Abstract
Background: Smoking is well-known as a risk factor for esophageal squamous cell carcinoma. However, little is known about the effect of this factor on survival. Methods: Esophageal cancer patients who underwent esophagectomy without any preoperative therapy were retrospectively reviewed. Patients’ postoperative overall and disease-free survivals were compared between 2 groups (non-heavy smokers and heavy smokers). Results: A total of 109 cases were evaluated in this study. The 5-year OS rate of the non-heavy smokers was 53.2% and 32.3% for the heavy group. The 5-year DFS rate of the non-heavy smokers was 51.1% and 27.4% for the heavy group. Kaplan-Meier survival analysis revealed that heavy smokers had significantly poorer OS (P=0.018) and DFS (P=0.009) than non-heavy smokers. In multivariate analysis, smoking was found to be an independent prognostic factor for OS (P=0.003; HR: 2.186; 95% CI: 1.309-3.650) and DFS (P=0.001; HR: 2.471; 95% CI: 1.467-4.163). Conclusion: Smoking was associated with survival among patients with ESCC, and it was recognized as an independent factor in both OS and DFS.
Keywords: Smoking, esophageal squamous cell carcinoma, prognosis
Introduction
Esophageal cancer is one of the most common malignant tumor in the digestive system. The latest data reveals that estimated 455,800 new esophageal cancer cases and 400,200 deaths occurred in 2012 worldwide [1]. The two main types of esophageal cancer are squamous cell carcinoma (ESCC) and adenocarcinoma (EAC). In Asian countries, the predominant histological type is squamous cell carcinoma. Although incidence rates of esophageal squamous cell carcinoma have been steadily declining in Northern America and Europe [2-4], they have been increasing in some Asian countries [5]. The increasing trend is mainly due to the increasing prevalence of established risk factors, such as alcohol and tobacco use, physical inactivity. Tobacco use as one of the primary risk factors for squamous cell carcinoma has been reported in many studies [6,7]. However, there are few reports regarding the prognostic value of smoking in esophageal cancer.
Therefore, in the current study, we aimed to assess the correlation between smoking and long-term postoperative outcome of ESCC through a retrospective study.
Materials and methods
Study population
We searched all of the esophageal cancer patients in the department of thoracic surgery at Qilu hospital from January to December 2008. All of the enrolled patients who underwent esophagectomy must have histological documentation of ESCC. Exclusive criteria of the present study: (1) patients who died within 30 days after operation; who underwent preoperative chemotherapy or radiotherapy; who could not be contacted during follow-ups. Consequently 109 patients were available for the present study.
Data collection
Demographic data and tumor associated data for all patients with esophageal cancer referred to our hospital for further analysis and treatment had been stored in a database by a specialized data manager in the record room, including gender, tobacco and alcohol use, tumor size and stage, treatment regimen, and so on. Tumor stage was based on the clinical TNM classification. Treatment regimens in this study included resection, postoperative radiotherapy, postoperative chemotherapy, a combination of radiotherapy and chemotherapy after resection.
Cumulative smoking dose was evaluated as pack-years; pack-years are calculated by multiplying the average number of packs of cigarettes smoked per day by the number of years a person has smoked. We divided the collected cases into two groups according to their PY: non- or light (non-heavy) smokers (PY<20) and heavy smokers (PY≥20) [8]. Alcohol intake cut-off point was 0.025 kg/d. The cut-off value was based on 2011 Chinese Inhabitant Dietary Guideline.
Follow-up time was calculated from the date of surgery to the event or October 2013.
Ethical standards
This study has been approved by the ethics committee of Qilu Hospital of Shandong University. All patients gave their informed consent prior to their inclusion in the study.
Statistical analyses
Analyses were performed using SPSS statistical software (version 20.0; SPSS Inc, Chicago, Ill). Student t test was used for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Survival analyses were performed by Kaplan-Meier curves with log-rank tests for significance. Univariable and multivariable logistic regression were used to estimate hazard ratio (HR) with 95% confidence intervals (CI) and P values. All P-values were based on two-sided tests of significance. P<0.05 were regarded as statistically significant.
Results
Clinicopathological characteristics of patients
The relationship between smoking and clinic pathological characteristics in ESCC is summarized in Table 1. The current study consisted of 109 patients, among whom 47 cases were non-heavy smokers and 62 cases were heavy smokers. The median age of the non-heavy group was 61 years (range, 38-79 years), while the heavy group was 59 years (range, 32-84 years). We found significant differences between groups according to patients’ gender (P=0.001) and alcohol use (P<0.001). There were no significant differences noted between groups in tumor diameter (P=0.152), TNM stage (P=0.068), and treatment regimen (P=0.565).
Table 1.
Patient characteristics
| Non-heavy smoker | Heavy smoker | P value | |
|---|---|---|---|
|
|
|||
| N=47 | N=62 | ||
| Gender | 0.001 | ||
| Male | 45 (95.7%) | 44 (71.0%) | |
| Female | 2 (4.3%) | 18 (29.0%) | |
| Median age (range) | 61 (38-79) | 59 (32-84) | 0.975 |
| Alcohol intake | <0.001 | ||
| Non or light | 19 (40.4%) | 48 (77.4%) | |
| Heavy | 28 (59.6%) | 14 (22.6%) | |
| Diameter | 0.152 | ||
| <3 cm | 11 (23.4%) | 8 (12.9%) | |
| ≥3 cm | 36 (76.6%) | 54 (87.1%) | |
| TNM stage | 0.068 | ||
| I | 7 (14.9%) | 7 (11.3%) | |
| II | 26 (55.3%) | 21 (33.9%) | |
| III | 12 (25.5%) | 30 (48.4%) | |
| IV | 2 (4.3%) | 4 (6.5%) | |
| Treatment regimen | 0.565 | ||
| Surgery only | 19 (40.4%) | 28 (45.2%) | |
| Surgery plus postoperative R | 13 (27.7%) | 14 (22.6%) | |
| Surgery plus postoperative C | 5 (10.6%) | 11 (17.7%) | |
| Surgery plus postoperative CRT | 10 (21.3%) | 9 (14.5%) | |
R: radiotherapy; C: chemotherapy; CRT: chemoradiotherapy.
Prognostic impact of smoking on OS and DFS
Kaplan-Meier curves of overall and disease-free survival (OS and DFS) based on smoking are shown in Figures 1 and 2. The 5-year OS rate of the non-heavy smokers was 53.2% and the heavy group was 32.3%. The 5-year DFS rate of the non-heavy smokers was 51.1% and 27.4% from the heavy group. Both overall and disease-free survival in non-heavy smokers were significantly better than those from the heavy smokers (OS, P=0.018; DFS, P=0.009).
Figure 1.
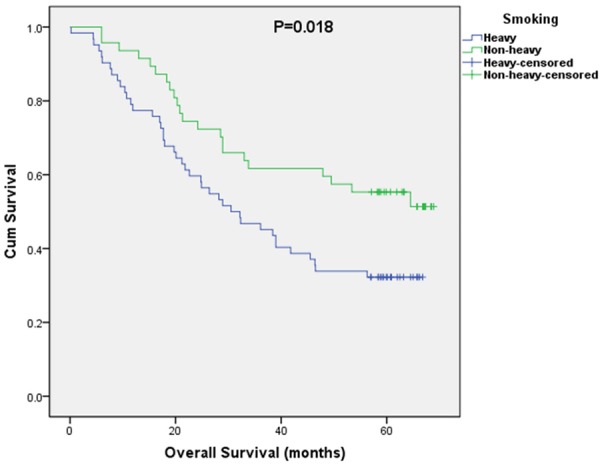
OS curves stratified by smoking history.
Figure 2.
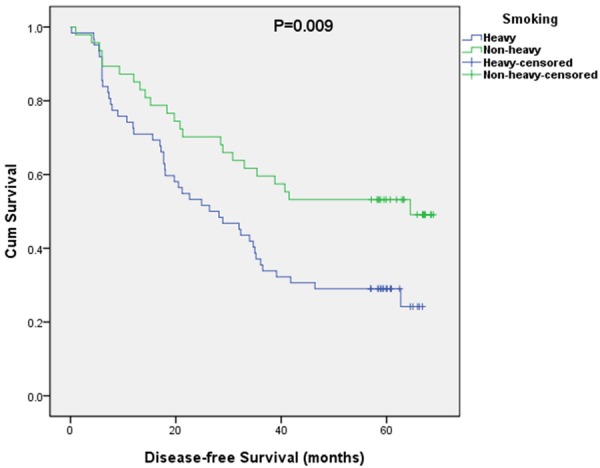
DFS curves stratified by smoking history.
Univariate and multivariate survival analyses
Table 2 shows the results of univariate analyses. The log-rank test revealed that factors significantly correlated with OS and DFS, including smoking, tumor diameter, TNM stage, and treatment regimen (all P<0.05).
Table 2.
Univariate analysis of factors associated with OS and DFS
| OS | DFS | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Gender | 0.624 | 0.439 | ||||
| Male | 1.000 | Ref. | 1.000 | Ref. | ||
| Female | 0.624 | 0.850 | 0.444-1.628 | 0.439 | 0.775 | 0.406-1.478 |
| Median age (range) | 0.763 | 0.996 | 0.967-1.025 | 0.768 | 0.996 | 0.969-1.024 |
| Smoking | 0.020 | 0.011 | ||||
| Non-heavy smoker | 1.000 | Ref. | 1.000 | Ref. | ||
| Heavy smoker | 0.020 | 1.850 | 1.103-3.106 | 0.011 | 1.933 | 1.166-3.206 |
| Alcohol intake | 0.747 | 0.940 | ||||
| Non or light | 1.000 | Ref. | 1.000 | Ref. | ||
| Heavy | 0.747 | 1.086 | 0.657-1.796 | 0.940 | 1.019 | 0.624-1.663 |
| Diameter | 0.040 | 0.033 | ||||
| <3 cm | 1.000 | Ref. | 1.000 | Ref. | ||
| ≥3 cm | 0.040 | 2.277 | 1.037-5.001 | 0.033 | 2.235 | 1.066-4.685 |
| TNM stage | 0.001 | <0.001 | ||||
| I | 1.000 | Ref. | 1.000 | Ref. | ||
| II | 0.069 | 3.039 | 0.917-10.075 | 0.064 | 3.106 | 0.937-10.294 |
| III | 0.002 | 1.328 | 1.929-20.761 | 0.001 | 7.704 | 2.356-25.199 |
| IV | 0.003 | 8.952 | 2.126-37.686 | <0.001 | 11.955 | 2.963-48.236 |
| Treatment regimen | <0.001 | 0.002 | ||||
| Surgery only | 1.000 | Ref. | 1.000 | Ref. | ||
| Surgery plus postoperative R | 0.291 | 1.424 | 0.739-2.747 | 0.194 | 1.516 | 0.809-2.839 |
| Surgery plus postoperative C | <0.001 | 4.292 | 2.181-8.445 | <0.001 | 3.726 | 1.903-7.293 |
| Surgery plus postoperative CRT | 0.067 | 1.935 | 0.954-3.923 | 0.054 | 1.961 | 0.989-3.890 |
OS: overall survival; DFS: disease-free survival; R: radiotherapy; C: chemotherapy; CRT: chemoradiotherapy.
>All of the above covariates were enrolled into the multivariable Cox regression analyses on OS and DFS. The results of multivariate analyses are shown in Table 3. In multivariate analysis, smoking was found to be an independent prognostic factor for OS (P=0.003; HR: 2.186; 95% CI: 1.309-3.650), as well as TNM stage (P=0.001; HR: 2.355; 95% CI: 1.417-3.915) and treatment regimen (P=0.032; HR: 1.786; 95% CI: 1.051-3.034). With respect to DFS, smoking was also an independent prognostic factor (P=0.001; HR: 2.471; 95% CI: 1.467-4.163). Besides, TNM stage (P<0.001; HR: 3.195; 95% CI: 1.959-5.213) was also an independent prognostic factors for DFS.
Table 3.
Multivariate analysis of factors associated with OS and DFS
| OS | DFS | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| P value | HR | 95% CI | P value | HR | 95% CI | |
| Smoking (Heavy) | 0.003 | 2.186 | 1.309-3.650 | 0.001 | 2.471 | 1.467-4.163 |
| Diameter (≥3 cm) | 0.212 | 1.669 | 0.746-3.734 | 0.236 | 1.583 | 0.741-3.385 |
| TNM stage (III/IV) | 0.001 | 2.355 | 1.417-3.915 | <0.001 | 3.195 | 1.959-5.213 |
| Treatment regimen (Surgery plus postoperative R/C/CRT) | 0.032 | 1.786 | 1.051-3.034 | 0.087 | 1.583 | 0.935-2.182 |
OS: overall survival; DFS: disease-free survival; R: radiotherapy; C: chemotherapy; CRT: chemoradiotherapy.
Discussion
In the present study, we retrospectively analyzed the impact of smoking history on prognosis of esophageal squamous cell carcinoma. Survival analysis revealed that both overall and disease-free survival rates were significantly worse in patients with a history of heavy smoking. More importantly, our result suggests smoking is an independent prognostic factor for esophageal squamous cell carcinoma.
Smoking is a well-established cause of esophageal squamous cell carcinoma, however, little is known about the effect of this factor on survival. During the past decades, most studies have been focus on the relationship between smoking and cancer incidence [9-12]. Recently, the inverse correlation between smoking and cancer prognosis has been studied. A nationwide case-control study in Sweden first reported that previous smokers (HR: 2.1, 95% CI: 1.0-4.4) had a worse outcome for ESCC [13], but current smoker status was not statistically significant (HR: 1.4, 95% CI: 0.7-2.8) among the patients with ESCC.
A study in Taiwan found that patients who regularly used alcohol, areca nut, and cigarette had 1.52 times the risk of early death (HR: 1.52, 95% CI: 1.02-2.27, P=0.04) compared with non-users [14]. Shitara Kand colleagues [8] conducted a study in Japan, and they found significantly worse survival rates in esophageal squamous cell cancer patients with a history of heavy tobacco smoking. Besides, they found heavy smoking only had a significant impact on the prognosis of patients treated by chemoradiotherapy, offering evidence that smoking interacts with major treatment modalities of esophageal cancer. The result of our study was consistent with former studies. In our study, we only evaluated the impact of smoking on postoperative patients. And for these patients, the treatment regimens were not related with smoking history (P=0.565).
Lifestyle factors, in particular tobacco smoking and alcohol, are well known risk factors for esophageal cancer. Thrift AP and colleagues [15] conducted a case-control study of esophageal cancer in Australia to evaluate the effect of tobacco smoking on survival. They found the risk of early death was greatest among current smokers who reported regularly consuming alcohol. In our study, we found the effect of smoking on survival after consideration of detailed demographic and lifestyle factors, as well as clinical data such as gender, age, alcohol intake, tumor stage, tumor diameter, and treatment. Alcohol intake was not significantly in the Cox regression analyses both for OS and DFS.
Lin Yand colleagues [16] observed a significant reduction in 3- and 5-year survival rates in smokers with lymph node-negative ESCC compared with those in non-smokers, and no significant difference was found between the survival rates of light and heavy smokers in this study. But this retrospective study was only for patients with lymph node-negative ESCC. Different from this previous study, in our cohort we enrolled both lymph node-negative and -positive patients, and survival rates between non-heavy smokers and heavy smokers was significant. But we did not found significant difference between non-smokers and smokers (OS P=0.689; DFS P=0.978, results not shown).
The exact mechanism through which smoking leads to a worse prognosis is still unknown. One of the possible explanations is that tobacco-specific chemical compounds cause genetic or epigenetic alterations, modulate expressions of large numbers of genes that include those that encode molecules related to proliferation, invasion and metastasis [17]. Another explanations is the over expression of DNA repair enzymes due to heavy smoking [18]. Taken together, these reports might explain the poor prognosis in patients with a heavy smoking habit in our study.
There are several limitations of this study. First, the present study was retrospective in which the comparison of non-heavy smokers and heavy smokers is subject to selection bias. Second, the sample size was not large enough. Further large cohort studies should now be conducted to validate and explicate the precise clinical value of smoking in patients with clear cell ESCC.
In summary, we have confirmed tobacco smoking as an independent factor for postoperative OS and DFS in patients with ESCC, and heavy smoking was strongly associated with poor prognosis. Further mechanistic studies are necessary to elucidate how smoking lead to an adverse outcome.
Acknowledgements
This study was supported by the Academics Independent Innovation Project of Jinan City (201401252).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Castro C, Bosetti C, Malvezzi M, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol. 2014;25:283–290. doi: 10.1093/annonc/mdt486. [DOI] [PubMed] [Google Scholar]
- 3.Otterstatter MC, Brierley JD, De P, Ellison LF, Macintyre M, Marrett LD, Semenciw R, Weir HK. Esophageal cancer in Canada: trends according to morphology and anatomical location. Can J Gastroenterol. 2012;26:723–727. doi: 10.1155/2012/649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855–859. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu CL, Lang HC, Luo JC, Liu CC, Lin HC, Chang FY, Lee SD. Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma, in Taiwan. Cancer Causes Control. 2010;21:269–274. doi: 10.1007/s10552-009-9458-0. [DOI] [PubMed] [Google Scholar]
- 6.Ishiguro S, Sasazuki S, Inoue M, Kurahashi N, Iwasaki M, Tsugane S, Group JS. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study) Cancer Lett. 2009;275:240–246. doi: 10.1016/j.canlet.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–346. [PubMed] [Google Scholar]
- 8.Shitara K, Matsuo K, Hatooka S, Ura T, Takahari D, Yokota T, Abe T, Kawai H, Tajika M, Kodaira T, Shinoda M, Tajima K, Muro K, Tanaka H. Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci. 2010;101:1001–1006. doi: 10.1111/j.1349-7006.2009.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramus JR, Gatenby PA, Caygill CP, Watson A, Winslet MC. The relationship between smoking and severe dysplastic disease in patients with Barrett’s columnar-lined oesophagus. Eur J Cancer Prev. 2012;21:507–510. doi: 10.1097/CEJ.0b013e328350b06f. [DOI] [PubMed] [Google Scholar]
- 10.Lee CH, Lee KW, Fang FM, Wu DC, Tsai SM, Chen PH, Shieh TY, Chen CH, Wu IC, Huang HL, Chen BH, Chang CH, Chen MK, Chou SH, Tsai YS, Chiang SL, Ko YC. The neoplastic impact of tobacco-free betel-quid on the histological type and the anatomical site of aerodigestive tract cancers. Int J Cancer. 2012;131:E733–743. doi: 10.1002/ijc.27401. [DOI] [PubMed] [Google Scholar]
- 11.Coleman HG, Bhat S, Johnston BT, McManus D, Gavin AT, Murray LJ. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett’s esophagus. Gastroenterology. 2012;142:233–240. doi: 10.1053/j.gastro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Tramacere I, La Vecchia C, Negri E. Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology. 2011;22:344–349. doi: 10.1097/EDE.0b013e31821092cd. [DOI] [PubMed] [Google Scholar]
- 13.Sundelof M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44:1566–1571. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Wu IC, Wu CC, Lu CY, Hsu WH, Wu MC, Lee JY, Chou SH, Lee JM, Chou YP, Wu DC, Wu MT. Substance use (alcohol, areca nut and cigarette) is associated with poor prognosis of esophageal squamous cell carcinoma. PLoS One. 2013;8:e55834. doi: 10.1371/journal.pone.0055834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, Whiteman DC Australian Cancer Study Clinical Follow-Up Study. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131:E759–768. doi: 10.1002/ijc.27420. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Su X, Su H, Lin P, Long H, Zhang L, Fu J, Rong T, Tan Z, Meng Y, Ma G. Prediagnostic smoking and postoperative survival in lymph node-negative esophagus squamous cell carcinoma patients. Cancer Sci. 2012;103:1985–1988. doi: 10.1111/cas.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshino I, Maehara Y. Impact of smoking status on the biological behavior of lung cancer. Surg Today. 2007;37:725–734. doi: 10.1007/s00595-007-3516-6. [DOI] [PubMed] [Google Scholar]
- 18.Nozoe T, Korenaga D, Kabashima A, Sugimachi K. Smoking-related increase of O(6)-methylguanine-DNA methyltransferase expression in squamous cell carcinoma of the esophagus. Cancer Lett. 2002;184:49–55. doi: 10.1016/s0304-3835(02)00188-x. [DOI] [PubMed] [Google Scholar]


