was significantly lower than that of the HI group after hypoxia for 6, 24 and 72 h (P < 0.05, Figure 1; Table 1).
Figure 1.

Expression of AQP-4 was detected using immunohistochemistry.
Table 1.
Effect of PROG on expression AQP-4 in brain tissue of neonatal rats (x̅ ± s)
| Immunohisochemistry | Western blot | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | HI | PROG | Control | HI | PROG | |
| 6 h | 0.15 ± 0.02 | 0.31 ± 0.04* | 0.26 ± 0.03 | 0.13 ± 0.01 | 0.23 ± 0.04 | 0.18 ± 0.03 |
| 24 h | 0.57 ± 0.06* | 0.42 ± 0.05Δ | 0.12 ± 0.03 | 0.45 ± 0.05* | 0.32 ± 0.06Δ | |
| 72 h | 0.86 ± 0.32* | 0.51 ± 0.08Δ | 0.13 ± 0.02 | 0.75 ± 0.13* | 0.35 ± 0.12Δ | |
| 7 d | 0.18 ± 0.05 | 0.16 ± 0.03 | 0.14 ± 0.02 | 0.20 ± 0.07 | 0.13 ± 0.08 | |
P < 0.05 vs. control group;
P < 0.05 vs. hypoxic-ischaemic group at the corresponding times.
Western blot
Western blot analysis showed low AQP-4 expression in the brain tissues of the control group. The grey-scale ratio of AQP-4 band and β-actin reference in the HI group was significantly higher than that of the control group after hypoxia for 6, 24 and 72 h, presenting a statistically significant difference (P < 0.05). AQP-4 protein expression in the drug prevention group was significantly decreased in comparison with that of the HI group (P < 0.05) (Table 1; Figure 2). This result was basically similar to the obtained immunohistochemistry results.
Figure 2.
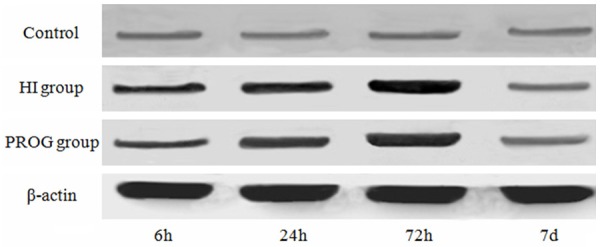
Expression of AQP-4 was detected using western blot.
BBB permeability and brain tissue water content
BBB permeability and encephaloedema began to rise in the HI group at 6 h, continued to rise within 24 h and reached the peak at 72 h. Compared with those in the control group, BBB permeability and cerebral oedema were significantly increased (P < 0.01). Compared with those in the HI group, BBB permeability and cerebral oedema in the PROG group were significantly decreased (P < 0.05, Table 2).
Table 2.
Effect of PROG on BBB permeability and brain tissue water content in brain tissue of neonatal rats (x̅ ± s)
| EB (μg/g) | WC (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | HI | PROG | Control | HI | PROG | |
| 6 h | 15.68 ± 5.31 | 48.56 ± 5.84* | 33.22 ± 7.08* | 85.67 ± 7.02 | 88.64 ± 5.26 | 87.49 ± 5.73 |
| 24 h | 95.47 ± 12.76* | 45.71 ± 6.27Δ | 92.75 ± 7.24* | 88.05 ± 8.43Δ | ||
| 72 h | 122.76 ± 8.90* | 96.08 ± 5.36Δ | 99.67 ± 8.07* | 91.19 ± 5.68Δ | ||
| 7 d | 23.64 ± 6.43 | 21.94 ± 4.82 | 87.15 ± 6.37 | 86.49 ± 6.58 | ||
P < 0.05 vs. control group;
P < 0.05 vs. hypoxic-ischaemic group at the corresponding times.
Correlation analysis
Correlation analysis was performed with AQP-4 expression as the dependent variable and EB and WC as the independent variables. AQP-4 was positively correlated with the contents of EB and WC (r = 0.535, 0.738, P < 0.05).
Discussion
The most evident histological and pathological changes during hypoxia-ischaemia include cerebral oedema and cerebral necrosis, with cerebral oedema being the most prominent. Cerebral oedema is mainly divided into vasogenic cerebral oedema and cytotoxic cerebral oedema. Cytotoxic cerebral oedema occurs in early ischaemia. Capillary endothelial cells in the ischaemic area and surrounding glial cells begin to swell within several minutes. The persistent swelling can induce BBB disruption and increase capillary permeability. At this moment, cytotoxic oedema is transited to vascular oedema. BBB is a diffusion barrier located between the peripheral blood and brain tissue. It is composed of cerebral capillary endothelial cells, perithelial cell and astrocyte foot plates. BBB damage is one of the most important pathophysiological mechanisms of ischaemic brain damage. Traumatic cerebral oedema is a type of mixed oedema that is closely related to the increase in BBB permeability [13,14]. The current study shows that EB and brain water contents in the brain tissue of HI group were significantly higher than those of the control group after hypoxia for 6, 24 and 72 h. Meanwhile, brain tissue water content was significantly increased compared with that in the control group. This finding suggests that the BBB structure was damaged after HI for 6, 24 and 72 h, and the permeability was increased, resulting in cerebral oedema. EB content and brain tissue water content in the PROG group were significantly lower than those in the HI group, suggesting that PROG can perform a neuroprotective function by reducing brain oedema.
AQP is a group of cell membrane transport proteins that are related to water permeability and perform a key function in water-mediated water transmembrane flow. AQP-4 is the main water channel protein. It is distributed in the central nervous system, mediates water molecule flow in brain tissue and is involved in the regulation of water channel activity. AQP-4 performs a crucial function in CSF metabolism and the regulation of water balance in brain. Studies have indicated increased AQP-4 expression in cerebral oedema caused by cerebral infarction, cerebral haemorrhage, brain tumour, inflammation, and brain injury in others’ reports [15,16]. AQP-4 may constitute the second diaphragm of BBB and adjust water transportation. The change in microenvironment results in the up regulation of AQP-4 expression after brain injury, changes the structure of the cell membrane, increases the permeability of BBB and enhances the water permeability [17]. This experiment also confirmed that AQP-4 protein expression in the cerebral cortex was increased in the HI group, and brain oedema was obvious. These results suggest that HIBD may increase the permeability of BBB through the up regulation of AQP-4 and, consequently, cause cerebral oedema. Verkman et al. also proved that AQP-4 gene knockout can significantly alleviate cerebral oedema caused by acute water intoxication or ischaemic stroke, suggesting that AQP-4 performs a key function in brain tissue oedema, restrains AQP-4 expression, and inhibits or alleviates cerebral oedema induced by AQP-4 [18]. Thus, inhibiting the expression of AQP-4 is the key to alleviating cerebral oedema.
Neurosteroid PROG is a type of steroidal compound produced by the central, peripheral nerve systems and glands and performs an important function in regulating the central nervous system. The protective effect of PRGO on brain injury and its mechanism is gradually being researched [19-22], but the molecular mechanism and pathway of PROG in alleviating cerebral oedema are unclear.
The experimental results show that AQP-4 expression in the brain tissue of rats increased after cerebral ischaemia. The AQP-4 expression and brain water content in the ischaemia group were higher than those in the control group. Both expression and water content began to increase after ischaemia for 6 h and reached the peak at 72 h. Brain oedema was the most obvious after ischaemia for 6 h to 72 h. The AQP-4 expression and brain water content decreased after PROG intervention. PROG was suggested to be possibly capable of alleviating ischaemic cerebral oedema through inhibiting AQP-4 expression after ischaemic cerebral damage. That is to say, AQP-4 expression, BBB and cerebral oedema in the brain tissue of neonatal rats were increased significantly after ischaemia for 6 h, continued to increase in 24 h, reached the peak at 72 h and recovered approximately to the level of the control group. Correlation analysis results show that the AQP-4 expression was synchronous with the process of cerebral oedema and that the two were positively related. PROG can relieve cerebral oedema by reducing the AQP-4 expression in the most evident stage of brain injury. This action may be one of the protective mechanisms of PROG on HIBD.
Acknowledgements
This study was supported by grants from the Education Department of Henan Science Research Program (No. 15A310007) and the Funding Program for Young Backbone Teachers in Colleges and Universities of Henan (2012GGJS-134).
Disclosure of conflict of interest
None.
References
- 1.Liu F, McCullough LD. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin. 2013;34:1121–1130. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasdorf E, Perlman JM. Hyperthermia, inflammation, and perinatal brain injury. Pediatr Neurol. 2013;49:8–14. doi: 10.1016/j.pediatrneurol.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Gu Q, Zhai L, Feng X, Chen J, Miao Z, Ren L, Qian X, Yu J, Li Y, Xu X, Liu CF. Apelin-36, a potent peptide, protects against ischemic brain injury by activating the PI3K/Akt pathway. Neurochem Int. 2013;63:535–540. doi: 10.1016/j.neuint.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Ji X, Zhang S, Ren H, Wang M, Guo C, Li Y. Hemin-mediated neuroglobin induction exerts neuroprotection following ischemic brain injury through PI3K/Akt signaling. Mol Med Rep. 2013;8:681–685. doi: 10.3892/mmr.2013.1523. [DOI] [PubMed] [Google Scholar]
- 5.Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG, Nisoli E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Su C. Progesterone-induced neuroprotection: factors that may predict therapeutic efficacy. Brain Res. 2013;1514:98–106. doi: 10.1016/j.brainres.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–294. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Zhang J, Yang Y, Dong W, Wang F, Wang L, Li X. Progesterone attenuates cerebral edema in neonatal rats with hypoxic-ischemic brain damage by inhibiting the expression of matrix metalloproteinase-9 and aquaporin-4. Exp Ther Med. 2013;6:263–267. doi: 10.3892/etm.2013.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30:47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Ikeda T. Progesterone and allopregnanolone exacerbate hypoxic-ischemic brain injury in immature rats. Exp Neurol. 2012;233:214–220. doi: 10.1016/j.expneurol.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Shahrokhi N, Khaksari M, Soltani Z, Mahmoodi M, Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure,and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88:414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi H, Andreasson K. The hypoxic-ischemic encephalopathy model of perinatal ischemia. J Vis Exp. 2008;19:3791–3792. doi: 10.3791/955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doi K, Sameshima H, Kodama Y, Furukawa S, Kaneko M, Ikenoue TK. Perinatal death and neurological damage as a sequential chain of poor outcome. J Matern Fetal Neonatal Med. 2012;25:706–709. doi: 10.3109/14767058.2011.587061. [DOI] [PubMed] [Google Scholar]
- 14.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011;69:743–758. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu YM, Wang CC, Chen L, Qian LB, Ma LL, Yu J, Zhu MH, Wen CY, Yu LN, Yan M. Both PI3K/Akt and ERK1/2 pathways participate in the protection by dexmedetomidine against transient focal cerebral ischemia/reperfusion injury in rats. Brain Res. 2013;494:1–8. doi: 10.1016/j.brainres.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang X, Wang C, Li Y, Dong L, Cui L, Wang L, Liu Z, Qiao H, Zhu C, Xing Y, Cao X, Ji Y, Zhao K. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: up-regulated pAkt, pGSK and pCREB, down-regulated NF-κB expression, ameliorated BBB permeability. Brain Res. 2012;1459:61–70. doi: 10.1016/j.brainres.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Tu J, Zhang Q, Lu D, Zhu Y, Zhang W, Yang F, Brann DW, Wang R. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3β signaling. Neurochem Int. 2011;59:749–758. doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Sun B, Chen L, Wei X, Xiang Y, Liu X, Zhang X. The Akt/GSK-3β pathway mediates flurbiprofen-induced neuroprotection against focal cerebral ischemia/reperfusion injury in rats. Biochem Biophys Res Commun. 2011;409:808–813. doi: 10.1016/j.bbrc.2011.05.095. [DOI] [PubMed] [Google Scholar]
- 19.Collino M, Aragno M, Castiglia S, Tomasinelli C, Thiemermann C, Boccuzzi G, Fantozzi R. Insulin reduces cerebral ischemia/reperfusion injury in the hippocampus of diabetic rats: a role forglycogen synthase kinase-3beta. Diabetes. 2009;58:235–242. doi: 10.2337/db08-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XY, Li XJ, Li DL, Li XJ, Zhu XQ, Guo XP. Neuroprotective effect of progesterone in newborn rats with hypoxic-ischemic encephalopathy. Int J Phys Sci. 2011;6:2894–2900. [Google Scholar]
- 21.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]


