Abstract
Objective: In the present study, the antiviral effects of polyphylla saponin I isolated from Parispolyphylla on influenza A virus are investigated both in vitro and in vivo. Methods: Column chromatography and reversed phase liquid chromatography separation technology were used to extract and purify polyphylla saponin I. The purity of polyphylla saponin I was assayed by high performance liquid chromatography. Methyl thiazolyl tetrazolium assay and analyses of cytopathic effects were performed to examine the antiviral activity of polyphylla saponin I upon MDCK cells infected with influenza A virus. Model mice were made by intranasal inoculation of influenza a virus. Mice infected with influenza A virus were orally administered polyphylla saponin I and oseltamivir twice a day for 5 days to study their antiviral efficacy in vivo. Results: Polyphylla saponin I had no cytotoxicity on MDCK cells at the concentration of 50 μg/mL. Polyphylla saponin I (6.25, 12.5, 25 and 50 μg/mL) and oseltamivir (40 μg/mL) had remarkable inactivation effects on influenza A virus, prevention effects on influenza A virus adsorption on MDCK cells, and inhibitory effects on the reproduction of influenza A virus in MDCK cells. In addition, polyphylla saponin I (5 and 10 mg/kg), and oseltamivir (3 mg/kg) significantly reduced viral hemagglutination titer, improved the pathologic histology of lung tissues, and decreased the mortality of mice infected with influenza A virus. Polyphylla saponin I (5 and 10 mg/kg) prolonged the survival time of mice from 8.5±0.3 days to 13.2±0.5 days, with the prolonged life rates being 49.4% and 55.3%, respectively. Conclusion: Polyphylla saponin I has antiviral activity on influenza A virus both in vitro and in vivo.
Keywords: Influenza A virus, polyphylla saponin I, oseltamivir, antiviral activity
Introduction
Influenza is a highly contagious and epidemic respiratory disease caused by influenza A virus. Human influenza A virus, a member of orthomyxoviridae, causes high morbidity and mortality, particularly in infants, elderly people, and those suffering from immunodeficiency [1]. The life cycle of influenza viruses is well documented and most viral proteins are potential therapeutic targets [2-6]. In short term, antiviral therapy is vital to control the spread of influenza. To date, only two classes of anti-influenza drugs have been approved: M2 ion channel inhibitors such as amantadine and rimantadine, or neuraminidase inhibitors such as oseltamivir or zanamivir [7]. However, influenza viruses undergo continuous genetic changes by means of mutation and recombination, forming drug-resistant strains. Viral resistance may be developed by modifications in amino acid composition of neuraminidase, or binding of haemagglutinin to receptors on cell surface.
Plants, with a long evolutionary history in which resistance against viruses is developed, have increasingly drawn concerns as potential sources of antiviral drugs [8,9]. Recently, several types of herbal medicines have been used for the treatment of infection by influenza virus. Parispolyphylla var. yunnanensis (named “Chonglou” in Chinese) is one of the most famous medicinal plants in China. The rhizome of this plant has been developed into traditional Chinese medicines such as “Yunnan Baiyao” and “Gongxuening”, which are used to treat dispersing blood stasis and hemostasis, to activate blood circulation, to alleviate pain, to reduce swelling, to stop bleeding and to reduce inflammation [10-12].
Although P. polyphylla has been recorded to have a stronger inhibitory effect on Asian influenza A virus, little has been systematically investigated in vitro and in vivo with respect to its anti-influenza effects. It is reported that polyphylla saponins are the main and active component in Paris polyphylla [13]. The purpose of this work is to extract polyphylla saponin I and examine whether it has inhibitory effects on influenza A virus in vitro or in vivo.
Materials and methods
Animals
Equal numbers of male and female BALB/c mice weighing 18-22 g were purchased from Gansu Cancer Hospital, China. Mice with clinical signs of disease or abnormalities were not used in the study. All mice were allowed for free access to water, and were fed with non-medicated rations. The mice were acclimated for at least 2 days prior to experimentation. All animal experiments were conducted according to the ethical guidelines of Gansu Center for Disease Control and Prevention.
Cells
Madin-Darby canine kidney (MDCK) cells were obtained from the Central Laboratory of the College of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, China. The cells were cultured in Dulbecco’s modified eagle medium, supplemented with 100 units/ml penicillin, 100 mg/ml streptomycin and 10% (volume ratio) fetal calf serum. Fetal calf serum was reduced to 2% (volume ratio) for viral infection.
Virus
Influenza A virus, specifically, a mouse-lung-adapted strain (A/PR/8/1934), was obtained from the Chinese Center for Disease Control and Prevention. It had been generated and amplified in 10-day-old chicken embryos and then preserved at -80°C. Titers of the virus were quantified on MDCK cell monolayers by determination of the 50% Tissue Culture Infective Dose (TCID50), according to Reed-Muench method [14]. The TCID50 value for influenza A virus was assayed to be 10-4.6/0.1 ml. A virus solution of 100 times of this TCID50 was used for the experiment in vitro. The median lethal dose (LD50) of influenza A virus was 10-3.5/0.05 ml in mice. Influenza A virus of 10 times of this LD50 was instilled into the nares of mice in the experiment (infection group). All tested mice were challenged with influenza A virus in P3 animal laboratory of the Gansu Center for Disease Control and Prevention, China.
Preparation of polyphylla saponin I
P. polyphylla was purchased from Chengdu Lotus Pond Traditional Chinese Medicine Market (Chengdu, Sichuan Province, China). P. polyphylla was identified as dried roots of P. polyphylla Smith var. yunnanensis (Franch) Hand. -Mazzby Gansu Institute for Food and Drug Control. Dried, crushed roots (500 g) of P. polyphylla were extracted with 95% ethanol by reflux twice (each of 2 hours) to remove pigments and small lipophilic molecules. The condensed solution was dissolved in distilled water and defatted with aetherpetrolei. The water fraction was further extracted by n-butyl alcohol, and then the n-butyl alcohol soluble fraction was condensed with a vacuum rotary evaporator to obtain crude extracts of total polyphylla saponins (48 g).
The crude extracts were then eluted by chloroform-methanol-water gradient elution on a silica gel column (800 g), and two main parts were collected through chromatograph. Four monomeric compounds were separated by reversed phase liquid chromatography (RPLC). Octadecylsilyl chemically modified porous glass column (150 mm × 4 mm) was used to detect the four compounds in the total saponins. At the same time, methanol-water (volume ratio of 80:20) was used as mobile phases. The detection wave length was 210 nm, and the column temperature was 35°C. The four compounds were identified as polyphylla saponin I, II, VI and VII, respectively, through infrared spectrometry (IR), hydrogen nuclear magnetic resonance (1HNMR), carbon-13 nuclear magnetic resonance (13CNMR) and fast atom bombardment mass spectrometry (FAB-MS).
High-performance liquid chromatography (HPLC)
HPLC was used to analyze the purity of polyphylla saponin I, II, VI and VII. Polyphylla saponin I, II, VI and VII was detected by Symmetry C18 column (4.6 mm × 150 mm, 5 μm) with acetonitrile-water (volume ratio of 85:15) as mobile phases. The detection wave length was 210 nm, the flow rate was 1.0 ml/min, the column temperature was 35°C and the injection volume was 20 μl [15]. The purities of polyphylla saponin I, II, VI and VII were 95.3%, 90.2%, 92.4% and 86.8%, respectively. Therefore, polyphylla saponin I was selected to study antiviral activity in mice.
Determination of cytotoxicity
The toxicity of polyphylla saponin I to MDCK cells was determined as described by a previous report [16]. MDCK cells were seeded into 96-well plates (2 × 104 cells/well; 100 μL). After 24 h of incubation at 37°C in the presence of 5% CO2, 100 μL solution of polyphylla saponin I or oseltamivir (H20065415, Yichang Yangtze River Pharmaceutical Co., Ltd., Yichang, China) was added and incubated sequentially for another 24 h. Eight different concentrations of polyphylla saponin I or oseltamivir solutions (200, 100, 50, 25, 12.5, 6.25, 3.13 and 1.56 μg/mL) were tested. At the end of this period, 100 μL medium was removed and 20 μL 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well. The plate was then wrapped in aluminium foil and incubated for another 3-4 h. The medium was then carefully removed and 150 μL DMSO was added to each well in order to dissolve the formed formazan crystals. MTT formazanproduct was determined by enzyme-linked immunosorbent assay (ELISA), measuring absorbance at 490 nm using a microplate reader (Multiskan MK3, Thermo Scientific, Waltham, MA, USA). Cell survival rate was calculated using the following Equation:
Cell survival rate=Aagent/Acell contro × 100%
All experiments were repeated for three times. The cytotoxicity curve was then generated by plotting cell viability percentages against polyphylla saponin I concentrations.
Calculation of cell inhibitory rates
To determine cell inhibitory rates, MDCK cells (2 × 104 cells/well; 100 μL) were seeded into 96-well plates and incubated overnight at 37°C in the presence of 5% CO2. Polyphylla saponin I or oseltamivirwas added to MDCK cells at the time of infection by influenza A virus (t=0), 2 h prior to infection by influenza A virus (t=-2), and 2 h after infection by influenza A virus (t=+2), and incubated at 37°C in 5% CO2 [17,18]. The degree of inhibition of virus was recorded by microscopic observations, comparing with normal cell controls and virus controls until the cytopathic effect (CPE) score in virus controls became +++ and cell controls were still normal. The absorbance value (A490nm) in MTT colorimetric assay was used to evaluate the direct inactivation effect of polyphylla saponin I on influenza A virus. The cell survival rate and the degree of inhibition (inhibitory rate) of influenza A virus by polyphylla saponin I were calculated using the following Equations:
Cell survival rate=Aagent/Acell contro × 100%
Inhibitory rate=(Aagent - AVirus control)/(Acell control - AVirus control) × 100%
The experiment was performed in triplicate. The effects upon CPE were observed and recorded as follows: CPE changes ≤25% were scored as +, CPE changes of 25-50% were scored as ++, CPE changes of 51-75% were scored as +++, and CPE changes of 76-100% were scored as ++++ [19].
Determination of mortality, mean survival time, and prolonged life rate of infected mice
One hundred Kunming mice were randomly divided into 5 groups of 20 mice, including normal control group, influenza A virus infection group, osetamivir group, lower dose polyphylla saponin I group (5 mg/kg, bw/day) and higher dose polyphylla saponin I group (10 mg/kg, bw/day). The dosage of polyphylla saponin I was 5 and 10 mg/kg according to the results of acute toxicity experiment (0.1 and 0.2 times of LD50). Except for the mice in the normal control group, all mice were challenged with intranasal inoculation of influenza A virus. After anesthetizing the mice with diethyl ether, 50 μL of 10 × LD50 influenza A virus were instilled into the nares. Each group was completely isolated from other groups. The mice in lower and higher dose polyphylla saponin I groups and the oseltamivir group were orally administered varying doses of polyphylla saponin I (5 mg/kg and 10 mg/kg body mass) or oseltamivir(3 mg/kg body mass), respectively. Both reagents were administered twice daily for 5 days, beginning 4 h after exposure to the virus. At the same time, the mice in influenza A virus infection group and normal group were orally administered distilled water. Then, mortality, mean survival time, and prolonged life rate were calculated.
Viral hemagglutination titration
The lungs of each mouse were isolated, and blotted up with filter paper. A portion of lung specimen was homogenized after adding normal saline at low temperature [liver weight (g)/normal saline (mL) =1/9 (w/v)] (4°C, 14000 g × centrifugation for 30 min). The supernatants obtained from centrifugation were used to analyze the viral hemagglutination titer in triplicate by traces of blood coagulation test [20].
Hematoxylin and eosin staining
All animals were sacrificed at the end of the experimental period. To assay the influence of polyphylla saponin I on lung tissue of mice infected with influenza A virus, the other portion of lung specimen fixed in 10% neutral-buffered formalin was embedded in paraffin, sliced into 4-μm thickness, and stained with hematoxylin and eosin. The pathological changes were assessed and photographed under an Olympus BX-51 microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data were analyzed using the statistical software SAS 8.0 for Windows (Cary, NC, USA). One-way Analysis of Variance (ANOVA) was used for multiple comparisons. Data were considered significantly different if P<0.05.
Results
Polyphylla saponin I has higher cytotoxicity than oseltamivir
To determine the concentrations of polyphylla saponin I and oseltamivir used in this study, we tested their cytotoxicity. The data showed that the cytotoxicity of polyphylla saponin I, in contrast to that of oseltamivir, was increased in a dose-dependent manner (1.56 to 200 μg/mL). The nontoxic dose for polyphylla saponin I (50 μg/mL) was higher than the corresponding value of oseltamivir (40 μg/mL), suggesting that the highest concentration of polyphylla saponin I used in this experiment should be 40 μg/mL, whereas the concentration of oseltamivir used should be 40 μg/mL. These results demonstrate that polyphylla saponin I has higher cytotoxicity than oseltamivir.
Polyphylla saponin I (40 μg/mL) has similar inhibition effect on influenza A virus in MDCK cells compared with the same concentration of oseltamivir
To test the effect of polyphylla saponin I on influenza A virus activity in MDCK cells, cell inhibitory rates were calculated. Polyphylla saponin I showed a certain direct inactivation effect on influenza A virus, as indicated by the cell inhibitory rates determined over a range of polyphylla saponin I concentrations. At polyphylla saponin I concentrations of 5 μg/mL, 10 μg/mL, 20 μg/ mL and 40 μg/mL, cell inhibitory rates were 62.4%, 68.2%, 78.0% and 84.2%, respectively. By comparison, in the presence of 40 μg/mL oseltamivir, the cell inhibitory rate was 84.4%, which was not significantly different from that of 40 μg/mL polyphylla saponin I (P>0.01) (Table 1). When cells were pre-incubated with polyphylla saponin I prior to exposure to influenza A virus, cell inhibitory rates were substantially increased. The cell inhibitory rates at concentrations of 5 μg/mL, 10 μg/mL, 20 μg/ mL and 40 μg/mL were 71.2%, 75.2%, 77.5%, and 80.8%, respectively, which were significantly lower than the cell inhibitory rate (84%) of oseltamivir (P<0.05) (Table 1). Furthermore, polyphylla saponin I was also effective in inhibiting the proliferation of influenza A virus in MDCK cells. Cell inhibitory rates of polyphylla saponin I at concentrations of 5 μg/mL, 10 μg/mL, and 20 μg/mL were 77.3%, 82.2% and 87.3%, respectively, which were significantly lower than the cell inhibitory rate (91.7%) of oseltamivir at 40 μg/mL (P<0.05). Of note, there was no significant difference between polyphylla saponin I and oseltamivir at the concentration of 40 μg/mL (Table 1). These results suggest that polyphyllasaponin I (40 μg/mL) has similar inhibition effect on influenza A virus in MDCK cells compared with oseltamivir (40 μg/mL).
Table 1.
Effect of polyphylla saponin I on MDCK cells
| Groups | ρ/μg/mL | Direct inactivation effect (%) (t=0) | Preventative effect (%) (t=-2) | Inhibition effect (%) (t=+2) |
|---|---|---|---|---|
| Polyphylla saponin I | 5 | 62.4** | 71.2** | 77.3** |
| 10 | 68.2** | 75.2** | 82.2** | |
| 20 | 78.0* | 77.5** | 87.3* | |
| 40 | 84.2 | 80.8* | 91.4 | |
| Oseltamivir | 40 | 84.4 | 84 | 91.7 |
| Influenza A virus | - | 0 | 0 | 0 |
| Normal cells | - |
Note: Polyphylla saponin I or oseltamivir was added to MDCK cells at the time of infection by influenza A virus (t=0), 2 h prior to infection by influenza A virus (t=-2), and 2 h after infection by influenza A virus (t=+2), and incubated at 37°C in 5% CO2. When the cytopathic effect (CPE) in wells containing influenza A virus reached +++ (CPE was evident in 50-75% of MDCK cells) and the cells of the normal cell group were normal, MTT assay was used to determine A490nm. Inhibitory rate (%) = (Areagent-Ainfluenza A virus)/(Anormal cells-Ainfluenza A virus) ×100%. Data are means ± SD (n=3).
P<0.01 compared with oseltamivir group;
P<0.05 compared with oseltamivir group.
The protective effect of polyphylla saponin I on mice infected with influenza A virus
To investigate the therapeutic effect of polyphylla saponin I on mice infected with influenza A virus, we determined the mortality, mean survival time, and prolonged life rate of the mice. The results showed that activity and food intake of mice were significantly reduced, while breathing and heart rate were increased, after infection by influenza A virus on day 6. The majority of mice infected with influenza A virus died during the 14-day experiment, with the mortality being 80%. The survival time of these mice were only 8.5±0.3 days, while the survival time of normal mice were 14 days. At the same time, the number of dead mice was significantly reduced in polyphylla saponin I groups, in which the survival time of mice reached 12.7±0.4 days, being longer than those of mice in the influenza A virus infection group (P<0.01). The prolonged life rates in lower and higher dose polyphylla saponin I groups were 49.4% and 55.3%, respectively, which were significantly higher than that in oseltamivir group (42.3%) (P<0.05) (Table 2). These results indicate that polyphylla saponin I has protective effects on mice infected with influenza A virus.
Table 2.
Protection effect of polyphylla saponin I on mice infected with influenza A virus
| Groups | Mortality (n) | Survival time (days) | Prolonged life rate (%) |
|---|---|---|---|
| Normal control | 0 | 14 | 100 |
| Influenza A virus infection | 8 | 8.5±0.3 | |
| Oseltamivir (3 mg/kg) | 2 | 12.1±0.7## | 42.3 |
| Polyphylla saponin I (5 mg/kg) | 2 | 12.7±0.4## | 49.4* |
| Polyphylla saponin I (10 mg/kg) | 2 | 13.2±0.5## | 55.3** |
Note: The mice infected with influenza A virus were given polyphylla saponin I (5 and 10 mg/kg), or oseltamivir (3 mg/kg), twice a day, for 5 days. On the 14th day, the mortality and survival time of these mice were calculated. Prolonged life rate (%) = (Survival time of drug group-survival time of model control group)/survival time of model control group × 100%.
P<0.01 compared with influenza A virus infection group;
P<0.01 compared with oseltamivir group;
P<0.05 compared with oseltamivir group.
Polyphylla saponin I reduces viral hemagglutination titer of lung tissues in mice infected with influenza A virus
To study the effect of polyphylla saponin I on viral hemagglutination titer of lung tissues, viral hemagglutination titration was performed. The viral hemagglutination titer of lung tissue of mice infected with influenza A virus was 2.94±0.06, but then significantly decreased to 1.57±0.09 after treatment of polyphylla saponin I (10 mg/kg) (P<0.01) (Table 3). These data suggest that polyphylla saponin I reduces viral hemagglutination titer of lung tissues in mice infected with influenza A virus.
Table 3.
Effect of polyphylla saponin I on viral hemagglutination titer of lung tissue in mice infected with influenza A virus
| Groups | Number | Doses (mg/kg-1) | Hemagglutination titer |
|---|---|---|---|
| Normal control | 10 | - | - |
| Influenza A virus infection | 10 | - | 2.94±0.06 |
| Oseltamivir | 10 | 3 | 1.61±0.13## |
| Polyphylla saponin I | 10 | 5 | 1.69±0.02## |
| 10 | 10 | 1.57±0.09## |
Note: The mice infected with influenza A virus were given polyphylla saponin I (5 and 10 mg/kg), or oseltamivir (3 mg/kg), twice a day, for 5 days. On day 6, lungs of each mouse were isolated, and blotted up with filter paper. A portion of lung specimen was homogenized after adding normal saline at low temperature [liver weight (g)/normal saline (mL) =1/9 (w/v)] (4°C, 14000 g centrifugation for 30 min). The supernatants obtained from centrifugation were used to analyze the viral hemagglutination titer in triplicate by traces of blood coagulation test.
P<0.01 compared with influenza A virus infection group.
Polyphylla saponin I ameliorates lung tissue injury induced by influenza A virus in mice
To examine the effect of polyphylla saponin I on lung tissue injury induced by influenza A virus in mice, we performed hematoxylin and eosin staining. In contrast to normal control group (Figure 1A), mice infected with influenza A virus displayed a histopathological pattern that was characterized by inflammatory cellular infiltration, interstitial and alveolar edema and hemorrhage (Figure 1B). Meanwhile, these features were significantly attenuated inoseltamivir group and polyphylla saponin I groups, in which less thickening of alveolar wall, and infiltrative inflammatory cells were observed (Figure 1C-E). These results indicate that polyphylla saponin I ameliorates lung tissue injury induced by influenza A virus in mice.
Figure 1.
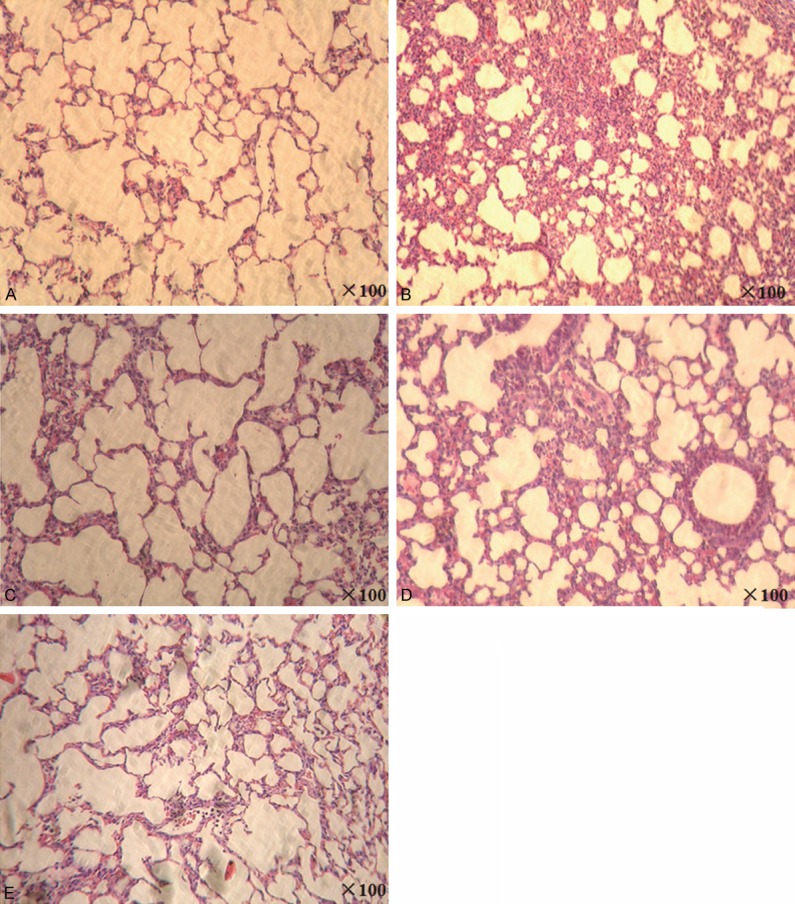
Effect of polyphylla saponin I on lung tissue injury induced by influenza a virus in mice. All mice were sacrificed at the end of the experiment. A portion of lung specimen fixed in 10% neutral-buffered formalin was embedded in paraffin, sliced into 4-μm thickness, and stained with hematoxylin and eosin. Histopathological changes were assessed and photographed under a microscope (× 100). A. Normal control group; B. Influenza A virus infection group; C. Oseltamivir group; D. Higher dose (10 mg/kg) polyphylla saponin I group; E. Lower dose (5 mg/kg) polyphylla saponin I group.
Discussion
In recent years, bird-to-human transmission of avian influenza viruses occurred in many countries, drawing more and more attentions to the prevention, control and treatment of respiratory infectious diseases [21]. Therefore, the development of anti-influenza A virus agents has become an urgent issue in epidemic-prone regions [22]. Despite success in the development of new antiviral agents such as oseltamivir in recent years, problems regarding chemotherapeutic drugs still exist, including adverse effects, emergent risk of viral resistance, and loss of efficacy due to serotype variation of viruses. Traditional Chinese medicine has played an important role in the healthcare of the Chinese people for several thousand years, and it provides unique contributions to the development of health science and medicine. The search for viral inhibitors with plant origins is a promising approach in the development of new antiviral agents. In this respect, a large number of extracts and pure substances have been tested, and some of them have been proven to have selective antiviral effects [23-25].
P. polyphylla Smith var. yunnanensis, commonly known as RhizomaParidis in China, has been documented in the “Chinese Pharmacopoeia” in 1985 for the first time. P. polyphylla has been used to treat fractures, parotitis, hemostasis, snake bites, and abscess in folk medicine for a long time. It is reported that paridissaponins are the main active components in P. polyphylla [13]. Our results showed that polyphylla saponin I could be prepared by column chromatography and reversed phase liquid chromatography separation technology. After HPLC purification, the purity of polyphylla saponin I reached 95.3%.
Oseltamivir is widely used in patients with influenza A or B infections in clinical settings all over the world [26,27]. It serves as a positive drug control in the present study. The cytotoxicity and anti-influenza A virus activity of polyphylla saponin I have been investigated by MTT assay, and the effects of polyphylla saponin I or oseltamivirupon influenza A virus-induced CPE have been observed using inverted light microscopy. The results showed that polyphylla saponin I had no cytotoxicity on MDCK cells under the concentration of 50 μg/mL (the corresponding concentration of oseltamivir was 40 μg/mL).
Anti-influenza A virus activities of polyphylla saponin I were tested with various concentrations (5, 10, 20 and 40 μg/mL) in accordance with the results of cytotoxicity tests [28]. Our initial objective is to obtain preliminary data relevant to the mechanism of action of polyphylla saponin I by exploring the effects of polyphylla saponin I addition at different time points in vitro (t=-2, 0, +2) in relation to the time of influenza A virus infection. Our MTT results showed that 40 μg/mL of polyphylla saponin I and oseltamivir had remarkable inactivation effects on influenza A virus and inhibitory effects on reproduction of influenza A virus in MDCK cells. The virus inhibitory rate of polyphylla saponin I reached 91.4% during the inhibition test of influenza A virus replication in MDCK cells, without significant difference compared with oseltamivir of the same concentrations (P>0.01).
The inhibitory effects of polyphylla saponin I added at the time of infection or 2 h after infection suggest that polyphylla saponin I could potentially be used in the inactivation and treatment of diseases caused by influenza A virus infection. Our study also indicates that polyphylla saponin I in vitro appears to be as effective as oseltamivir of the same concentration.
However, these results still need to be further proven by detailed evaluation of the antiviral effects of polyphylla saponin I on influenza A virus in vivo. Therefore, we investigated the therapeutic effect of polyphylla saponin I on mice infected with influenza A virus. The results showed that polyphylla saponin I (5 and 10 mg/kg) and oseltamivir (3 mg/kg) significantly reducedviral hemagglutination titer, improved the pathologic histology of lung tissues, and decreased the mortality of mice infected with influenza A virus. In addition, polyphylla saponin I (5 and 10 mg/kg) prolonged the survival time of mice from 8.5±0.3 days to 13.2±0.5 days (P<0.01), with the prolonged life rates being 49.4% and 55.3%, respectively. These effects might be related to anti-inflammatory and immunological enhancement by polyphylla saponin I.
In conclusion, polyphylla saponin I (5 μg/ml-40 μg/mL) exhibit clear antiviral effects on MDCK cells infected with influenza A virus, indicating that polyphylla saponin I can effectively reduce the infectivity of influenza A virus in vitro. Moreover, the antiviral effects of polyphylla saponin I are further confirmed in vivo on mice infected with influenza A virus.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81260070), The Natural Science Foundation of Gansu Province (1112RJZA021) and the Doctor Foundation Project of Lanzhou University of Technology (BS08200902).
Disclosure of conflict of interest
None.
References
- 1.Monto AS. Epidemiology of influenza. Vaccine. 2008;26(Suppl 4):D45–48. doi: 10.1016/j.vaccine.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 2.Krug RM, Aramini JM. Emerging antiviral targets for influenza A virus. Trends Pharmacol Sci. 2009;30:269–277. doi: 10.1016/j.tips.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das K, Aramini JM, Ma LC, Krug RM, Arnold E. Structures of influenza A proteins and insights into antiviral drug targets. Nat Struct Mol Biol. 2010;17:530–538. doi: 10.1038/nsmb.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du J, Cross TA, Zhou HX. Recent progress in structure-based anti-influenza drug design. Drug Discov Today. 2012;17:1111–1120. doi: 10.1016/j.drudis.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das K. Antivirals targeting influenza A virus. J Med Chem. 2012;55:6263–6277. doi: 10.1021/jm300455c. [DOI] [PubMed] [Google Scholar]
- 6.Vanderlinden E, Naesens L. Emerging antiviral strategies to interfere with influenza virus entry. Med Res Rev. 2014;34:301–339. doi: 10.1002/med.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boltz DA, Aldridge JR Jr, Webster RG, Govorkova EA. Drugs in development for influenza. Drugs. 2010;70:1349–1362. doi: 10.2165/11537960-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawai R, Kuroda K, Shibata T, Gomyou R, Osawa K, Shimizu K. Anti-influenza virus activity of Chaenomeles sinensis. J Ethnopharmacol. 2008;118:108–112. doi: 10.1016/j.jep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Ehrhardt C, Hrincius ER, Korte V, Mazur I, Droebner K, Poetter A, Dreschers S, Schmolke M, Planz O, Ludwig S. A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral Res. 2007;76:38–47. doi: 10.1016/j.antiviral.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.He J, Zhang S, Wang H, Chen C, Chen S. Advances in studies on and uses of Paris polyphylla var. yunnanensis. Acta Botanica Yunnanica. 2006;28:271–276. [Google Scholar]
- 11.Fan YM, Li YM, Cheng QM. Introduction and domestication research of Paris polyphylla var. yunnanensis. Chinese Chemistry. 2005;36:1102–1104. [Google Scholar]
- 12.Liu G, Xu Q, Wang T. The essentials of traditional Chinese herbal medicine. Beijing: Foreign Languages Press; 2003. [Google Scholar]
- 13.Yan L, Zhang Y, Gao W, Man S. China Journal of Chinese Materia Medica. 2008;33:2057. [Google Scholar]
- 14.Yin Z, Liu JH. Animal virology. Beijing: Science Press; 1997. [Google Scholar]
- 15.Yu Q, Sheng ZB, Wang CZ, Tian WH. The development of study on Hypericum perforatum. Chem Indus Forest Prod. 2000;34:22–27. [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang XY, Liu ZS, Jiang HZ, Liu LH, Lan AJ. Inhibitory effect of monomeric hypericin on human cytomegalovirus in vitor. Med J Wuhan Univ. 2006;27:303–306. [Google Scholar]
- 18.Liu XL, Zhang Y, Liu XL. Anti-coxsackievirus B3 effect of Sophora flavescens alkaloids in vitro. J Shenyang Pharma Univ. 2006;23:724–730. [Google Scholar]
- 19.Chong HT, Kamarulzaman A, Tan CT, Goh KJ, Thayaparan T, Kunjapan SR, Chew NK, Chua KB, Lam SK. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001;49:810–813. doi: 10.1002/ana.1062. [DOI] [PubMed] [Google Scholar]
- 20.Experimental technology of common virus disease. Beijing: Science Press; 1978. Sciences IoepacoCaom. [Google Scholar]
- 21.Poovorawan Y. Epidemic of avian influenza A (H7N9) virus in China. Pathog Glob Health. 2014;108:169–170. doi: 10.1179/2047772414Z.000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres E, Leiva R, Gazzarrini S, Rey-Carrizo M, Frigole-Vivas M, Moroni A, Naesens L, Vazquez S. Azapropellanes with anti-influenza a virus activity. ACS Med Chem Lett. 2014;5:831–836. doi: 10.1021/ml500108s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CT. Plants as a source of potential antiviral agents, Econ. Med Plant Res. 1991;5:167–251. [Google Scholar]
- 24.Vanden Berghe D, Vhetinck A, Van Hoof L. Plant products as potential antiviral agents. France: Bulletin de l’Institut Pasteur; 1986. [Google Scholar]
- 25.Yang CH, Tan DH, Hsu WL, Jong TT, Wen CL, Hsu SL, Chang PC. Anti-influenza virus activity of the ethanolic extract from Peperomia sui. J Ethnopharmacol. 2014;155:320–325. doi: 10.1016/j.jep.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burch J, Corbett M, Stock C, Nicholson K, Elliot AJ, Duffy S, Westwood M, Palmer S, Stewart L. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:537–545. doi: 10.1016/S1473-3099(09)70199-9. [DOI] [PubMed] [Google Scholar]
- 27.Tisoncik-Go J, Cordero KS, Rong L. Analysis of oseltamivir resistance substitutions in influenza virus glycoprotein neuraminidase using a lentivirus-based surrogate assay system. Virol Sin. 2013;28:81–91. doi: 10.1007/s12250-013-3307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YF, Chou CT, Lei HY, Liu CC, Wang SM, Yan JJ, Su IJ, Wang JR, Yeh TM, Chen SH, Yu CK. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J Virol. 2004;78:7916–7924. doi: 10.1128/JVI.78.15.7916-7924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


