Abstract
As a kind of autoimmune encephalitis which was just identified, the clinical manifestations of the anti-N methyl-D aspartate (anti-NMDA) receptor encephalitis are complex, diverse and in severe condition. The immunotherapy has shown good effect on the treatment but in generally, the diagnosis and treatment are still in the experience accumulation stage. More clinical research in different population is necessary, for example, in the Chinese population. This study was completed in anti-NMDA receptor encephalitis patients who were diagnosed in Beijing Xuan Wu Hospital (China) during the time from 2011 to 2013. Total 33 patients were involved with the average age of 29.7 years old when the diseases were onset. With diverse clinical manifestations, most patients displayed positively by NMDAR antibody test and 63.6% of them were associated with elevated CSF-lgA. Patients also showed abnormal MRI and EEG. Only three patients had teratomas. With hormone therapy, gamma globulin treatment or plasma exchange, more than three quarters of patients fully recovered and the others had moderate symptoms. Based on our results, we suggest that NMDAR antibody test would be helpful to make a timely diagnosis and to administer immunotherapy.
Keywords: Anti-NMDAR, Chinese population, clinical manifestations, treatment
Introduction
Autoantibody from serum and cerebrospinal fluid was distinguished in young female patients who had memory disorders, psychiatric symptoms, consciousness loss, respiratory disorders and benign teratoma [1]. This autoantibody was recognized to be involved in anti-N-methyl-D-aspartate receptor (anti-NMDA) encephalitis. It is believed that this encephalitis is a type of paraneoplastic encephalitis in which neuropsychiatric symptoms arise from cytotoxic effects caused by an autoimmune reaction primarily against the NR1/NR2. The anti-NMDAR encephalitis are potentially lethal, but the clinical manifestations are complex and diverse [1-4]. For example, it can be autoimmune without malignancy or paraneoplastic. Significantly, an early diagnosis will ensure timely immunotherapy and/or tumor resection [2,5,6].
Epidemiological studies have shown that anti-NMDA receptor encephalitis is the second most common immune-mediated type of encephalitis after acute disseminated encephalomyelitis [7]. However, due to the complex and diverse of the clinical manifestations, the diagnosis and treatment are still different. In China, the NMDA receptor antibody detection was not conducted until 2011 [8,9]. This study aims to analyze the clinical data of Chinese anti-NMDAR encephalitis patients who were diagnosed and hospitalized in Beijing Xuan Wu hospital during the time from 2011 to 2013. We summarized the clinical features and prognosis of Chinese anti-NMDA receptor encephalitis. This study will contribute to the understanding of this novel encephalitis in Chinese population.
Methods
Patients
The study was approved by Ethics Committee from Beijing Xuan Wu Hospital of Capital Medical University. We identified and reviewed inpatients in the Neurology Department of Beijing Xuan Wu Hospital who were diagnosed with anti-NMDAR encephalitis during the period from January 2011 to December 2013. All patients were positive for anti-NMDAR encephalitis by testing NMDAR antibodies from cerebrospinal fluid. The clinical manifestations were divided into eight groups: mental and behavioral abnormalities, cognitive disorders, language disorders, epilepsy, movement disorders, disturbance of consciousness, autonomic dysfunction, central hypoventilation, and sleep disorders. The modified Rankin scale (mRS) was used to estimate the neurological status: mRS = 0 corresponding to fully restoration; mRS = 1-2 corresponding to significant improvement; mRS > 2 corresponding to partial improvement. First-line treatment was used alone or in combination with hormones, intravenous immunoglobulin, and/or plasma exchange. Second-line treatment was used alone or in combination with cyclophosphamide or azathioprine. Demographics, clinical manifestations, laboratory tests, imaging and EEG findings, treatment response and prognosis were analyzed. Follow-ups were also conducted.
Laboratory tests
In serologic tests, TSH levels, T3 levels, T4 levels, anti-TPO antibody and anti-TG antibody were evaluated using an electro-chemiluminescence immunoassay (UniCel DX1800, Beckman Coulter). In CSF tests, protein levels, IgG, IgA, IgM, IgG synthesis rate were completed by immunoturbidimetric assays and immunofixation electrophoresis. Myelin basic protein levels (MBP) were detected by ELISA. AMP, Ma2, Ri, Yo and Hu were evaluated by protein immunoblot. The measurements of sera anti-NMDAR (IgG) and CSF anti-NMDAR (IgG) were carried out using the immunohistochemistry method. Sera tumor markers (carcino-embryonic antigen (CEA), Carbohydrate antigen 125, 153, 724, 199 (CA125, CA153, CA724, CA199), Cytokeratins 19 fragments (Cyfra21-1), neuron-specific enolase (NSE), Total Prostate Specific Antigen (tPSA), and free prostate specific antigen (fPSA) were examined by electro-chemiluminescence immunoassay (UniCel DX1800, Beckman Coulter). EEG examination was completed by the Da Vinci cerebral video EEG monitoring system (Micromed, ITALY).
MRI examination
Regular MRI series including axial T2 weighted image (T2W1), T1 weighted image (T1W1), fluid attenuated inversion recovery image (FLAIR), and diffusion weighted image (DWI) were conducted. All images were acquired using Siemens Trio Tim 3.0 T scanner (12-channel coil, 45 T/m). Scan sequences included: T2W1: TR/TE = 3830/98 ms, FOV: 230×218 mm, matrix 179×218; TlWI: TR/TE = 155/2.81ms, FOV 230×186 mm, matrix 156×320; FLAIR: TR/TE = 8500/87 ms, FOV: 230×201, matrix 224×256; DWI: TR/TE = 3000/9I ms, FOV: 240×240, matrix = 160×160, b value (0.500, 1000 s/mm2). All above parameters were completed at the same location, thickness and space (z = 5.0 mm, space = 1 mm).
Antiretroviral therapy was mainly used at the initial phase of hospitalization in combination with hormones (methylprednisolone, prednisone, and dexamethasone. Intravenous gamma globulin and/or plasma exchange therapy was used as first-line immunotherapy.
Results
Demographic and clinical data
This study included a total of 33 patients (21 females, 12 males) who had been diagnosed with anti-NMDA receptor encephalitis (age of onset: 14-60 with average of 29.7 year-old). The average interval from appearing the clinical symptoms to confirmation as anti-NMDAR encephalitis was 70.1 days (11-549 days). Initially all the 33 patients were diagnosed with other diseases including viral encephalitis (16 cases, 48.5%), mental disorders (7 cases, 21.2%), respiratory infections (4 cases, 12.1%), epilepsy (1 case, 3%), seizures caused by low calcium level (1 case, 3%), depression (1 case, 3%), ischemic cerebrovascular disease (1 case, 3%), myelin disease (1 case, 3%), and peripheral neuropathy (1 case, 3%) (Figure 1). The prodromata included fever (23 cases, 67.9%), non-specific flu-like symptoms (9 cases, 27.3%), and headache (13 cases, 39.4%). In the subsequent course of the disease, the most common clinical symptoms included mental and behavioral abnormalities (31 cases, 93.9%), visual or auditory hallucinations (17 cases, 51.5%), epilepsy (25 cases, 75.8%), movement disorders (21 cases, 63.6%), disturbances of consciousness (17 cases, 51.5%), cognitive disorders (20 cases, 60.6%), and sleep disorders (12 cases, 36.4%). The other symptoms included autonomic dysfunction (11 cases, 33.3%, including sweating, too slow or too fast heart rate, repeated fever), language disorders (8 cases, 24.2%), hypoventilation (6 cases, 18.2%), and paresthesia (1 case, 3%).
Figure 1.
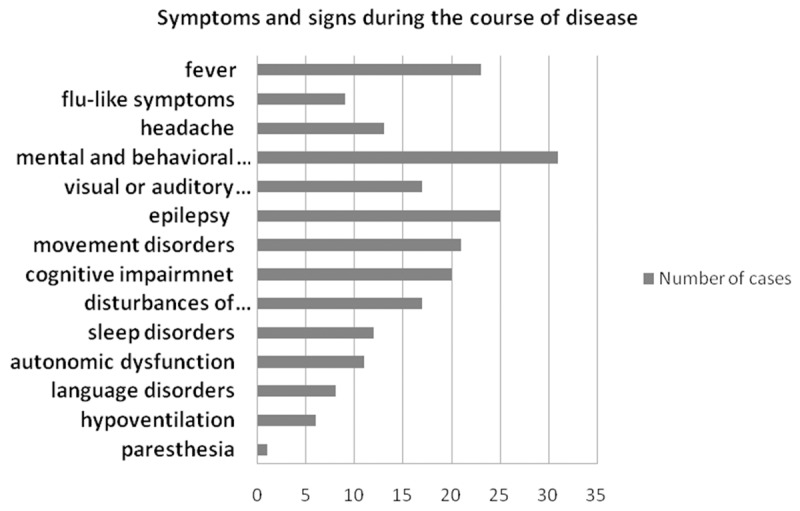
Symptoms and signs of 33 patients with Anti-NMDA receptor encephalitis.
Serologic and CSF studies
In our study, there were 14 patients (42.4%) with increased lumbar puncture pressure, 10 (34.8%) with increased CSF protein levels (average 73 mg/dl, range 46~121 mg/dl, normal 15~45 mg/dl); 23 (69.7%) with increased IgA (average 0.78 g/l, the range of 0.21~3.83 g/l, normal 0~0.2 g/l); 11 (33.3%) with elevated IgG (average 17.9 g/l, range 5.91~112 g/l, normal 0.48~5.85 g/l); 17 (51.5%) had leukocyte count within the normal range (<10×106/L) and remained 16 (48.5%) had increased (10~255/µl), primary lymphocytes. 18 patients (54.5%) were positive in anti-NMDA receptor from both the CSF and the serum, while 15 patients (45.5%) were positive in serum anti-NMDA receptor but positive in CSF anti-NMDA receptor. There was no abnormal serum tumor marker in 24 patients while elevated serum tumor marker was found in 9 cases of patients. The antibody detection of Hu, Yu, and Ri as paraneoplastic indicators was all negative (Table 2).
Table 2.
Laboratory findings of 33 patients with Anti-NMDAR encephalitis
| Test | Number (%) of patients/total number of patients tested |
|---|---|
| Serologic findings | |
| TSH levels elevated | 3 (9.1)/33 |
| T3 levels elevated | 0/33 |
| T4 levels elevated | 7 (21.2)/33 |
| Anti-TPO antibody elevated | 10 (30.3)/33 |
| Anti-TG antibody elevated | 7 (21.2)/33 |
| CSF findings | |
| WBC | 16 (48.5)/33 |
| Protein levels elevated | 10 (30.3)/33 |
| IgG | 11 (33.3)/33 |
| IgA | 23 (69.7)/33 |
| IgM | 6 (18.2)/33 |
| Oligoclonal bands positive | 9 (27.3)/33 |
| Myelin basic protein levels elevated | 6 (18.2)/33 |
| IgG synthesis rate elevated | 6 (18.2)/33 |
| Serum Anti-NMDAR (IgG) | 18 (54.5)/33 |
| CSF Anti-NMDAR (IgG) | 33 (100)/33 |
| AMP | 0/33 |
| Ma2 | 0/33 |
| Ri | 0/33 |
| Yo | 0/33 |
| Hu | 0/33 |
Notes: Abbreviations: TPO, thyroid peroxidase; TG, thyroglobulin; WBC, white blood cell.
Electroencephalogram (EEG)
All patients completed EEG examinations (Table 1). The first round examination showed that the signals in 14 patients (39.1%) were normal or slightly abnormal, 18 patients (54.5%) were moderate or more severe abnormal; in 15 patients (45.5%) were primarily diffuse or localized in medium-high amplitude slow waves, without typical epileptiform discharges. The EEG in 3 patients (9.1%) had paroxysmal discharge. The second exam in the course showed abnormal EEG with still primarily diffuse or localized medium-high amplitude slow waves in 15 patients (45.5%), 5 of which (15.2%) had epileptiform discharges.
Table 1.
Serologic, CSF, MRI and EEG
| No. | CSF | MRI T2/FLAIR | EEG abnormalities | |
|---|---|---|---|---|
|
| ||||
| WBC/µL | Protein mg/dL | |||
| 1 | 1 | 42 | + | Edge state |
| 2* | 255 | 70 | bilateral media temporal lobe, hippocampus | Severe |
| 3* | 2 | 20 | left hippocampal atrophy | |
| 4* | 0 | 32 | + | + |
| 5* | 68 | 82 | + | Mildly |
| 6* | 18 | 45 | left medial temporal lobe, hippocampus, thalamus, PLIC, midbrain cerenral pedumcle | + |
| 7* | 158 | 98 | left temporal lobes | Mildly |
| 8 | 3 | 15 | + | Mildly Moderately |
| 9 | 22 | 15 | right hippocampus, Hyperintensity of left rear lateral ventricles, no enhancement | |
| 10 | 15 | 39 | right thalamus | Moderately |
| 11* | 1 | 31 | + | Moderately |
| 12* | 1 | 32 | The left medulla oblongate, pons, cerebral peduncle | Moderately |
| 13* | 8 | 13 | + | Epileptiform |
| 14 | 20 | 65 | thickened bilateral cortical between the cortex and medulla of frontal lobes | Mildly |
| 15 | 0 | 5 | + | |
| 16 | 0 | 65 | right temporal lobe-hippocampus-corpus callosum | Mildly |
| 17 | 100 | 18 | cingulated and corpus callosum | Moderately |
| 18 | 6 | 55 | bilateral hippocampus | Moderately |
| 19 | 14 | 15 | + | Moderately |
| 20 | 4 | 46 | + | Moderately |
| 21 | 8 | 22 | + | Moderately |
| 22 | 14 | 43 | + | Moderately |
| 23* | 0 | 31 | left temporal cortex, bilateral frontal-temporal areas | Moderately |
| 24* | 32 | 10 | bilateral insula and frontal lobes | Moderately |
| 25 | 6 | 15 | + | |
| 26 | 10 | 22 | Punctate abnormal signal in right | Mildly |
| 27* | 13 | 76 | bilateral frontal-parietal lobes | Mildly |
| 28 | 5 | 52 | bilateral temporo-occipital lobes | Moderately |
| 29 | 47 | 124 | bilateral temporal and insular lobes | Moderately. Mild dysrhythmia |
| 30 | 15 | 39 | right thalamus | + |
| 31* | 4 | 14 | + | N/A |
| 32* | 12 | 33 | bilateral thalamus | Moderately |
| 33 | 2 | 15 | bilateral cingulated cortex | + |
means negative of IgG NMDAR-Antibodies test in Serum before treatment.
+ means normal (in MRI and/or EEG).
Magnetic resonance imaging (MRI)
All patients underwent brain MRI examination. 13 patients (39.4%) were normal while 20 patients showed T2 or FLAIR high signal (almost no change in strength) in different anatomical structures primarily involving in behavioral and mental impairments, cognitive impairment and seizures: frontal (3/33, 9% patients), temporal (8/33, 24.2%), occipital lobes (1/33, 3%), hippocampus (6/33, 18%), insula (2/33, 6%), corpus callosum (2/33, 6%), cingulated gyrus (2/33, 6%), periventricular (2/33, 6%), post internal capsule (1/33, 3%), thalamus (4/33, 12.1%), cerebral peduncle (2/33, 6%), pons (1/33, 3%), and medulla oblongata (1/33, 3%). Among them, 14 patients involved two or more parts of above structures and 6 patients involved in one part alone. Narrowed abnormal signal were shown in 11 patients and ipsilateral hippocampal atrophy in 3 patients. In addition, the 3rd case underwent a PET examination and showed increased diffuse signal in the left cerebral cortex, basal ganglia, thalamus and right cerebellum.
Treatment and follow-up
The average hospitalization time of the 33 patients was 36 days (in a range of 3-128 days). All patients were followed up by telephone or appointment. The average follow-up time was 7.8 months (in a range of 0.5-22 months). Of the patients, 24 patients (72.7%) were fully recovered, and 9 patients (27.3%) kept mild symptoms. In addition to anti-epileptic and supportive treatment, 20 patients received antiretroviral therapy at the initial phase of hospitalization. All patients except two received first-line immunotherapy of hormones, gamma globulin or plasma exchange, 3 of whom received the combination of hormones, intravenous gamma globulin and plasma exchange therapy, and their conditions were gradually stabilized and the psychiatric symptoms were relieved; 11 of whom received the combination of hormone and gamma globulin that their mental symptoms were relieved; 15 of whom also got improvement after the use of hormone therapy alone. The third case also received second-line immunosuppressant azathioprine to stop seizures and eliminate hallucinations except for restlessness. The 7th case showed positive for herpes simplex virus by a PCR test. Thus, this patient received only antivirus anti-epileptic treatment without the first-line treatment which resulted in improved psychiatric symptoms but remained mild loss in memory abilities. Only 3 in 21 female patients had concomitant ovarian teratoma. One had resection before the onset of the disease; the other two had resection after being diagnosed as anti-NMDA receptor encephalitis. The CSF NMDA antibody titers decreased in 14 cases during the treatment (Table 3).
Table 3.
Treatments and follow-up
| No. | yr/Sex | Dur. | Treatment | IgG NMDAR-Ab | Follow-up period (months) | Clinical course | mRS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Hormones | Immunoglob | Plasmaph | Azathioprine | Anti-epilep | Acyclovir | Anticonv | Ventilation | Teratoma res | Serum | CSF | ||||||||
|
| ||||||||||||||||||
| B | A | B | A | |||||||||||||||
| 1 | 25/F | 2 m | X | X | X | X | X | X | +++ | + | +++ | ++ | 0.5 | G | 4 | |||
| 2* | 29/F | 9 d | X | X | X | - | ++ | 2 | G | 3 | ||||||||
| 3 | 20/F | 1 y | X | X | X | X | - | ++ | 6 | G | 3 | |||||||
| 4 | 14/M | 1 m | X | X | X | X | - | ++ | 15 | F | 0 | |||||||
| 5 | 50/F | 15 d | X | X | X | - | ++ | 5 | F | 0 | ||||||||
| 6 | 18/F | 7 d | X | - | ++ | 6 | F | 0 | ||||||||||
| 7 | 43/M | 1 m | X | - | + | 10 | F | 0 | ||||||||||
| 8 | 19/M | 18 d | X | X | X | + | ++ | 11 | F | 0 | ||||||||
| 9 | 14/M | 2 m | X | X | X | + | - | ++ | + | 5 | P | 2 | ||||||
| 10 | 60/F | 43 d | X | X | ++ | +++ | 7 | F | 0 | |||||||||
| 11 | 17/F | 2 m | X | X | - | ++ | 21 | F | 0 | |||||||||
| 12 | 24/M | 5 d | X | X | - | ++ | 17 | F | 0 | |||||||||
| 13 | 25/F | 7 m | X | X | X | X | - | ++ | 7 | P | 3 | |||||||
| 14 | 33/F | 7 d | X | X | X | + | - | ++ | + | 13 | F | 0 | ||||||
| 15 | 33/F | 1 m | X | X | X | + | ++ | 16 | F | 0 | ||||||||
| 16 | 25/M | 40 d | X | X | + | ++ | 15 | F | 0 | |||||||||
| 17 | 16/F | 1 m | X | X | X | + | ++ | 12 | F | 0 | ||||||||
| 18 | 31/F | 6 d | X | X | X | X | X | X | + | - | ++ | - | 11 | F | 0 | |||
| 19* | 34/F | 20 d | X | X | X | X | X | + | - | +++ | + | 3 | F | 0 | ||||
| 20 | 19/F | >1 m | X | X | X | X | + | ++ | 10 | F | 0 | |||||||
| 21* | 23/F | 10 d | X | X | X | X | X | X | X | + | - | +++ | + | 12 | G | 3 | ||
| 22 | 22/F | 15 d | X | X | X | X | X | ++ | ++ | 6 | F | 0 | ||||||
| 23 | 35/F | 11 d | X | X | X | X | - | - | ++ | + | 2 | F | 0 | |||||
| 24 | 31/M | 39 d | X | X | X | - | - | ++ | ++ | 10 | G, P | 2 | ||||||
| 25 | 25/F | 11 d | X | X | X | X | ++ | ++ | 4 | P | 3 | |||||||
| 26 | 27/F | 36 d | X | X | X | ++ | ++ | 4 | P | 2 | ||||||||
| 27 | 32/M | 1.5 y | X | - | ++ | 3 | F | 0 | ||||||||||
| 28 | 17/F | 40 d | X | X | +++ | ++ | +++ | +++ | 3 | P | 3 | |||||||
| 29 | 57/F | 36 d | X | X | + | ++ | 6 | P | 3 | |||||||||
| 30 | 60/F | 43 d | X | X | X | ++ | +++ | 8 | F | 0 | ||||||||
| 31 | 39/F | 16 d | X | X | - | + | 11 | P | 1 | |||||||||
| 32 | 48/M | 20 d | X | - | ++ | 0.5 | P | 3 | ||||||||||
| 33 | 15/M | 2 m | X | X | X | + | +++ | 2 | F | 0 | ||||||||
Notes: NMDAR antibodies +++ strong positive, ++ positive, + weak positive, - negative, immunoglob = immunoglob; plasmaph = plasmapheresis; anti-epilep = anti-epileptic; anticonv = anticonvulsant; teratoma res = teratoma resection; X means the treatment was used on the patient. NMDAR-Ab = NMDAR antibodies; B = before treatment; A = after treatment; F = fully restored; G = gradual recovery; P = part of improvement; mRS = the modified Rankin scale;
denotes the patient who had concomitant ovarian teratoma.
Discussion
The clinical manifestations of anti-NMDA receptor encephalitis varied in different races and areas. There was only few reports of anti-NMDA receptor encephalitis in China [8,9] and there was low proportion of concomitant tumor detected [10]. This study retrospectively analyzed the clinical data, laboratory, EEG, imaging characteristics and prognosis of 33 Chinese cases with anti-NMDA receptor encephalitis in order to explore the characteristics of the NMDA receptor encephalitis. Overall, there were some clinical features of anti-NMDAR encephalitis that were different from the other types of encephalitis. In our study, anti-NMDAR encephalitis were found in population of all ages, gender, although a higher incidence in young women, which is consistent with previous literatures [2,3,11,12]. Due to complex clinical symptoms, a large part of patients was initially diagnosed with other diseases, including viral encephalitis, schizophrenia, upper respiratory tract infection, etc. The main clinical manifestations in patients with anti-NMDAR encephalitis included fever, non-specific flu-like symptoms, and headache as prodromal symptoms, abnormalities, seizures, consciousness loss, and motor dysfunction. The other less common symptoms included cognitive disorders, sleep disorders, autonomic dysfunction, central hypoventilation, etc.
Large part of patients (66.7%) showed prodromal symptoms of fever, headache, etc. in early onset, which was similar to viral infections. According to the research, viral infections like prodromal symptoms might neglect a part of the early immune response activation [13], or results as part of the non-specific infection pushing through the blood-brain barrier by means of immune response [14]. After the prodromal period, almost all patients had significant diverse and recurrent psychiatric symptoms, of which the most prominent symptoms were psychomotor excitement and hallucinations [15-18]. Seven patients in our study had been misdiagnosed initially. Seizure was another prominent anti-NMDAR encephalitis symptom [1,2,19]. In our study, 25 patients (75.8%) showed generalized or partial seizures, which occurred at any stage of the disease without significant efficacy when using a variety of antiepileptic drug. Additionally, abnormal movement disorders were characterized in patients with anti-NMDAR encephalitis [20,21]. In our study, 21 patients had motor dysfunction, mainly in the mouth, face, trunk or limbs, and to a lesser extent, nystagmus, dystonia and ataxia when made a lateral view. The motor dysfunctions, especially involuntary movements, are recurrent, and often overlap with seizures that can easily cause overlook of existing seizures or excessive use of anti-epileptic drugs for the treatment of involuntary movements [4,20]. Cognitive dysfunctions are shown in patients with anti-NMDAR encephalitis, and they still persist over an extended period after the acute phase of recovery, which is a main long-term sequela of anti-NMDAR encephalitis patients. The cognitive functions could recover if early treatment is given [2,22,23]. In our group there were 60.6% patients who had memory disorders showing varied degrees of improvement after treatment. Some patients still presented with mild cognitive decline at their time of discharge. On the one hand, this might be due to the lack of knowledge of the disease. On the other hand, the assessment of memory might be disrupted and the treatment might be delayed due to lack of precise information from patients with psychiatric symptoms and language barriers. In addition to the aforementioned symptoms, there were some cases of serious conditions, for example, significant autonomic dysfunctions, impaired consciousness or even hypoventilation associated with respiratory failure that required mechanical ventilation and intensive care, thus prolonging the hospitalization [24]. In addition, the rate of positivity of anti NMDAR Ab was higher in the CSF as compared to the serum, especially when 32/33 patients presented with fever and flu-like illness prior to their clinical presentation.
Although the EEG examination on patients revealed no consistent results, the EEG signal were primarily diffuse or localized medium-high amplitude slow waves, with untypical epileptiform discharges [19,25]. Strengthening long-term video-EEG dynamically monitoring can contribute to making the diagnosis and determining the appropriate treatment for NMDAR encephalitis [26].
The MRI tests revealed no specific results, either. It could be completely normal or high signals changed in frontal lobes, temporal lobes and hippocampus, while lesions were not limited to the limbic system and were not necessarily closely related to the clinical manifestations [2,27]. The patients in our group had high ratio of abnormal MRI results in that 54.5% of the patients had abnormal signals mainly in the frontal lobes, temporal lobes, hippocampus, cingulate, and involved different parts of the thalamus, cerebral peduncle, pons and medulla etc in midbrain. The ranges of abnormal MRI signals were narrow in the follow-up scan after treatment, however part of the patients showed atrophy of the hippocampus. This cannot be critical but might be a supportive method for treatment since it is consistent with the case of cognitive impairment that when the condition improved, the corresponding images were gradually restored, while when the disease was persistent, the corresponding images showed brain atrophy.
Routine examination of cerebrospinal fluid of patients in our group revealed a slightly increased number of lymphocytes and CSF protein levels, and elevated immunoglobulin lgA. This might suggest a process of immune and inflammatory caused by this disease [4,11]. The final diagnosis of anti-NMDAR encephalitis depends on the anti-NMDAR antibodies detected in serum and CSF, which is high specificity. All 33 patients were NMDAR antibody positive in CSF, only 18 of which also had antibody positive in their serum. The serum titer was significantly lower than the corresponding CSF titer. The diagnosis of the disease, however, is not only dependent on the detection of NMDAR antibodies although it contributes to the development of immune therapy and prognosis [2,5].
The patients received supportive treatments for psychotic symptoms and epilepsy, as well as first-line immune therapies including corticosteroids and immunoglobulins, of which four patients in severe condition received plasma exchange, and 3 patients with combined teratoma underwent surgical resection. These experiences were in line with the results of the literature: the effect of combination therapy is better than that of monotherapy [6]. This also prompts us that it is very important to have an early and aggressive immunotherapy, while the choice and the dosage of immunotherapy still need further exploration.
According to the literature, females who had anti-NMDAR encephalitis often had ovarian teratoma, a disease that can significantly improve after tumor resection [2,5,6]. However, in our group, only 3 patients had ovarian teratoma out of the 21 female patients, of which one patient suffered from anti-NMDAR encephalitis after surgery of cervical cancer, and there were no combined tumor in male patients. Whether there is lower proportion of concomitant tumor in anti-NMDAR encephalitis patients in China, still needs to be further explored with expanded samples.
There are still some limitations to our study. This study is a retrospective analysis with a somewhat small size. In the future, studies will be conducted in multi-centers with a larger sample to explore in-depth population incidence of Chinese anti-NMDA receptor encephalitis, treatment strategies, and the follow-up observations.
Conclusions
This study summarized the clinical manifestations, laboratory, imaging, EEG characteristics and prognoses of Chinese patients with anti-NMDA receptor encephalitis in 33 patients. Most patients have a better response to frontline immunotherapy such as hormone, globulin or plasma exchange. We suggest that the diagnosis of anti-NMDA receptor encephalitis should be considered, i.e., perform NMDAR antibody testing and do tumor follow-up investigation.
Acknowledgements
This report was supported by National Key Department of Neurology funded by Chinese Health and Family Planning Committee; National Science and Technology Major Project (sub) Chinese Clinical Repository establishment and application of neuropsychiatric disorders (No. 2011ZX09307001-003); The National Science Foundation of China (No. H0906); Beijing 215 Talent Project of Academic Leaders.
Disclosure of conflict of interest
None.
References
- 1.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery HE, Day GS, Dunn S, Fritzler MJ, Pruss H, De Souza C, Doja A, Mossman K, Resch L, Xia C, Sakic B, Belbeck L, Foster WG. Anti-NMDA receptor encephalitis. The disorder, the diagnosis and the immunobiology. Autoimmun Rev. 2012;11:863–872. doi: 10.1016/j.autrev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, Dalmau J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MP, Irani SR, Vincent A, Brown DW, Crowcroft NS UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 8.Xu CL, Liu L, Zhao WQ, Li JM, Wang RJ, Wang SH, Wang DX, Liu MY, Qiao SS, Wang JW. Anti-N-methyl-D-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol. 2011;11:149. doi: 10.1186/1471-2377-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song L, Liu AH. [Anti-N-methyl-D-aspartic acid receptor encephalitis: clinical analysis of 7 cases] . Zhonghua Yi Xue Za Zhi. 2013;93:2508–2510. [PubMed] [Google Scholar]
- 10.Guan W, Fu Z, Zhang H, Jing L, Lu J, Zhang J, Lu H, Teng J, Jia Y. Non-tumor-Associated Anti-N-Methyl-d-Aspartate (NMDA) Receptor Encephalitis in Chinese Girls With Positive Anti-thyroid Antibodies. J Child Neurol. 2015;30:1582–5. doi: 10.1177/0883073815575365. [DOI] [PubMed] [Google Scholar]
- 11.Luca N, Daengsuwan T, Dalmau J, Jones K, deVeber G, Kobayashi J, Laxer RM, Benseler SM. Anti-N-methyl-D-aspartate receptor encephalitis: a newly recognized inflammatory brain disease in children. Arthritis Rheum. 2011;63:2516–2522. doi: 10.1002/art.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day GS, High SM, Cot B, Tang-Wai DF. Anti-NMDA-receptor encephalitis: case report and literature review of an under-recognized condition. J Gen Intern Med. 2011;26:811–816. doi: 10.1007/s11606-011-1641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairweather D, Frisancho-Kiss S, Rose NR. Viruses as adjuvants for autoimmunity: evidence from Coxsackievirus-induced myocarditis. Rev Med Virol. 2005;15:17–27. doi: 10.1002/rmv.445. [DOI] [PubMed] [Google Scholar]
- 14.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci U S A. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka T, Sakai F. [Anti-nMDA receptor encephalitis--clinical manifestations and pathophysiology] . Brain Nerve. 2008;60:1047–1060. [PubMed] [Google Scholar]
- 16.Nasky KM, Knittel DR, Manos GH. Psychosis associated with anti-N-methyl-D-aspartate receptor antibodies. CNS Spectr. 2008;13:699–703. doi: 10.1017/s109285290001378x. [DOI] [PubMed] [Google Scholar]
- 17.Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2011;231:86–91. doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Barry H, Hardiman O, Healy DG, Keogan M, Moroney J, Molnar PP, Cotter DR, Murphy KC. Anti-NMDA receptor encephalitis: an important differential diagnosis in psychosis. Br J Psychiatry. 2011;199:508–509. doi: 10.1192/bjp.bp.111.092197. [DOI] [PubMed] [Google Scholar]
- 19.Bayreuther C, Bourg V, Dellamonica J, Borg M, Bernardin G, Thomas P. Complex partial status epilepticus revealing anti-NMDA receptor encephalitis. Epileptic Disord. 2009;11:261–265. doi: 10.1684/epd.2009.0266. [DOI] [PubMed] [Google Scholar]
- 20.Ferioli S, Dalmau J, Kobet CA, Zhai QJ, Broderick JP, Espay AJ. Anti-N-methyl-D-aspartate receptor encephalitis: characteristic behavioral and movement disorder. Arch Neurol. 2010;67:250–251. doi: 10.1001/archneurol.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamelou M, Plazzi G, Lugaresi E, Edwards MJ, Bhatia KP. The distinct movement disorder in anti-NMDA receptor encephalitis may be related to Status Dissociatus: a hypothesis. Mov Disord. 2012;27:1360–1363. doi: 10.1002/mds.25072. [DOI] [PubMed] [Google Scholar]
- 22.Poloni C, Korff CM, Ricotti V, King MD, Perez ER, Mayor-Dubois C, Haenggeli CA, Deonna T. Severe childhood encephalopathy with dyskinesia and prolonged cognitive disturbances: evidence for anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. 2010;52:e78–82. doi: 10.1111/j.1469-8749.2009.03542.x. [DOI] [PubMed] [Google Scholar]
- 23.Finke C, Kopp UA, Pruss H, Dalmau J, Wandinger KP, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry. 2012;83:195–198. doi: 10.1136/jnnp-2011-300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchino A, Iizuka T, Urano Y, Arai M, Hara A, Hamada J, Hirose R, Dalmau J, Mochizuki H. Pseudo-piano playing motions and nocturnal hypoventilation in anti-NMDA receptor encephalitis: response to prompt tumor removal and immunotherapy. Intern Med. 2011;50:627–630. doi: 10.2169/internalmedicine.50.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkpatrick MP, Clarke CD, Sonmezturk HH, Abou-Khalil B. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav. 2011;20:392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Gitiaux C, Simonnet H, Eisermann M, Leunen D, Dulac O, Nabbout R, Chevignard M, Honnorat J, Gataullina S, Musset L, Scalais E, Gauthier A, Hully M, Boddaert N, Kuchenbuch M, Desguerre I, Kaminska A. Early electro-clinical features may contribute to diagnosis of the anti-NMDA receptor encephalitis in children. Clin Neurophysiol. 2013;124:2354–2361. doi: 10.1016/j.clinph.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Iizuka T, Yoshii S, Kan S, Hamada J, Dalmau J, Sakai F, Mochizuki H. Reversible brain atrophy in anti-NMDA receptor encephalitis: a long-term observational study. J Neurol. 2010;257:1686–1691. doi: 10.1007/s00415-010-5604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


