Abstract
This study is to investigate the effects of brucea javanica oil emulsion (BJOE) combined with transcatheter hepatic arterial chemoembolization (TACE) on primary liver cancer (PLC) and the related mechanisms. Totally 64 PLC patients were divided into the TACE monotherapy and BJOE/TACE combination therapy groups. The short- and long-term efficacies, and the toxicity and tolerability profiles, of these treatments were evaluated. The serum levels of soluble Fas (sFas) and soluble Fas ligand (sFasL) were detected with ELISA. For the short-term efficacy, the response rate (RR) in the TACE monotherapy and BJOE/TACE combination therapy groups were 50% (16/32) and 78.12% (25/32), respectively. Survival analysis showed that, the combination therapy significantly elevated the 1-, 2-, and 3-year survival rates of PLC patients, compared with the monotherapy. No significant differences were observed in the toxicity and tolerability profiles between these therapies. ELISA showed that, the serum sFas/sFasL levels were significantly increased in PLC patients. At 1 m after the combination therapy, the serum sFas/sFasL levels were significantly higher than before treatment. At 3 m and 6 m after treatment, the serum sFas/sFasL levels were gradually declined. The short- and long-term efficacies of the BJOE/TACE combination therapy for PLC are superior to the TACE monotherapy. The combination therapy could promote liver cancer cell apoptosis by regulating the expression of sFas/sFasL. Serum sFas/sFasL levels might be used as the predictive marker for the disease pathogenesis and prognosis, and the treatment efficacy.
Keywords: Primary liver cancer (PLC), brucea javanica oilemulsion (BJOE), transcatheter hepatic arterial chemoembolization (TACE), combination therapy, sFas/sFasL
Introduction
Primary liver cancer (PLC) is one of the common malignant tumors in China, which is characterized by the occultonset, high malignancy, rapid progression, short survival time, and difficulty of treatment [1]. In recent years, the incidence of PLC in China has been continuously going up, and the annual new-onset cases in this country account for 55% of the world’s total amount [2]. Moreover, up to 300 thousand PLC patients die in China each year, accounting for more than half of all the PLC-induced deaths world wide [3]. Currently, the transcatheter hepatic arterial chemoembolization (TACE) represents one of the major treatment options for patients with advanced PLC, who are not suitable for the surgical resection [4]. However, the outcomes of TACE are always unsatisfactory due to the high recurrence rate. Therefore, numerous experimental and clinical studies are trying to improve the treatment outcome of advanced PLC, mainly focusing on controlling the clinical symptoms and reducing the adverse effects.
The Fas system plays an important role in regulating cell apoptotic process [5]. Fas, also known as Apo1 or CD95, is a type I transmembrane glyco protein belonging to the tum or necrosis factor receptor (TNFR)/nerve growth factor receptor (NGFR) family. Fas antigen mainly exists in the form of membrane receptors. However, soluble Fas (sFas) could also be detected in the cytoplasm and serum due to the lack of the transmembrane domain resulting from mRNA splicing. The sFas could bind to Fasligand (FasL) and block the interaction between Fas and FasL, there after inhibiting cellular apoptosis. It has been shown that the expression level of Fas is down-regulated in liver cancer cells, while the serum sFas level is elevated in patients with PLC [6].
Brucea javanica oil emulsion (BJOE) is a traditional Chinese medicine. It has been shown that BJOE could directly kill the cancer cells by up-regulating the tumor suppress or genes [7]. Moreover, BJOE has also been found to reverse the tumor cell resistance to chemotherapy and improve the body immunity, without significant adverse effects [8]. The therapeutic effects of BJOE on PLC, especially in combination with TACE, have not yet been fully elucidated. In this study, the effects of BJOE/TACE combination therapy on PLC patients and the related mechanisms were investigated. These patients were treated with TACE monotherapy or BJOE/TACE combination therapy, and the short- and long-term efficacies, as well as the toxicity and tolerability profiles, of these treatments were evaluated. The serum sFas/sFasL levels were also determined and analyzed.
Materials and methods
Patients and grouping
Totally 64 patients with advanced PLC (40 cases of primary cancer and 24 cases of recurrence) were included in this study, who had been admitted to our hospital, from Jan 2008 to Dec 2010. PLC was confirmed by biopsy and clinical diagnosis (at least two kinds of imaging examination, combined with the detection of tumor-specific marker a-fetoprotein, AFP), excluding other malignant metastatic tumors. The inclusion criteria were as follows: (1) unresectable PLC cases; (2) although resectable, unwilling to or could not take the surgery (due to advanced age, cardiopulmonary dysfunction, and severe cirrhosis, etc.); (3) non-disseminated cancer, with no more than 4 tumors detected with CT or digital subtraction angiography (DSA) (maximum diameter of no more than 10 cm); (4) with good general condition, i.e., KPS ≥70; (5) class A or B, according to Child-Pugh classification of liver function; (6) without inferior venacavaor portal vein thrombosis (PVTT); (7) without extra hepatic metastases, jaundice, orascites; (8) with available follow-up data. Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of the Yidu Central Hospital of Weifang.
These PLC patients were divided into (1) the TACE monotherapy group (including 20 cases of primary cancer and 12 cases of recurrence), in which subjects received TACE treatment alone, and (2) the BJOE/TACE combination therapy group (including 20 cases of primary cancer and 12 cases of recurrence), in which subjects received the BJOE/TACE combination therapy. Another 30 healthy subjects were used as the normal control group.
Therapeutic treatments
With the support of the DS Adevice, PLC patients were subjected to the percutaneous femoral artery puncture using the Seldinger technique. 4-5-FRH, Yashiro, or Cobra catheter was inserted into the hepatic artery. The information of tumor size, position, feeding artery distribution, and with or without arteriovenous fistula, was obtained with the DSA angiography. Amicro-catheter was inserted into the target vessel, and the following substances were slowly injected through the catheter under fluoroscopic monitoring: 5-20 mL ultra liquid iodized oil, 4-10 mg mitomycin C (MMC), 25-100 mg carboplatin (CBP), 10-30 mg 4’-epi-adriamycin (E-ADM), and gelatin sponge or PVA particle suspension. For the combination therapy group, 30-60 mL BJOE was also injected. The administration dosage depended on the tumor size and the liver function status. When the angiography following embolization indicated that the majority of tumor blood vessels were blocked, the catheters heath was removed, and the puncture site was bandaged. For the combination therapy group, the daily intravenous infusion of BJOE started at 2 d after TACE, and lasted for 15 d. For the TACE monotherapy group, PLC patients did not receive the arterial embolism and intravenous infusion of BJOE. Aftert reatments, these patients were subjected to regular monitoring of the liver/kidney function and AFP, as well as the CT scan. The treatment was repeated every 3-4 w. Each patient received to at least 3 cycles of treatment. Inaddition, these PLC patients also received supportive treatments, including the prophylactic antiemetic treatment before TACE, postoperative antiemetic treatment (for 1-3 d) depending on the gastrointestinal condition, and conventional liver treatment (for 7-10 d). For the patients with viral hepatitis, antiviral therapy was needed to keep the virus under 1×103 copies/mL.
Enzyme linked immunosorbent assay (ELISA)
The serum sFas/sFasL levels were detected with ELISA kits (Senxiong Biotech, Shanghai, China), according to the manufacturer’s instructions, at 1 m, 3 m, and 6 m after treatments.
Liver function assessment
The liver function was assessed by the measurement of serum levels of albumin (ALB), alanineamino transferase (ALT), and total bilirubin (TBIL) with the bromocresol green method, enzymatic assay, and vanadate method, respectively, using the automatic biochemical analyzer (AU5400; Olympus Optical Co, Tokyo, Japan).
Therapeutic efficacy evaluation
The therapeutic efficacy was evaluated with telephone and clinic follow-up, starting from the first treatment of TACE and/or BJOE in Mar 2013, and ending at death or until the follow-up deadline. No death cases resulted from the treatments. The death cases were mainly caused by the tumor rupture-induced bleeding, distant metastasis, organ failure, or gastrointestinal bleeding due to portal hypertension. After all the treatments, the blood routine examination, blood biochemistry examination, AFP level determination, and unenhanced/enhanced CT scan or DSA were regularly performed. The patient’s condition was reviewed every three months in the first year of follow-up, every month in the second year, and twice a month in the third year.
The short-term efficacy was evaluated according to the Response Evaluation Criteriain Solid Tumors (RECIST) guide line from the World Health Organization (WHO) [9]: (1) complete response (CR) was defined as complete tumor necrosis or disappearance of all tumors, confirmed at ≥4 w; (2) partial response (PR) was defined as tumor necrosis ≥50% or the decline in the product of the longest diameter and the greatest perpendicular diameter ≥50%; (3) improvement was defined as tumor necrosis or regression ≥25%, while ≤50%; (4) stable disease (SD) was defined as tumor necrosis or regression <25%, or tumor increase <25%; (5) progressive disease (PD) was defined as tumor increase >25%, or new lesions. Response rate (RR) was calculated as the sum of CR and PR. On the other hand, the long-term efficacy was evaluated by observing the 1-, 2-, and 3-year survival rates.
The treatment toxicity was observed and assessed, including fever, abdominal pain, nausea, vomiting, elevated transaminase level, and bone marrow suppression [10]. Moreover, whether serious complications occurred or not was also observed, including acute liver failure, liver abscess, acute pancreatitis, gall bladder perforation, abdominal bleeding, pneumothorax, and hemothorax.
Statistical analysis
Data were expressed as mean ± SD. The SPSS17.0 software was used for statistical analysis. The χ2 test and t-test were performed for the group comparison. The Kaplan-Meier method was applied for the survival estimation, and the differences were analyzed with the log-rank test. P<0.05 was considered as statistically significant.
Results
Short-term efficacy assessment of monotherapy and combination therapy for PLC
The baseline characteristics of all the patients with PLC were listed in Table 1, including sex, age, tumor number and size, liver function (Child-Pugh classification), serum AFP level, and HBsAg (the tumor with largest diameter was observed for patients with multiple lesions). No significant differences were noted between the TACE monotherapy and BJOE/TACE combination therapy groups (P>0.05), indicating that the grouping of these patients was suitable for the investigation. At 3 m after treatments, the short-term efficacy was assessed with unenhanced/enhanced CT scan or DSA. As shown in Table 2, the response rate (RR) in the TACE monotherapy group was 50% (16/32), while the RR in the BJOE/TACE combination therapy group was 78.12% (25/32) (compared with the monotherapy group, P<0.05). These results suggest that the short-term efficacy of the combination therapy for PLC patients is superior to the TACE monotherapy.
Table 1.
Baseline characteristics of the PLC patients
| Clinical indexes | BJOE/TACE combination therapy | TACE monotherapy | χ2 | P |
|---|---|---|---|---|
| Case | 32 | 32 | - | - |
| Average age (y) | 50.9 ± 9.5 | 56.1 ± 12.5 | - | 0.097 |
| Sex (male/female) | 26/6 | 24/8 | 1.012 | 0.314 |
| HBsAg (negative/positive) | 29/3 | 30/2 | 0.024 | 0.878 |
| Child-Pugh classification (A/B) | 19/13 | 22/10 | 0.049 | 0.825 |
| Tumor number (single/multiple) | 23/9 | 21/11 | 1.102 | 0.294 |
| Lesion diameter (≤5 cm/>5 cm) | 4/28 | 8/24 | 0.079 | 0.778 |
| Serum AFP (ng/mL, ≤400/>400) | 22/10 | 19/13 | 1.820 | 0.177 |
| Okuda (1985) staging (phase I/II) | 20/12 | 23/9 | 0.076 | 0.783 |
Abbreviations: BJOE, bruceajavanica oil emulsion; TACE, transcatheter hepatic arterial chemoembolization.
Table 2.
Short-term efficacies of monotherapy and combination therapies [% (case)]
| Group | CR | PR | SD | PD | RR |
|---|---|---|---|---|---|
| Combination therapy | 12.5% (4) | 68.8% (22) | 21.9% (7) | 3.13% (1) | 81.3% (26) |
| Monotherapy | 3.13% (1) | 43.8% (14) | 25% (8) | 18.8% (6) | 46.9% (15) |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RR, response rate.
Survival analysis of PLC patients after monotherapy and combination therapy
The survival analysis of PLC patients subjected to TACE monotherapy and BJOE/TACE combination therapy was performed, and the 1-, 2-, and 3-year survival rates were calculated. Our results showed that, the 1-, 2-, and 3-year survival rates in the TACE monotherapy group were 59.4%, 20.3%, and 6.8%, respectively, with the median survival time of 15 m and the average survival time of 16.5 m (Table 3). On the other hand, the 1-, 2-, and 3-year survival rates in the BJOE/TACE combination therapy group were 79.7%, 52.3%, and 23.0%, respectively, with the median survival time of 25 m and the average survival time of 24.8 m (Table 3). Significant differences in the survival rates were observed between these two treatments (P<0.05). Similar results were obtained from the survival curves (Figure 1). These results suggest that the BJOE/TACE combination therapy could significantly elevate the survival rates of PLC patients compared with the TACE monotherapy.
Table 3.
Survival analysis of PLC patients after treatment
| Group | Median survival time (m) | Average survival time (m) | Survival rate | Log-rank test | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 1-year | 2-year | 3-year | ||||
| Combination therapy | 25 | 24.8 | 79.7% | 52.3% | 23.0% | χ2=6.827 |
| Monotherapy | 15 | 16.5 | 59.4% | 20.3% | 6.8% | P=0.009 |
Figure 1.
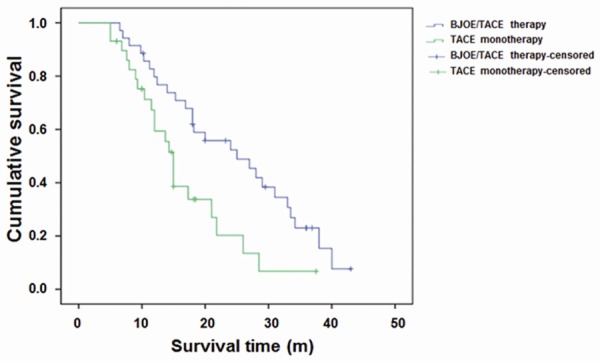
Survival curves for the PLC patients after the TACE monotherapy and the BJOE/TACE combination therapy. Kaplan-Meier survival curve was displayed. The differences in survival time were analyzed by Log-Rank test.
Toxicity and tolerability profiles of monotherapy and combination therapy for PLC
The toxicity and tolerability profiles of the TACE monotherapy and BJOE/TACE combination therapy in the PLC patients were next investigated. As shown in Table 4, all these 64 PLC patients had been reported with treatment-related adverse reactions, mainly including fever, gastrointestinal reactions (nausea, vomiting, fatigue, anorexia, abdominal pain), liver damage (as indicated by the elevated transaminase level), and bone marrow suppression. These adverse reactions would be attenuated after proper treatments, such as pyretolysis, liver and stomach protection, and leukogenicaction. However, no severe complications, like acute liver failure, acute pancreatitis, liver abscess, gall bladder perforation, abdominal bleeding, pneumothorax, and hemothorax, were observed during the treatments. No significant differences in the toxicity and tolerability profiles were observed between the TACE monotherapy and the BJOE/TACE combination therapy.
Table 4.
Toxicity and tolerability profiles of monotherapy and combination therapies [% (case)]
| Group | Fever | Gastrointestinal reactions | Elevated transaminase level | Bone marrow suppression |
|---|---|---|---|---|
| Combination therapy | 31.13% (10) | 50% (16) | 34.4% (11) | 62.5% (20) |
| Monotherapy | 37.5% (12) | 62.5% (20) | 37.5% (12) | 56.3% (18) |
| χ2 | 0.195 | 2.705 | 0.092 | 0.029 |
| P | 0.659 | 0.100 | 0.762 | 0.866 |
Serum sFas/sFasL levels in patients with PLC
The serum sFas/sFasL levels, as well as albumin (ALB), alanineamino transferase (ALT), and total bilirubin (TBIL), in these PLC patients were investigated. As shown in Table 5, compared with the normal control group, the serum sFas/sFasL levels were significantly increased in patients with primary cancer (compared with the control group, P<0.05 for sFas and P<0.01 for sFasL), which were further elevated in patients with recurrence (compared with the control group, both P<0.01; compared with the primary cancer group, both P<0.01). As shown in Table 6, when these PLC patients were treated with the BJOE/TACE combination therapy, at 1 m after treatment, the serum sFas/sFasL levels were significantly higher than before treatment (P<0.01). At 3 m after treatment, the serum sFas/sFasL levels were significantly decreased compared with 1 m after treatment (both P<0.05). The serum sFasL level was comparable to before treatment, while the serum sFas level was even lower than before treatment. At 6 m after treatment, the serum sFas/sFasL levels were further declined, which were significantly lower than before treatment (both P<0.05). On the other hand, no significant differences were observed in the serum levels of ALB, ALT, and TBIL in PLC patients between before and after the combination treatment. These result ssuggest that the BJOE/TACE combination therapy could regulate the serum sFas/sFasL levels in these PLC patients, which might contribute to the therapeutic effects on the disease.
Table 5.
Serum levels of sFas and sFasL in normal controls and PLC patients
| Group | Case | sFas (µg/L) | sFasL (µg/L) |
|---|---|---|---|
| Normal control | 30 | 22.56 ± 7.82 | 27.56 ± 7.86 |
| Primary cancer | 40 | 52.18 ± 21.98* | 87.12 ± 27.56** |
| Recurrence | 24 | 108.12 ± 26.78**,## | 318.56 ± 140.12**,## |
Note: Compared with the normal control group;
P<0.05;
P<0.01.
Compared with the primary cancer group;
P<0.01.
Table 6.
Serum levels of sFas, sFasL, ALB, ALT, and TBIL in PLC patients after treatment
| Group | Case | sFas (µg/L) | sFasL(μg/L) | ALB (g/L) | ALT (U/L) | TBIL (µmol/L) | |
|---|---|---|---|---|---|---|---|
| Before treatment | 40 | 52.26 ± 23.12 | 87.18 ± 27.12 | 34.26 ± 5.89 | 93.18 ± 60.12 | 52.18 ± 21.36 | |
| After BJOE/TACE combination therapy | 1 m | 40 | 103.68 ± 27.12** | 152.36 ± 28.12** | 35.12 ± 3.59 | 89.12 ± 36.78 | 43.26 ± 19.12 |
| 3 m | 40 | 31.28 ± 6.78*,## | 83.56 ± 14.89## | 37.12 ± 5.36 | 67.28 ± 43.12 | 37.12 ± 18.86 | |
| 6 m | 40 | 22.16 ± 3.12**,##,&& | 35.16 ± 5.18**,##,&& | 34.15 ± 4.51 | 59.18 ± 18.56 | 32.12 ± 20.18 | |
| After BJOE monotherapy | 1 m | 40 | 82.76 ± 25.16** | 140.36 ± 21.61** | 35.89 ± 3.96 | 91.28 ± 35.88 | 49.64 ± 20.08 |
| 3 m | 40 | 46.23 ± 10.96*,## | 81.28 ± 14.89## | 36.08 ± 5.16 | 82.68 ± 42.04 | 43.28 ± 19.92 | |
| 6 m | 40 | 40.22 ± 6.18**,##,&& | 70.08 ± 6.86**,##,&& | 34.28 ± 5.02 | 60.58 ± 19.86 | 39.58 ± 19.28 | |
| After TACE monotherapy | 1 m | 40 | 92.58 ± 23.06** | 146.72 ± 26.08** | 36.28 ± 3.08 | 90.12 ± 36.78 | 50.36 ± 18.66 |
| 3 m | 40 | 42.36 ± 7.26*,## | 80.36 ± 13.78## | 35.36 ± 5.36 | 80.22 ± 38.66 | 45.32 ± 18.86 | |
| 6 m | 40 | 38.28 ± 3.06**,##,&& | 62.28 ± 5.96**,##,&& | 34.69 ± 4.51 | 62.27 ± 20.36 | 42.38 ± 19.22 | |
Abbreviations: sFas, soluble Fas receptor; sFasL, soluble Fas ligand; ALB, albumin; ALT, alanine aminotransferase; TBIL, total bilirubin. Note: Compared with before treatment;
P<0.05;
P<0.01.
Compared with 1 m after treatment;
P<0.01.
Compared with 3 m after treatment;
P<0.01.
Discussion
Currently, surgical resection is still the first choice for the treatment of PLC [11]. For the surgical treatment, a better prognosis would be expected for early diagnosis. However, many patients might have been found to be associated with extra hepatic metastasis and portal vein tumor thrombus formation at the initial diagnosis of PLC, thus missing the good chance for the surgical treatment. Although advanced imaging technique (such as CT and MRI) can help to confirm the small lesions at the early stage, the resection rate for liver cancer is still only 10%-30% [12]. That is because even small liver cancer might also be accompanied by multiple local lesions, early vascular invasion, and other liver parenchyma diseases such as cirrhosis. On other hand, the natural survival time for patients with unresectable advanced liver cancer is only 3 to 6 months [13]. The vascular interventional therapy TACE has currently been used for the treatment of unresectable advanced liver cancer [14]. However, the long-term efficacy is still not satisfactory due to the incomplete embolization, hepatic arterial variation, liver’s dual blood supply, and collateral circulation. The 3-year survival rate is even less than 20% [13]. In recent years, Chinese medicine has gradually become a promising option for the treatment of advanced PLC and the improvement of the disease symptoms, especially for the brucea javanica oil emulsion (BJOE) and transcatheter hepatic arterial chemoembolization (TACE) combination therapy.
Liver tumorigenesis and development is associated with the deficient cellular apoptosis. The pathogenesis and development of PLC could be attributed to the imbalance between cell proliferation and apoptosis regulation. The interaction between Fas and FasL is one of the important pathways to induce cellular apoptosis. Fas mainly exist as the membrane receptors, which bind to extracellular FasL and deliver the apoptotic signal into the cells. However, the mRNA splicing process during the Fas gene transcription might lead to the deletion of the transmembrane domain, ending up with soluble Fas (sFas) in the tissue fluid and/or serum. The serum sFas could bind to FasL to fight against FasL-mFas-mediated cellular apoptosis [15]. FasL is the natural ligand for Fas, which is a type II transmembrane protein belonging to the TNF family. FasL is mainly expressed in activated T cells, NK cells, several cancer cells, and some immune privilege areas (such as the anterior chamber and Sertoli cell surface).Membrane-bound FasL (mFasL) could be cleaved into soluble FasL (sFasL) by metalloenzyme. The binding of sFas and sFasL could also induce cellular apoptosis, although with a less potent inducing effect as mFasL.
It has been shown that the expression level of Fas was down-regulated in cancer cells from PLC patients, while the serum sFas level was elevated in these patients. In line with this, our results showed that, compared with the control group, the serum sFas/sFasL levels were significantly increased in the PLC patients (either with primary cancer or recurrence). These results suggest that the elevated serum sFas/sFasL levels might be associated with the disease pathogenesis. Tumor-specific antigen might induce the over-expression of Fas in T cells. The down-regulated expression or absence of Fas in liver cancer cells would block the effects of FasL (expressed by activated T cells) on cancer cells. Instead, they act on T cells over-expressing Fas and induce apoptosis, so that the cancer cells could escape from the immune surveillance [16]. Moreover, the Fas-mediated cellular apoptosis might be influenced by various factors and enzymes, including Fas-associated phosphatase I on cancer cells and Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (vFLIP), which bind to Fas and inhibit the Fas-mediated signal transduction, thereby inhibiting theapoptosis of liver cancer cells.
At present, TACE is the one of the major options for the treatment of PLC, especially for the patients with unresectable advanced PLC. However, complete tumor necrosis occurs in only 5% of the patients treated with TACE, while in the other 95% of patients, surviving cancer cells could be observed within and outside the tumor capsule, which might lead to recurrence and severe liver damage [17]. Therefore, combination therapy has always been applied in the treatment of the disease to improve the efficacy. Chinese medicine has been characterized by the long-term efficacy,as well as the survival-prolonging, immunity-enhancing, liver function-improving, and chemotherapy-induced embolism-reducing effects. BJOE is an oil-in-water emulsion made from petroleum ether extract and refined soybean lecithin. BJOE is an anti-tumor medicine first developed in China, whose main active ingredients include oleic acid and linoleic acid. Brucea javanica has been well known for its heat-clearing and detoxifying, excrescence-breaking up, hard mass-softening and -resolving, and dysentery- and malaria-preventing effects. It has been shown by numerous experimental and clinical studies that, as the cell cycle-non specific anti-tumor agents, BJOE could directly inhibit and kill the cancer cells in the G0, G1, S, G2, and M phases, and significantly inhibit the DNA synthesis in cancer cells [8].
In this study, totally 64 PLC patients were subjected to TACE monotherapy or BJOE/TACE combination therapy, and the short-term efficacy was evaluated with unenhanced/enhanced CT scan at 3 m after treatment. Our results showed that, the RR (CR+PR) in the TACE monotherapy group was 46.9% (15/32), while the RR in the BJOE/TACE combination therapy group was 81.3% (26/32), with statistically significant differences between these two groups. For the long-term efficacy, the 1-, 2-, and 3-year survival rates in the TACE monotherapy group were 59.4%, 20.3%, and 6.8%, respectively, with the median survival time of 15 m and the average survival time of 16.5 m. On the other hand, the 1-, 2-, and 3-year survival rates in the BJOE/TACE combination therapy group were 79.7%, 52.3%, and 23.0%, respectively, with the median survival time of 25 m and the average survival time of 24.8 m. Taken together, these results suggest that the both the short- and long-term efficacies of the BJOE/TACE combination therapy are superior to the TACE monotherapy.
Based on all the above results, we suppose that, in the combination therapy, the arterial embolization and intravenous infusion of BJOE would reduce the resistance of cancer cells to anti-cancer drugs, and improve the apoptosis of liver cancer cells, exerting a synergistic effect with the chemotherapeutic drugs. It has been shown that, the cellular apoptosis could be induced by the Fas/FasL, P53, and Bcl-2 pathways. Some anti-cancer drugs could elevate the expression of Fas/FasL and enhance the Fas/FasL death signaling, and then inhibit the cancer cells. In this study, our results showed that, the serum sFas/sFasL levels were both elevated in PLC patients (with primary cancer or recurrence) treated with the BJOE/TACE combination therapy, particularly at 1 m after treatment. At 3 m and 6 m after treatment, the serum sFas/sFasL levels were gradually decreased. These results suggest that, the BJOE/TACE combination therapy could induce the expression of apoptosis-associated sFasL and the binding between Fas and FasL, inhibiting the proliferation and promoting the apoptosis of liver cancer cells.
In summary, our results showed that both the sort- and long-term efficacies of the BJOE/TACE combination therapy were superior to the TACE monotherapy. Moreover, serum sFas/sFasL levels had been altered in PLC patients subjected to the BJOE/TACE combination therapy. The BJOE/TACE combination therapy could amendment the body immunity, reduce the adverse effects of chemotherapeutic drugs, prolong the survival time, and improve the quality of life of PLC patients. The combination therapy could synergistically regulate the expression and release of sFas/sFasL, promote the apoptosis of liver cancer cells, and inhibit the tumor growth. These findings suggest that serum sFas/sFasL levels might be used as the predictivemarker for the disease pathogenesis and prognosis, and the treatment efficacy.
Acknowledgements
We thank Director Jianyi Niu from the Yidu Central Hospital of Weifang for his valuable assistance during this work.
Disclosure of conflict of interest
None.
References
- 1.Zhao J, Greene CM, Gray SG, Lawless MW. Long noncoding RNAs in liver cancer: what we know in 2014. Expert Opin Ther Targets. 2014;18:1207–1218. doi: 10.1517/14728222.2014.941285. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Song X. An Evaluation on Incident Cases of Liver Cancer in China. China Cancer. 2005;14:28–31. [Google Scholar]
- 4.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz A, Sanwald J, Thomas M, Feuer R, Sawodny O, Ederer M, Borner C, Humar M, Merfort I. Interleukin-1beta enhances FasL-induced caspase-3/-7 activity without increasing apoptosis in primary mouse hepatocytes. PLoS One. 2014;9:e115603. doi: 10.1371/journal.pone.0115603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Bassiouny AE, El-Bassiouni NE, Nosseir MM, Zoheiry MM, El-Ahwany EG, Salah F, Omran ZS, Ibrahim RA. Circulating and hepatic Fas expression in HCV-induced chronic liver disease and hepatocellular carcinoma. Medscape J Med. 2008;10:130. [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Z, Zhang B, Huang Y, Qiu H, Chen P, Guo GF. Involvement of autophagy inhibition in oil emulsion-induced colon cancer cell death. Oncol Lett. 2015;9:1425–1431. doi: 10.3892/ol.2015.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han F, Cai D, Wu X, Zhai X, Wang L. Research progress of Brucea javanica antitumor mechanism. Journal of Modern Oncology. 2013;212:669–671. [Google Scholar]
- 9.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu XZ. A new classification system of anticancer drugs-based on cell biological mechanisms. Med Hypotheses. 2006;66:883–887. doi: 10.1016/j.mehy.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J, Gores GJ. Surgical resection versus transplantation for early hepatocellular carcinoma: clues for the best strategy. Hepatology. 2000;31:1019–1021. doi: 10.1053/he.2000.6959. [DOI] [PubMed] [Google Scholar]
- 12.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 13.Ye S. Present status and evaluation of interventional therapy for primaryhepatocellular carcinoma. Chin J Hepatol. 2002;10:165–6. [PubMed] [Google Scholar]
- 14.Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1–15. doi: 10.1046/j.1365-2036.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 15.Fabregat I, Roncero C, Fernandez M. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 2007;27:155–162. doi: 10.1111/j.1478-3231.2006.01409.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Chen Z, Tian L, Liu F, Liu L, You Y, Zou P. Inhibitory Effect of Anti-Fas Ribozyme on Apoptosis of Mouse T Cells. Chin J Cancer. 2005;24:520–524. [PubMed] [Google Scholar]
- 17.Ahrar K, Gupta S. Hepatic artery embolization for hepatocellular carcinoma: technique, patient selection, and outcomes. Surg Oncol Clin N Am. 2003;12:105–126. doi: 10.1016/s1055-3207(02)00089-3. [DOI] [PubMed] [Google Scholar]


