Abstract
Increasing evidences have demonstrated that serum interleukin-35 (IL-35) levels are closely associated with the development, progression, and poor prognosis of a variety of cancers. However, the relationship between IL-35 and the progression of human clear cell renal cell carcinoma (ccRCC) are poorly understood. The aim of present study was to assess the expression of IL-35 and determine its clinical significance in human ccRCCs. Enzyme-linked immunosorbent assay (ELISA) was performed to examine the serum IL-35 levels in 132 patients with ccRCC and 100 healthy controls. The association of IL-35 levels with clinicopathological parameters and prognosis of ccRCC patients was statistically analyzed. Serum IL-35 levels in patients with ccRCC (25.86±11.78 pg/ml) were significantly higher than those in healthy controls (10.05±9.47 pg/ml, P<0.001). High serum IL-35 levels were significantly correlated with pathologic stage (P<0.001), fuhrman grade (P<0.001), tumor size (P=0.012), T stage (P=0.007), N classification (P=0.002), metastasis (P<0.001) and recurrence (P=0.001). The Kaplan-Meier survival analysis demonstrated that high serum IL-35 levels were significantly associated with poor overall survival (log-rank, P<0.001). Multivariate analysis demonstrated that serum IL-35 levels (HR=2.919, 95% CI =1.871-4.830, P=0.001) and pathologic stage (HR=2.541, 95% CI =1.227-3.987, P=0.002) were an independent prognostic factor for the overall survival of ccRCC patients. In conclusion, high serum IL-35 levels are associated with poor prognosis in patients with ccRCC. IL-35 may represent a promising and useful prognostic biomarker for ccRCC.
Keywords: Interleukin-35, clear cell renal cell carcinoma, prognosis, biomarker
Introduction
Clear cell renal cell carcinoma (ccRCC) is the second most common cancer in urinary system worldwide, with incidence and mortality rates increasing approximately 2-3% per decade [1]. About 70% of patients were present with localized or locally advanced renal cell carcinoma, unfortunately, 20-40% of these patients would experience recurrence and metastasis [2]. Although there have been immense improvements in the treatment of ccRCC during recent years, the 5 year survival rate of patients with metastatic RCC is less than 10% [3]. Some environmental and genetic factors have been found to be associated with ccRCC, however, the molecular mechanisms involved in the initiation and progression of ccRCC remain unclear. Therefore, it is essential to identify novel diagnostic and prognostic markers of ccRCC for the personalized care of patients and to aid in more accurate prediction of clinical outcome of patients with ccRCC.
Interleukin (IL)-35 is a novel immune-suppressing cytokine [4]. IL-35 is a heterodimer protein composed of an IL-12α chain and an IL-27β chain, which are encoded by the IL-12 p35 and Epstein-Barr virus-induced gene 3 (EBI3) genes, respectively [4]. IL-35 signals through a unique heterodimer of receptor chains IL-12Rβ2 and gp130 or the homodimers of each chain in target cells [5]. IL-35 is first shown to be predominantly secreted by CD4+CD25+Foxp3+ regulatory T cells (Tregs) and is required for Treg-mediated immunosuppression. IL-35 can suppress the immuneresponse through the expansion of regulatory T cells and suppression of Th1, Th2 and Th17 cell development and responses [6,7].
Recent evidences demonstrated that IL-35 play an important role in the pathogenesis of tumor development, progression, and prognosis. Jin et al reported that circulating IL-35 was significantly increased in pancreatic ductal adenocarcinoma patients, and IL-35 expression levels were associated with lymph node metastasis and advanced tumor stage [8]. Incolorectal cancer, Zeng et al. reported high expression of IL-35 in CRC tissues, which also recruited Treg cells into the tumor microenvironment, in favor of tumor growth [9]. In addition, Gu et al showed that, in NSCLC patients, the plasma levels of IL-35 were significantly higher relative to healthy volunteers, plasma IL-35 correlated positively with tumor TNM stage, lymph node metastases and poor overall survival [10]. Furthermore, Wang et al. reported that tumor derived IL-35 induces CD11b+Gr1+ myeloid cell accumulation in the tumor microenvironment, and thereby, promotes tumor growth [11]. Together, these evidences suggest that IL-35 is a novel anti-inflammatory cytokine which contributes to tumor development and metastasis.
However, to the best of our knowledge, the plasma concentration of IL-35 has not yet been systematically evaluated in patients with ccRCC. The aim of present study was, therefore, to investigate the plasma IL-35 levels and clinical significance of IL-35 in ccRCC and explored the association between IL-35 expression level and prognosis status.
Materials and methods
Patients and follow-up
The study was approved by the Institutional Review Board of our hospital. Written informed consent was obtained from all of the patients and controls before the research according to the committee’s regulations. 132ccRCC patients in the Department of Nephrology, The Second Affiliated Hospital of Wenzhou Medical University were enrolled into the study as the patient group between March 2009 and March 2013. The criteria for study enrollment were as follows: patients with histologically confirmed ccRCC who were newly diagnosed, untreated without a history of other tumors, and subsequently underwent radical nephrectomy. 100 age- and sex-matched healthy volunteers were taken as the control group. The clinicopathologic features of patients are shown in Table 1. There was no significant difference in age (P>0.05) and sex (P>0.05) between ccRCC patients and healthy controls. The disease stage was classified according to 7th edition of the American Joint Committee on Cancer (AJCC) staging system and the future of TNM [12]. Clinical follow-up was performed for all patients every 3 months (median, 34 months; range, 5-58 months), and the results of patients who were lost to follow-up or died from other etiology instead of ccRCC were regarded as censored data.
Table 1.
Correlation between serum IL-35 levels and clinicopathological features of 132 ccRCC patients
| Characteristics | Case n (%) | Mean ± SD (pg/ml) | P value |
|---|---|---|---|
| Age (years) | |||
| ≤50 | 50 (37.8) | 25.11±12.07 | 0.742 |
| >50 | 72 (62.2) | 26.29±11.22 | |
| Gender | |||
| Male | 81 (61.4) | 27.27±12.27 | 0.311 |
| Female | 51 (38.6) | 23.14±10.36 | |
| Pathologic stage | |||
| I | 78 (59.1) | 12.04±6.89 | <0.001 |
| II | 32 (24.2) | 16.37±8.27 | |
| III | 12 (9.1) | 23.97±12.10 | |
| IV | 10 (7.6) | 29.14±10.24 | |
| Fuhrman Grade | |||
| I | 37 (28) | 15.17±9.14 | <0.001 |
| II | 77 (58.3) | 17.24±9.27 | |
| III | 12 (9.1) | 24.74±11.11 | |
| IV | 6 (4.6) | 28.04±12.34 | |
| Tumor size (cm) | |||
| ≤7 | 100 (75.8) | 22.14±11.09 | 0.012 |
| >7 | 32 (24.2) | 30.07±12.37 | |
| T stage | |||
| T1 | 85 (64.4) | 20.33±9.41 | 0.007 |
| T2 | 30 (22.7) | 26.29±12.17 | |
| T3, T4 | 17 (12.9) | 30.30±13.07 | |
| N stage | |||
| N0 | 113 (37.8) | 23.17±10.39 | 0.002 |
| N+ | 19 (37.8) | 31.34±13.27 | |
| Metastasis | |||
| No | 112 (85.6) | 21.97±10.11 | <0.001 |
| Yes | 20 (14.4) | 32.04±11.77 | |
| Recurrence | |||
| No | 110 (83.3) | 24.20±11.11 | 0.001 |
| Yes | 22 (16.7) | 29.87±13.04 |
Measurement of serum IL-35 levels
Blood samples were collected in EDTA-K2 tubes and processed within 1 hour of collection prior to surgery. Cell and nucleic acids free plasma was isolated from all blood samples using a 2-step centrifugation protocol (3000 g for 10 min and 12000 g for 5 min, all at 4°C). All the serum samples were frozen immediately after collection and stored at -80°C until analysis. Serum IL-35 concentrations were quantified by using a commercial human IL-35 heterodimer ELISAkit (Biolegend, San Diego, CA, USA) according to the manufacturer’s protocol. All samples were assayed in duplicate. The mean concentration was determined for each sample.
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 statistical software (SPSS, Chicago, IL, USA). The results are expressed as mean ± standard deviation (SD). Clinical characteristics were compared by Chi-squared test for categorical variables, and for continuous variables, the Kolmogorov-Smirnov test was employed to examine whether the acquired data was normally distributed. For nonparametric data, comparisons between the groups were performed using the Mann-Whitney U test or the Kruskal-Wallis test. One-way ANOVA or student t-test was used for parametric data. Survival curves were plotted using the Kaplan-Meier product-limit method, and differences between survival curves were tested using the log-rank test. The prognostic value of IL-35 was studied using univariate and multivariate Coxproportional hazard models. P value <0.05 was considered statistically significant.
Results
IL-35 was upregulated in ccRCC patients
Sandwich ELISA was used to examine the levels of IL-35 in serum of 132 patients with ccRCC and 100 healthy controls. As shown in Figure 1, the levels of IL-35 in serum samples from ccRCC patients were (25.86±11.78) pg/ml, which was 2-fold higher than that in healthy controls (10.05±9.47) pg/ml (P<0.001).
Figure 1.
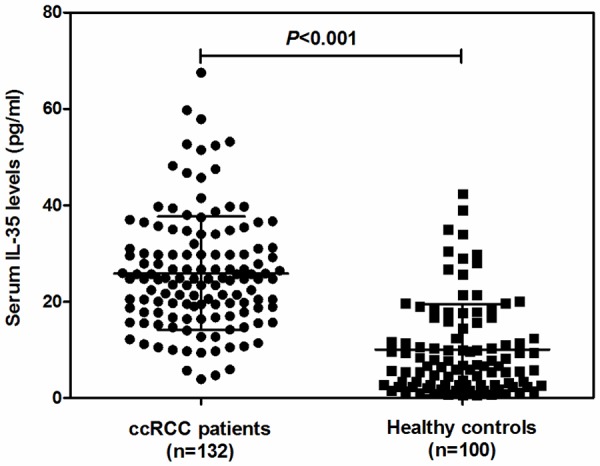
Serum IL-35 concentrations in 132 patients with ccRCC and 100 healthy controls detected by Enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± SD.
Correlations between the levels of IL-35 and the clinicopathological factors in ccRCC patients
The association of serum IL-35 levels with clinicopathological features of the patients with ccRCC was evaluated. As shown in Table 1, high levels of IL-35 were significantly correlated with pathologicstage (P<0.001), Fuhrman grade (P<0.001), tumor size (P=0.012), T stage (P=0.007), N classification (P=0.002), metastasis (P<0.001) and recurrence (P=0.001), respectively. However, other clinical characteristics, including age and gender were not directly related to the high levels of serum IL-35, suggesting that serum IL-35 levels may be closely associated with the development and progression of ccRCC.
Association between IL-35 levels and clinical outcomes of ccRCC patients
Based on the medianvalue of serum IL-35 levels, 24.72 pg/mL was selected as a cutoff value to divide all patients into groups with high (n=66) and low (n=66) IL-35 levels. Kaplan-Meier analysis and the log rank test were applied to assess the relationships between IL-35 levels in ccRCC and prognosis status. As shown in Figure 2, patients with higher levels of IL-35 had poorer overall survival rates than those with lower IL-35 levels. The group of high IL-35 levels patients’ mean overall survival time was 35.5 months, but the low levels group’s mean overall survival time was 53 months. The log-rank test showed the overall survival rates were significantly different between these two groups (log-rank, P<0.001).
Figure 2.
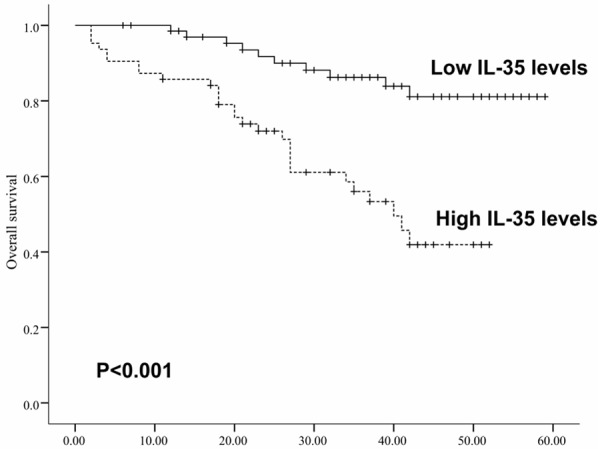
Kaplan-Meier survival curves for ccRCC patients with high (n=66) or low (n=66) serum levels of IL-35.
As shown in Table 2, univariate Cox regression analysis showed that pathologic stage (P<0.001), fuhrman grade (P=0.005), tumorsize (P=0.009), T stage (P=0.019), N stage (P=0.007), metastasis (P=0.002), recurrence (P=0.001) and high IL-35 levels (P<0.001) were significantly related to overall survival. Furthermore, the multivariate Cox regression analysis revealed that only pathologic stage (HR=2.541, 95% CI =1.227-3.987, P=0.002) and high serum IL-35 levels (HR=2.919, 95% CI =1.871-4.830, P=0.001) was independent rognostic factors for the overall survival of ccRCC patients, whereas the others factors were not independently related to the survival of ccRCC patients.
Table 2.
Univariate and multivariate analyses of factors associated with survival and recurrence of ccRCC patients
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age (≤50 vs. >50 years) | 1.067 | 0.814-1.742 | 0.742 | 0.874 | 0.514-1.502 | 0.836 |
| Gender (male vs. female) | 1.349 | 0.631-2.505 | 0.534 | 1.107 | 0.387-1.724 | 0.511 |
| Pathologic stage (I-II vs. III-IV) | 2.827 | 1.947-4.133 | <0.001 | 2.541 | 1.227-3.987 | 0.002 |
| Fuhrman Grade (I-II vs. III-IV) | 1.459 | 0.992-2.315 | 0.005 | 1.087 | 0.347-1.884 | 0.874 |
| Tumor size (≤7 vs. >7 cm) | 2.110 | 1.091-2.872 | 0.009 | 1.532 | 0.551-2.142 | 0.353 |
| T stage (T1-T2 vs. T3-T4) | 1.834 | 1.199-2.634 | 0.019 | 0.991 | 0.411-1.397 | 0.697 |
| N stage (N0 vs. N+) | 2.232 | 1.608-3.247 | 0.007 | 1.754 | 0.918-2.203 | 0.247 |
| Metastasis (yes vs. no) | 2.509 | 1.359-4.011 | 0.002 | 1.741 | 0.957-2.357 | 0.189 |
| Recurrence (yes vs. no) | 2.677 | 1.547-3.977 | 0.001 | 1.547 | 0.879-2.671 | 0.247 |
| Serum IL-35 levels (high vs. low) | 3.544 | 2.417-5.371 | <0.001 | 2.919 | 1.871-4.830 | 0.001 |
Discussion
IL-35 is a newly discovered suppressive cytokine secreted by regulatory T cells (Tregs) and may have therapeutic potential in several inflammatory disorders, autoimmune diseases and allograft rejection [13,14]. Recently evidences demonstrate that IL-35 expression is closely associated with the development, progression, and poor prognosis of a variety of tumors. However, there lationship between IL-35 and the progression of human ccRCC are poorly understood.
To the best of our knowledge, our study provides the first evidence for the greater increase in protein levels of IL-35 in serum of ccRCC patients than in healthy controls. Recently, a high incidence of IL-35 expression in several cancers and a significant association with tumor progression and metastasis has been reported, which was consistent with our findings in ccRCC. Jin et al reported that circulating IL-35 was significantly increased in pancreatic ductal adenocarcinoma patients [8]. In colorectal cancer, Zeng et al. reported high expression of IL-35 in CRC tissues [9]. In addition, Gu et al showed that, in NSCLC patients, the plasma levels of IL-35 were significantly higher relative to healthy volunteers [10]. Although the plasma concentrations of IL-35 that they reported in cancer patient were not consistent, and this may due to the different cancer types and the sensitivity of commercial IL-35 ELISA kit, these evidences together suggest that IL-35 may be involved in the development and progression of human cancers.
In the tumor microenvironment, other than Tregs, tumor-infiltrating dendritic cells (DCs) and tumor cells are also considered to be the primary IL-35 producer [15-17]. Tumor-derived IL-35 has been found to increase CD11b+Gr1+ myeloid cell accumulation in the tumor microenvironment and promotes tumor angiogenesis [11]. Also, Olson and colleagues identified a population of CD8+CTLA-4+ IL-35-secreting tumor Ag-specific Tregs in prostate cancer patients, which prevents Ag-specific effector responses by an IL-35-dependent mechanism [18]. In addition, IL-35 has been found to potently inhibit antitumor T cell responses, and thereby, promotes tumor development [6]. While these findings are important and exciting, the exact impact of IL-35 on human ccRCC progress and metastasis is yet to be fully addressed. Then we analyzed the correlation between the serum IL-35 levels and the clinical features of patients, and the results indicated that the high levels for IL-35 were significantly correlated with pathologic stage, fuhrman grade, tumor size, T stage, N classification, metastasis and recurrence. Moreover, according to Kaplan-Meier analysis, serum IL-35 levels in ccRCC were significantly correlated with overall survival; patients with high serum IL-35 levels had a shorter survival time than those with low levels. Our findings were in consistent with published data in pancreatic ductal adenocarcinoma, colorectal cancer and NSCLC [8-10]. Given the role of IL-35 played in protection of tumor cells against immunity, these observations further supports that higher levels of IL-35 are likely to be a prerequisite to ensure tumor progression and metastasis. Therefore, our data revealed that there were positively correlations between the serum IL-35 levels and clinic-pathological parameters and may be a potential prognostic marker and therapeutic target for ccRCC.
Our findings that high IL-35 levels were associated with aggressive tumor progression and metastasis prompt us to investigate its possible prognostic value in ccRCC patients. According to the univariate and multivariate analyses, we identified high serum IL-35 levels as an independent predictor for overall survival, which was in agreement with recent findings in NSCLC and pancreatic ductal adenocarcinoma, suggesting that the detection of serum IL-35 levels might help identifycc RCC patients with a poor prognosis, and could therefore be an ovel prognostic marker for ccRCC patients.
As far as we know, this is the first study to evaluate the value of serum IL-35 as a clinically potential indicator for ccRCC progression, as well as a prognostic marker for patient survival. However, it should admitted that our study was a single hospital-based, retrospective study, and multi-centers or community-based prospective studies are required, and further investigation of the mechanism by which the tumor prompting roles of IL-35 in ccRCC is needed.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 3.Spiess PE. Surgical management of locally recurrent renal cell carcinoma post-renal cryoablation: importance of stringent selection criteria. Urol Oncol. 2010;28:241–242. doi: 10.1016/j.urolonc.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 5.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Dong C, Yue Y, Xiong S. In vivo delivery of interleukin-35 relieves coxsackievirus-B3-induced viral myocarditis by inhibiting Th17 cells. Arch Virol. 2014;159:2411–2419. doi: 10.1007/s00705-014-2098-z. [DOI] [PubMed] [Google Scholar]
- 8.Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol. 2014;75:29–33. doi: 10.1016/j.humimm.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Zeng JC, Zhang Z, Li TY, Liang YF, Wang HM, Bao JJ, Zhang JA, Wang WD, Xiang WY, Kong B, Wang ZY, Wu BH, Chen XD, He L, Zhang S, Wang CY, Xu JF. Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol. 2013;6:1806–1816. [PMC free article] [PubMed] [Google Scholar]
- 10.Gu X, Tian T, Zhang B, Liu Y, Yuan C, Shao L, Guo Y, Fan K. Elevated plasma interleukin-35 levels predict poor prognosis in patients with non-small cell lung cancer. Tumour Biol. 2015;36:2651–6. doi: 10.1007/s13277-014-2887-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol. 2013;190:2415–2423. doi: 10.4049/jimmunol.1202535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Leung PS, Bowlus C, Gershwin ME. IL-35 and Autoimmunity: a Comprehensive Perspective. Clin Rev Allergy Immunol. 2015;49:327–32. doi: 10.1007/s12016-015-8468-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Wu Y, Wang Y, Cai Y, Hu B, Bao G, Fang H, Zhao L, Ma S, Cheng Q, Song Y, Zhu Z, Chang H, Yu X, Sun A, Zhang Y, Vignali DA, Wu D, Liu H. IL-35 mitigates murine acute graft-versus-host disease with retention of graft-versus-leukemia effects. Leukemia. 2015;29:939–46. doi: 10.1038/leu.2014.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niedobitek G, Pazolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198:310–316. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- 16.Dixon KO, van der Kooij SW, Vignali DA, van Kooten C. Human tolerogenic dendritic cells produce IL-35 in the absence of other IL-12 family members. Eur J Immunol. 2015;45:1736–47. doi: 10.1002/eji.201445217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. IL-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PLoS One. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012;189:5590–5601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]


