Abstract
This study aims to investigate the extravascular lung water index (EVLWI) in lung water scavenging of sepsis patients with acute kidney injury (AKI) by renal replacement therapy (RRT). 57 septic acute kidney injury patients with EVLWI > 7 ml/kg were selected and randomly divided into two groups: the treatment group with continued RRT for 24 h per day, the control group with RRT for ≤8 h per day. Fluid resuscitation and RRT were performed simultaneously. After fluid resuscitation, EVLWI, hemodynamics, oxygenation index, blood lactate, and intensive care unit (ICU) stay were determined. The values of EVLWI, pulmonary vascular permeability index (PVPI), and blood lactate decreased and the intrathoracic blood volume index (ITBVI) increased significantly at 24 h, 48 h and 72 h, after RRT, compared with those before RRT in the two groups (P < 0.05). The values of EVLWI at 48 h and 72 h after RRT in the treatment group were significantly lower than that in the control group (P < 0.05). The cardiac index (CI) at 48 h and 72 h after RRT in the treatment group was significantly higher than that before RRT (P < 0.05). The values of PVPI, ITBVI, CI, blood lactate, transcutaneous oxygen saturation pulse (SPO2), oxygenation index (PO2/FiO2) and arterial oxygen (PO2) before and 24 h, 48 h, and 72 h after RRT. The 28d mortality had no significant difference in the two groups (P > 0.05). The average ICU stay for the treatment group was significantly shorter than that of the control group (P < 0.05). EVLWI monitoring of septic patients with AKI in RRT time had clinical reference value.
Keywords: Sepsis, extravascular lung water index, acute kidney injury, renal replacement therapy, pulse indicating continuous cardiac output, fluid resuscitation
Introduction
Sepsis is a systemic inflammatory response syndrome (SIRS) caused by infection. The disease progresses rapidly, with some patients developing septic shock and multiple organ dysfunction syndrome (MODS). According to incomplete statistics, 2-3 million patients worldwide suffer from sepsis each year, and about 1,400 people die from sepsis daily. Sepsis has become the leading cause of death in intensive care unit (ICU) patients [1-3], the mortality rate reaching as high as 30-50% [1,4-6]. It has been reported that 64.4% of patients with septic shock had acute kidney injury (AKI) within 24 h secondary to hypotension [7]. MODS and AKI caused by sepsis were primary reasons for prolonged hospitalization and increased mortality [8].
Severe sepsis easily leads to heart, lung, kidney, and other organ damage. In addition to active anti-infection treatment, early fluid resuscitation was key to effectively improving tissue perfusion. Septic patients have massive release of inflammatory cytokines and increased vascular permeability, resulting in an increase in lung water and decreased oxygenation index after fluid resuscitation. Therefore, full fluid resuscitation, avoiding pulmonary edema, was difficult. The central venous pressure (CVP), pulmonary capillary wedge pressure (PAWP), intrathoracic blood volume (ITBV), dedicated end-diastolic volume (GEDV), and other indicators were commonly used to guide fluid resuscitation in traditional treatment, however their accuracy was questioned [9,10].
Pulse indicator continuous cardiac output (PICCO) was a monitoring technology combining pulmonary thermodilution with arterial pulse curve analysis, of which, extravascular lung water index (EVLWI), pulmonary vascular permeability index (PVPI), pulmonary edema, and pulmonary vascular permeability assessment had high clinical values. In this paper, the impact of different renal replacement therapy (RRT) times on pulmonary edema of high EVLWI patients were analyzed to investigate the scavenging of extravascular lung water at different RRT time points in patients with septic AKI.
Materials and methods
General information
This study followed standard procedures in line with medical ethics. Subjects or their families gave written informed consent for the implementation of PICCO monitoring before RRT. Fifty-seven septic acute kidney injury patients with EVLWI > 7 ml/kg were collected from Nanjing Hospital of Nanjing Medical University during December 2012 and December 2014.
Inclusion criteria: ① age 18 years or older; ② meet the diagnostic criteria ACCP/SCCM for sepsis [11]; and ③ AKI meet the KDIGO standards of 2012 [12]. Sepsis diagnostic criteria: compliance with SIRS diagnostic criteria with clear evidence of an infection. The diagnostic criteria for SIRS is 2 or more of the following: (1) body temperature > 38.2°C or < 36°C; (2) heart rate > 90 beats/min; (3) respiration > 20 breaths/min, or carbon dioxide partial pressure (PaCO2) < 32 mmHg (1 mmHg = 0.133 kPa); (4) white blood cell count > 12×109/L, or < 4×109/L, rod-shaped nucleus ratio > 0.10. Severe sepsis diagnosis meant inadequate tissue perfusion and organ dysfunction appeared in patients.
Exclusion criteria: ① younger than 18 years old; ② did not complete treatment; ③ admission creatinine > 707 μmol/L and routine dialysis; ④ severe cardiopulmonary dysfunction, including atrial septal defect, severe heart valve insufficiency, aortic aneurysm, after lobectomy, severe pneumothorax, severe arrhythmia, and massive pulmonary embolism; ⑤ the presence of contraindications for a femoral artery or central venous catheter; and ⑥ severe coagulation disorders.
Patients were randomly divided into two groups: patients with continued RRT (CRRT) for 24 h per day were the treatment group, while patients with RRT ≤ 8 h each time were the control group. The treatment group had 29 cases, 19 male and 10 females aged 61.21±18.23 years of age, with APACHE-II scores 26.31±8.06 points. The control group had 28 patients, 18 males and 10 females, aged 60.57±19.64 years, with APACHE-II scores 25.57±6.29 points. Age, gender, site of infection, and APACHE-II scores of the treatment group were not significantly different from the control group. After being sent to ICU, all patients underwent bedside X-ray or computed tomography (CT) scan, sputum culture, blood culture, and other bacteriological examinations. The clinical characteristics of 57 severe sepsis cases with AKI are shown in Table 1. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Nanjing First Hospital. Written informed consent was obtained from all participants.
Table 1.
Clinical features of 57 severe septic patients with AKI
| Items | Count | |||
|---|---|---|---|---|
|
|
||||
| Treat group (n = 29) | Control group (n = 28) | t/x 2 | P value | |
| Age (Year) | 61.21±18.23 | 60.57±19.64 | T = 0.152 | 0.880 |
| Male/Female | 19/10 | 18/10 | x 2 = 0.009 | 0.922 |
| APACHE II | 26.31±8.06 | 25.57±6.29 | T = 0.385 | 0.702 |
| ICU stay | 7.10±2.06 | 8.52±2.94 | T = -2.110 | 0.039 |
| 28 d mortality | 27.59% | 32.14% | x 2 = 0.141 | 0.707 |
| Source | x 2 = 0.144 | 0.707 | ||
| Medical patients | 19 | 17 | ||
| Surgical patients | 10 | 11 | ||
| Underground disease | ||||
| High blood pressure | 11 | 9 | x 2 = 0.210 | 0.647 |
| Coronary heart disease | 7 | 6 | x 2 = 0.059 | 0.807 |
| Diabetes | 5 | 3 | x 2 = 0.503 | 0.478 |
| Chronic obstructive pulmonary disease | 3 | 4 | x 2 = 0.205 | 0.650 |
| Cancer | 1 | 0 | ||
| Others | 5 | 5 | x 2 = 0.004 | 0.951 |
| Infection site | ||||
| Respiratory tract | 21 | 20 | x 2 = 0.007 | 0.934 |
| Urinary tract | 2 | 3 | x 2 = 0.259 | 0.610 |
| Blood | 2 | 1 | x 2 = 0.316 | 0.574 |
| Digestive tract | 1 | 1 | x 2 = 0.001 | 0.980 |
| Skin and soft tissue | 1 | 2 | x 2 = 0.390 | 0.532 |
| Mixed infection | 2 | 1 | x 2 = 0.316 | 0.574 |
General treatment
Conventional treatment, according to the International Sepsis Treatment Guidelines in 2012 [13], was performed for patients in both groups after admission, including timely and proper anti-infective treatment, early goal-directed fluid resuscitation, rational use of vasoactive drugs to maintain mean arterial pressure > 65 mmHg (or average base arterial pressure level), aggressive treatment of underlying diseases, nutritional support, other symptomatic treatment, and mechanical ventilation therapy based on the patient’s condition.
RRT
RRT was immediately implemented in the fluid resuscitation of patients with AKI.
Equipment
Bedside blood purification machine (Germany GAMBRO Prismaflex Company); France M100 set blood filter (model prismaflex M100set), polysulfone membrane filter; and dialysis tubing (US BARD double-lumen tube, model 5553200). Temporary blood pathways were established with central venous (femoral vein, internal jugular vein) catheters using Seldinger technology.
Replacement fluid formulations
Saline, 3000 ml; 5% glucose, 500 ml; sterile water injection, 500 ml; 5% sodium bicarbonate, 250 ml; 25% magnesium sulfate, 3.2 ml; 10% potassium chloride, 10~15 ml (adjusted according to the patient conditions potassium input); the synchronization input 10% calcium chloride, 10-20 ml (central intravenous infusion).
Anticoagulation
Ordinary heparin 12500 IU plus saline 3000 ml pre-shoot filters and piping were used before treatment. In RRT, low molecular weight heparin was used for anticoagulant; the first dose was 60-80 IU/kg, followed by 1-10 IU/(kg/h) continuous infusion for maintenance. Patients with bleeding or bleeding tendencies were treated with local anticoagulation with free heparin or unfractionated heparin (before filter 1000-1666 u/h, protamine heparin for anticoagulant after filter, the ratio of protamine: unfractionated heparin was 1 mg: 100-150 u, adjusted the amount according to APTT.
Treatment parameters
The treatment mode was CVVHDF, blood flow velocity was 150-200 ml/min, dose was 35-40 ml/(kg/h), the temperature was set at 38°C, and the filters were promptly replaced when clotting or a transmembrane pressure of ≥ 400 mmHg appeared.
PICCO monitoring
Equipment
All patients underwent PICCO catheterization (internal jugular vein or subclavian vein and femoral artery pathways). PICCO catheters and systems (Germany Pulsion company), central venous catheter (Medical Technology Co., Ltd. Guangdong Lily), and Philips monitors (Germany Philips the company) were used.
Monitoring methods
The average results of 3 consecutive measurements of central venous catheter rapid injection (over 5 s) with 10 ml of 0-4°C 0.9% sodium chloride solution was obtained. Each error was less than 10%. EVLWI, PVPI, chest volume index (ITBVI), CI, and other parameters were recorded.
Measurement outcome
The patient’s general conditions such as gender, age, and APACHE-II score on admission were collected.
Considering the limits of PICCO placement time and increased pulmonary edema, fluid resuscitation often occurred within 72 h. This study recorded EVLWI, PVPI, ITBVI, CI values, blood lactate, transcutaneous pulse oxygen saturation (SPO2), arterial partial pressure of oxygen (PO2), and oxygenation index (PO2/FiO2) value after fluid resuscitation for patients with RRT before treatment, or 24 h, 48 h, and 72 h after.
The ICU stay and 28 d mortality of the 2 groups were analyzed.
Statistical methods
SPSS 19.0 software package was used. Measurement data were expressed as mean ± standard deviation (x̅ ± s). The self comparisons of the EVLWI, PVPI, ITBVI, CI, SPO2, PO2, PO2/FiO2, and blood lactate values before and after were carried out using Student’s t test. Independent sample t tests were used to compare the two groups during ICU stay. The comparisons of mortality count data was performed using χ2 test. Taking α = 0.05, the inspection standards were set to be P < 0.05 representing statistical significance.
Results
PICCO monitoring of patients in the two groups
EVLWI of 24 h, 48 h, and 72 h after RRT in the treatment group was lower than that before RRT (P < 0.01); EVLWI of 48 h and 72 h after RRT were lower than that of 24 h after RRT (P < 0.05 and P < 0.01, respectively); and EVLWI of 72 h after RRT was lower than that of 48 h after RRT (P < 0.01). In the control group, EVLWI of 24 h, 48 h, and 72 h after RRT were lower than that before EGDT treatment (P < 0.05, P < 0.01, and P < 0.01, respectively); EVLWI of 48 h and 72 h after RRT were lower than that of 24 h after EGDT (P < 0.01); and EVLWI of 72 h after RRT was less than that of RRT after 48 h (P < 0.01). In the comparisons of treatment group and control group, EVLWI before RRT and 24 h after treatment had no significant difference (P > 0.05), and the EVLWI of 48 h and 72 h after RRT treatment in the treatment group were significantly lower than the control group (P < 0.05, Table 2).
Table 2.
EVLWI, PVPI, ITBVI, CI values of the two groups
| Renal replacement time | EVLWI (ml/kg) | PVPI | ITBVI (ml/m2) | CI | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment group | Control group | Treatment group | Control group | Treatment group | Control group | Treatment group | Control group | |
| 0 h | 12.77±3.73 | 12.20±2.36 | 3.32±1.27 | 3.26±1.01 | 761.3±145.1 | 735.7±143.1 | 3.11±1.16 | 3.23±0.71 |
| 24 h | 10.30±2.55## | 11.06±2.38## | 2.76±0.89## | 2.80±0.52## | 938.7±197.8## | 927.6±134.0## | 3.39±1.09 | 3.43±0.67 |
| 48 h | 8.52±4.11*,## | 9.77±2.03## | 2.23±0.89## | 2.33±0.74## | 954.5±235.5# | 932.8±76.1# | 3.61±0.83# | 3.54±0.60 |
| 72 h | 6.64±1.9*,## | 7.10±1.53## | 1.91±0.96## | 2.03±0.93## | 944.1±97.3## | 921.5±58.2## | 3.73±0.73## | 3.58±0.52## |
Note: EVLWI: extravascular lung water index, WVLWI: PVPI: pulmonary vascular permeability index, ITBVI: intrathoracic blood volume index, CI: cardiac index. Compare with the control group;
P < 0.05;
Compared with before treatment;
P < 0.05;
P < 0.01.
PVPI of 24 h, 48 h, and 72 h after RRT in the treatment group was lower than that before RRT treatment (P < 0.01). PVPI of 48 h and 72 h after RRT were less than that of 24 h after RRT (P < 0.05 and P < 0.01, respectively). PVPI of 72 h after RRT treatment was lower than that of 48 h after RRT (P < 0.05). In the control group, PVPI of 24 h, 48 h, and 72 h after RRT were lower than that before RRT (P < 0.01). PVPI of 48 h and 72 h after RRT was less than that of 24 h after RRT (P < 0.05 and P < 0.01, respectively). PVPI of 72 h after RRT treatment was less than that of 48 h after RRT (P < 0.01). In the comparisons of treatment group and control group, PVPI before, and 24 h, 48 h, 72 h after RRT treatment had no significant difference (P > 0.05). ITBVI before RRT of the control group was lower than 24 h, 48 h, and 72 h after RRT treatment group (P < 0.01, P < 0.05, and P < 0.01, respectively). ITBVI of 24 h, 48 h, and 72 h after RRT treatment had no significant differences (P > 0.05). ITBVI before RTT in the control group were lower than that of 24 h, 48 h, and 72 h after RRT (P < 0.01). ITBVI of 24 h, 48 h, and 72 h after treatment had no significant differences (P > 0.05). In the comparisons before and 24 h, 48 h, and 72 h after RRT between the treatment group and the control group, there was no significant difference (P > 0.05).
CI of 24 h after RRT treatment was higher than that before RRT, but there was no significant difference between the two (P > 0.05). CI of 48 h and 72 h after RRT was significantly higher than before the RRT (P < 0.05 and P < 0.01, respectively). CI of 72 h after RRT was higher than that of 24 h and 48 h after RRT, but with no significant difference (P > 0.05). The CI of 24 h and 48 h after RRT in the control group was higher than before RRT with no significant difference (P > 0.05). CI of 72 h after RRT was significantly higher than that before RRT (P < 0.05). The comparisons of the CI in RRT treatment 24 h, 48 h, and 72 h after RRT between the two groups showed no statistically significant difference (P > 0.05). EVLWI trends before and 24 h, 48 h, 72 h after RRT in the two groups are shown in Figure 1.
Figure 1.
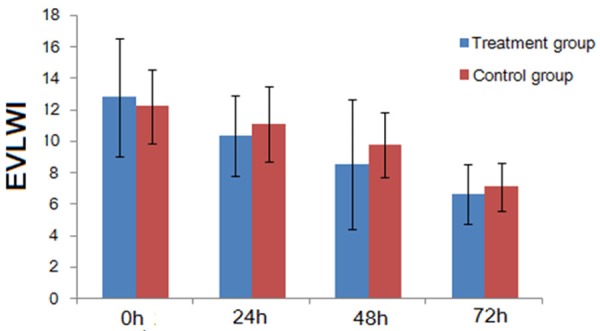
Comparison of the EVLWI between the two groups.
Hemodynamics and tissue perfusion of patients in the two groups
Blood lactate of 24 h, 48 h, and 72 h after RRT in the treatment group were significantly lower than that before treatment (P < 0.01). The blood lactate of before RRT, and 24 h, 48 h, and 72 h after of the treatment group had no significant difference compared with the control group (P > 0.05). Blood lactate of 24 h, 48 h, and 72 h after RRT in the control group were significantly lower than that before treatment (P < 0.01). Blood lactate of 48 h and 72 h after RRT were significantly lower than that of 24 h after RRT (P < 0.01). Blood lactate of 72 h after RRT was significantly lower than that of 48 h after RRT (P < 0.01). Blood lactate before, and 24 h, 48 h, and 72 h after RRT in the treatment group had no significant difference compared with the control group (P > 0.05, Table 3).
Table 3.
Tissue perfusion and oxygenation of patients in the two groups
| Renal replacement time | Blood lactate (mmol/L) | SPO2 (%) | PO2 (mmHg) | PO2/FiO2 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment group | Control group | Treatment group | Control group | Treatment group | Control group | Treatment group | Control group | |
| 0 h | 5.34±3.77 | 5.26±2.41 | 94.4±5.4 | 94.2±4.7 | 91.3±33.2 | 90.2±21.1 | 168.7±91.5 | 163.8±71.6 |
| 24 h | 2.98±2.48## | 3.15±1.22## | 93.5±5.8 | 93.2±2.2 | 81.5±28.1 | 73.8±15.0 | 152.0±78.7 | 140.5±74.7 |
| 48 h | 2.23±1.88## | 2.01±0.55## | 94.7±7.7 | 94.1±1.8 | 98.3±32.6# | 90.6±17.7## | 197.2±82.5 | 206.2±85.8 |
| 72 h | 1.96±1.90## | 1.42±0.46## | 95.3±8.0 | 95.4±1.6 | 102.5±26.3## | 95.6±20.3## | 246.3±108.2 | 251.4±98.2 |
Note: SPO2: transcutaneous pulse oxygen saturation, PO2: arterial partial pressure of oxygen, PO2/FiO2: oxygenation index. Compared with the control group, compared with before treatment;
P < 0.05;
P < 0.01.
After RRT treatment, SPO2 in the treatment group showed a gradual upward trend with the progress of time, but there was no significant difference before and after the treatment (P > 0.05). In the control group, SPO2 of 48 h after RRT was higher than that of 24 h after RRT (P < 0.01), and SPO2 of 72 h after RRT was higher than that of 48 h after RRT (P < 0.01). SPO2 before, and 24 h, 48 h, and 72 h after RRT had no significant difference compared with the control group (P > 0.05).
PO2 of 24 h after RRT in the treatment group was less than that of 0 h after RRT, the two groups were significantly different (P < 0.01). The PO2 of 48 h and 72 h after RRT treatment were significantly higher than that of 24 h after RRT, with a statistically significant difference (P < 0.05 and P < 0.01, respectively). In the control group, PO2 of 24 h after RRT showed a transient decrease, and there was a significant difference compared with that before RRT (P < 0.01). PO2 increased significantly 48 h and 72 h after RRT, with a significant difference, compared with that of 24 h after RRT (P < 0.01). PO2 before, and 24 h, 48 h, and 72 h after RRT in the treatment group had no significant difference compared with that of the control group (P > 0.05).
PO2/FiO2 of 24 h after RRT in the treatment group showed a transient decrease. PO2/FiO2 of 24 h after RRT was lower than that of 48 h and 72 h after RRT (P < 0.05 and P < 0.01, respectively). PO2/FiO2 of 72 h after RRT was higher than that of 0 h and 48 h after RRT (P < 0.01). In the control group, PO2/FiO2 of 72 h after RRT was higher than that before, and 24 h, and 48 h after RRT (P < 0.01). PO2/FiO2 of 48 h after RRT was higher than that of 24 h after RRT (P < 0.01). PO2/FiO2 before, and 24 h, 48 h, and 72 h after RRT had no significant difference in the comparison with the control group (P > 0.05).
ICU stay of patients in the two groups
ICU stay of patients in the treatment group was 7.10±2.06 days, compared with 8.52±2.94 days for the control group. There was a significant difference between the two groups (t = -2.110, P < 0.05).
Comparisons of extent and prognosis for critically ill patients
APACHE-II score of the treatment group on admission was 26.31±8.06, and 25.57±6.29 for the control group. There was no significant difference between the two groups (t = 0.38, P > 0.05). The 28d mortality of the treatment group was lower than that of the control group (27.59% vs 32.14%), but there were no significant difference (x 2 = 0.14, P > 0.05). The 28 d mortality for the treatment group was 27.59%-8 patients died. The mortality rate for the control group was 32.14% - 9 patients died.
Discussion
Sepsis was defined as over-expression of inflammatory mediators caused by infection and serious imbalance between the body’s pro-inflammatory and anti-inflammatory responses, and immune dysfunction [8,14,15]. It was believed that the occurrence of septic AKI was related with the following mechanisms: ① renal ischemia/reperfusion injury; ② peritubular capillary microcirculation; ③ endotoxin-induced inflammatory internal factors, such as tumor necrosis factor (TNF), interleukin (IL), platelet activating factor, leukotriene release, and inflammation cascade; and ④ the nitric oxide and nitric oxide synthase induction theory.
For a long time, it was considered that the incidence of AKI was primarily due to the reduction of renal blood flow caused by hemodynamic changes. Therefore, the treatment focused on enhancing cardiac output and perfusion pressure to improve renal blood flow. However, the infectious AKI animal model subverted this traditional concept. In septic shock, renal medullary and cortical blood flow was not reduced, but rather was increased. Septic AKI was different from non-septic AKI, where apoptosis in the former may play a more important role than necrosis [16]. In the septic AKI autopsy of patients by Lerolle et al. [17], it was found that apoptosis and leukocyte infiltration were the main pathogenic mechanisms.
Recent studies confirmed that RRT has roles in clearing sepsis-related inflammatory mediators (such as TNF, IL-1, IL-6 and IL-8), blocking the inflammatory cascade, preventing the progress of sepsis and other effects, as well as advantages such as a stable internal environment, improving hemodynamics, controlling the balance of capacity, and protection of organ function. RRT has shown some potential applications in the treatment of sepsis and has been identified as an effective mean of treatment [18]. Recent randomized trial showed RRT was the preferred treatment of septic AKI with unstable hemodynamics [19], which can significantly improve the prognosis of severe AKI [20]. However, randomized controlled trials also showed that CRRT had no more advantages than IHD [21,22]. In this study, the use of 35-40 ml/(kg/h) therapeutic dose was consistent with the literature [23,24].
Due to trauma, infection, shock, and other stress inflammation, patients with severe sepsis had vasodilation, capillary leak, and pulmonary alveolar-capillary barrier performance changes which caused lung water increase, adversely affecting lung capacity, respiratory mechanics and gas exchange. For patients with severe sepsis, resuscitation should be promptly performed to provide adequate and effective circulating blood volume, and to keep mean arterial pressure 60~65 mmHg. Low-dose dopamine was not used to prevent AKI, and loop diuretics were not used to increase urine output [25]. In the actual treatment of patients with severe sepsis, it was often found that in the fluid resuscitation for guaranteeing tissue perfusion, the incidence of pulmonary edema significantly increased.
How to perform full fluid resuscitation and avoid or reduce pulmonary edema were the difficulties of hemodynamic management in Critical Care Medicine. Traditional indicators such as CVP, because of the ease, were widely used for evaluating effective circulating blood volume to guide fluid therapy [11]. With the development of hemodynamic monitoring technology, due to vulnerable to various factors, accuracy decrease affected by factors such as positive pressure ventilation, pulmonary venous pressure and heart rate, the use of CVP for evaluating the effective circulating blood volume values were often questioned [26-28]. PICCO was a technology integrated thermodilution technique with arterial pulse contour analysis. Its capacity index was less affected by positive-pressure ventilation and heart rate. In addition to the continuous determination of the patient’s cardiac output, EVLWI has proven to be a sensitive indicator for evaluating the severity of pulmonary edema [29], which can effectively monitor the extent of pulmonary edema after early fluid resuscitation. At present, more than 2-fold EVLWI was generally believed to affect ventilation and diffusing capacity of the lung. Joint RRT helped controlling and timely giving adequate rehydration for patients, as well as reducing the incidence of lung injury.
Despite fluid resuscitation improving tissue perfusion in patients with severe sepsis, it was often accompanied by fluid retention in patients with early sepsis. For example, patients with AKI caused more prominent increase of lung water, affecting the alveolar-capillary gas exchange. Fluid management in RRT treatment was especially important. This study showed that although EVLWI of patients in both groups after RRT gradually decreased, the degree of decrease in EVLWI for 48 h and 72 h after CRRT in the treatment group was significantly greater than that of the control group, indicating that CRRT treated severe sepsis with AKI was better for decreasing lung water. The oxygenation index increase and full supply of tissue oxygen, cause by the reduction of lung water, lead to organ damage mitigation, avoiding or reducing the incidence of MODS, further reducing ICU stay and patient’s 28 d mortality. The results of the study showed that early fluid resuscitation using PICCO monitoring can timely detect pulmonary edema, providing guidance for fluid resuscitation and improving recovery speed. EVLWI can, not only evaluate the degree of pulmonary edema of septic AKI patients after early fluid resuscitation, but also provide a reference for septic fluid management and RRT time.
Patients with severe sepsis had vasodilation, capillary leak, and alveolar-capillary barrier effect change due to inflammatory mediator reactions, leading to increased permeability of the lungs. In this study, PICCO monitoring showed that PVPI increase was very significant in the early stages of sepsis, but PVPI of the treatment and control groups gradually decreased after RRT. On one hand, PVPI decline represented removing inflammatory mediators by RRT, while PVPI decline was also related to the release of inflammatory mediators due to effectively controlling infection. Although the PVPI of the treatment group decreased more quickly, there was no significant difference between the two groups. Therefore, clearing inflammatory mediators alone to extend RRT time for sepsis patients was not enough.
Before early EGDT and RRT of the patients in the two groups, effective blood volumes were significantly less. The ITBVI in both groups were lower than normal levels, and quickly returned to normal after fluid resuscitation. Therefore, ITBVI can effectively reflect the patient’s condition of fluid resuscitation. Whether CRRT or IRRT, ITBVI changes were not significantly different, indicating that adequate fluid resuscitation will quickly recover effective blood volume regardless of the RRT time.
It has been reported that early CI values of some patients with severe sepsis and septic shock were normal or even slightly elevated, which was consistent with highly ranked low resistance hemodynamic performance of septic shock patients. However, CI decreased as the disease progressed, indicating different degrees of myocardial depression [30]. The results were different from that reported in the literature. Earlier in severe sepsis, CI was lower, which may be associated with myocardial depression factor increase, the older age of patients in the group, cardiovascular disease, stress cardiomyopathy, or other factors. In this study, the values at each time point for RRT in the treatment for patients in the two groups showed no significant difference, indicating that the daily RRT treatment for 8 h and 24 h had little effect on cardiac output. It was worth noting that in the RRT process, blood temperature may have some influence on the PICCO determination, as blood flow and temperature changes during the RRT will affect the measurement of thermal dilution cardiac output. The study found that the measurement error mainly occurred before and after RRT treatment. Therefore cardiac output measurement did not depend on the RRT condition, and cardiac output measured by interrupting RRT was generally not recommended. If interrupted, the measurement needed to be performed after the blood temperature reached a steady state [31]. All patients in this study were measured when the blood temperature reached a steady state, thus avoiding errors generated in the measurements.
This study showed that blood lactate values decreased rapidly after fluid resuscitation and RRT. Blood lactate between the two groups showed no significant difference at the same time point, indicating that fluid resuscitation and tissue perfusion improvement were the main reasons for the decline of blood lactate, but were unrelated to the use of CRRT or IRRT mode.
SPO2, PO2, PO2/FiO2 of patients in the two groups after fluid resuscitation showed transient decrease trends, which may be related to the alveoli-blood fine vascular permeability of patients with sepsis, and the increase of lung water after fluid resuscitation. The treatment group returned to normal within 48 h, and the control group within 72 h. The results suggested that CRRT treatment of lung water clearance was better than IRRT, but the comparisons before, and 24 h, 48 h, and 72 h after RRT treatment of the two groups failed to show statistically significant difference. This may be related to the small number of samples in this study.
High mortality and prolonged ICU stay in patients with septic AKI has been reported [8]. In this study, patients in the treatment group had continuous RRT under EVLWI monitoring. ICU stay was significantly shorter, indicating that PICCO guided treatment can more quickly and effectively restore circulating blood volume, reduce tissue and organ ischemia and hypoxia time, and provide therapeutic time window for the treatment of primary disease. EVLWI monitoring can also avoid an increase of lung water in fluid resuscitation, thereby improving the prognosis of patients. The results also showed that the superiority of CRRT therapy to IRRT treatment may be due to its better effect of continuously clearing water and sustained inflammatory mediators. Lung water reduction of the patients quicker, thus ensuring the supply of oxygen to reduce the tissue and organ damage caused by lack of oxygen. Twenty-eight days mortality of the patients in the treatment group was lower than that of the control group (27.59% vs 32.14%), but there was no significant difference between the two groups, suggesting that daily RRT time was not a major cause of death in septic AKI patients.
In summary, the EVLWI had a clinical reference value for RRT time selection of sepsis patients with AKI. The sample size of the study was small, and the study subjects were older patients, which did not have a broad representation and underlying diseases of patients in this group are greater. A further stratified study was not performed due to the small number of cases, which may have some impact on the experiment.
Disclosure of conflict of interest
None.
References
- 1.Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma X, Ai Y, Xu Y, Liu D, An Y, Wu D, Sun R, Li S, Hu Z, Cao X, Zhou F, Jiang L, Lin J, Mao E, Qin T, He Z, Zhou L, Du B. Epidemiology and Outcome of Severe Sepsis and Septic Shock in Intensive Care Units in Mainland China. PLoS One. 2014;9:e107181. doi: 10.1371/journal.pone.0107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Atalan HK. Epidemiology of severe sepsis in the intensive care unit. Br J Hosp Med (Lond) 2008;69:442–443. doi: 10.12968/hmed.2008.69.8.30739. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez F, Barrera L, De La Rosa G, Dennis R, Dueñas C, Granados M, Londoño D, Molina F, Ortiz G, Jaimes F. The epidemiology of sepsis in Colombia: a prospective multicenter cohort study in ten university hospitals. Crit Care Med. 2011;39:1675–1682. doi: 10.1097/CCM.0b013e318218a35e. [DOI] [PubMed] [Google Scholar]
- 4.Medve L, Antek C, Paloczi B, Kocsi S, Gartner B, Marjanek Z, Bencsik G, Kanizsai P, Gondos T. Epidemiology of acute kidney injury in Hungarian intensive care units: a multicenter, prospective, observational study. BMC Nephrol. 2011;12:43. doi: 10.1186/1471-2369-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 6.Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care. 2010;14:R134. doi: 10.1186/cc9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 8.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi A, Kunisawa T, Kurosawa A, Sasakawa T, Ueno M, Takahata O, Iwasaki H. Utility of SVV (stroke volume variation) during abdominal aortic surgery. Masui. 2010;59:197–201. [PubMed] [Google Scholar]
- 10.Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, Della Rocca G, Grounds RM, Rhodes A. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78:527–533. [PubMed] [Google Scholar]
- 11.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 12.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares MO, Welton NJ, Harrison DA, Peura P, Shankar-Hari M, Harvey SE, Madan JJ, Ades AE, Palmer SJ, Rowan KM. An evaluation of the feasibility,cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta-analysis and value of information analysis. Health Technol Assess. 2012;16:1–186. doi: 10.3310/hta16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis DH, Chan DL, Pinheiro D, Armitage-Chan E, Garden OA. The immunopathology of sepsis: pathogen recognition, systemic inflammation, the compensatory anti-inflammatory response, and regulatory T cells. J Vet Intern Med. 2012;26:457–482. doi: 10.1111/j.1939-1676.2012.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs R, Honore PM, Joannes-Boyau O, Boer W, De Regt J, De Waele E, Collin V, Spapen HD. Septic acute kidney injury: the culprit is inflammatory apoptosis rather than ischemic necrosis. Blood Purif. 2011;32:262–265. doi: 10.1159/000330244. [DOI] [PubMed] [Google Scholar]
- 17.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced renal injury: apotosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 18.Wald R, Shariff SZ, Adhikari NK, Bagshaw SM, Burns KE, Friedrich JO, Garg AX, Harel Z, Kitchlu A, Ray JG. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study. Crit Care Med. 2014;42:868–877. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 19.Honore PM, Jacobs R, Joannes-Boyau O, De Regt J, Boer W, De Waele E, Collin V, Spapen HD. Septic AKI in ICU patients. diagnosis, pathophysiology, and treatment type, dosing, and timing: a comprehensive review of recent and future developments. Ann Intensive Care. 2011;1:32. doi: 10.1186/2110-5820-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;68:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 22.Lins RL, Elseviers MM, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24:512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 23.Joannes-Boyau O, Honoré PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Rozé H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39:1535–1546. doi: 10.1007/s00134-013-2967-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Yang Y, Lv R, Zhang Y, Xie W, Chen J. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant. 2012;27:967–973. doi: 10.1093/ndt/gfr486. [DOI] [PubMed] [Google Scholar]
- 25.Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, Ronco C, Schetz MR, Woittiez AJ. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology, ESICM. Intensive Care Med. 2010;36:392–411. doi: 10.1007/s00134-009-1678-y. [DOI] [PubMed] [Google Scholar]
- 26.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 29.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, McLaughlin B, Elborn JS, McAuley DF. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med. 2010;38:114–120. doi: 10.1097/CCM.0b013e3181b43050. [DOI] [PubMed] [Google Scholar]
- 30.Repessé X, Charron C, Vieillard-Baron A. Evaluation of left ventricular systolic function revisited in septic shock. Crit Care. 2013;17:164. doi: 10.1186/cc12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heise D, Faulstich M, Mörer O, Bräuer A, Quintel M. Influence of continuous renal replacement therapy on cardiac output measurement using thermodilution techniques. Minerva Anestesiol. 2012;78:315–321. [PubMed] [Google Scholar]


