Abstract
Aim: We aimed to evaluate the hemostatic effects and the clinical outcomes of preoperative and postoperative cryoceutical treatment (C-tx) following total knee arthroplasty. Patients and method: 42 patients received C-tx both preoperatively, and postoperatively. In the control group, 45 patients did not receive any C-tx. Amount of bloody drainage and verbal rating pain scores were noted. Results: We found significant difference in both the preoperative and postoperative hemoglobin levels and blood drainage (P<0.001). However, there was no significant difference in the average verbally rated pain scores (P>0.05). Conclusion: C-tx performed preoperatively and postoperatively for total knee arthroplasty is effective in decreasing perioperative and postoperative hemorrhage. However, it had no superior effect on the control of postoperative pain.
Keywords: Cryotherapy, total knee arthroplasty, pain management, postoperative hemorrhage
Introduction
It has been well documented that increased postoperative bloody drainage brings about many potential disadvantages. It’s supposed to cause more morbidity, longer stay in hospital, increased wound infection risk and higher hospitalization costs including risks of blood transfusion as well [1,2]. In order to reach satisfactory recovery following total knee arthroplasty, postoperative early mobilization and rehabilitation are extremely important [3]. Total knee arthroplasty is associated with serious postoperative blood loss even without perioperative anticoagulation [4,5]. Coagulopathies, tourniquet use, duration of surgery, postoperative bloody drainage are some of the well-known factors contributing to overall blood loss following total knee arthroplasty [6-8]. Increased postoperative bloody drainage may cause serious medical problems especially in the elderly patients, besides it’s also an economical concern due to longer stay in hospital [6]. Furthermore, blood transfusion has its potential risks such as allergic reactions, immune hemolytic reactions fever, transfusion related acute lung injury, delayed hemolytic reaction, iron overload, graft versus host disease and blood borne infections [9-18]. Cryoceutical treatment (C-tx) has become popular, and started to be used in some orthopedics clinics in recent years [19].
First generation cold therapy included gel packs and crushed ice in plastic bags, second generation included cold therapy with circulating ice water like a Cryo/Cuff (Aircast, Vista, CA, USA) or GameReady (Coolsystems Inc, Concod, CA, USA) and third generation devices which enabled computerized control of continous cold therapy (cTreatment, Waegener, Breese, Belgium). The purpose of the current study is to evaluate the hemostatic effects and the clinical outcomes of C-tx on postoperative pain and bloody drainage and to compare the results to standard cryotherapy.
Materials and methods
A prospective randomized clinical trial was performed in 92 consecutive patients undergoing cemented primary unilateral total knee arthroplasty without tourniquet control. Simple randomization [20] was performed to maintain complete randomness of each subject to a particular group. Patients who had been lost to follow-up (n: 5) were excluded from the final analysis. All of the patients included to the study had advanced gonarthrosis (Grade IV). Patients with rheumatoid arthritis, coagulopathies, neurologic problems, patients with a history of thrombophlebitis in the lower extremities and patients who had active systemic disease such as hepatitis, AIDS were not included to the study. Each case had one drain placed according to standard practice and all received 4000 IU of enoxaparin sodium (Clexane) subcutaneously every 24 hours, starting 12 hours after the operation. In order not to bring any bias to the study, all of the operations were made at the same hour of the day, particularly in the morning. All of the Patients who received c-treatment and patients who did not receive c-treatment were homogeneous with respect to age, gender and weight. Demographic features of the patients are summarized at Table 1.
Table 1.
Demographic details of the patients
| Control Group | Treatment Group | Value | p | |
|---|---|---|---|---|
| 1AgeMean ± SD | 65.36 ± 6.98 | 65.14 ± 4.06 | 0.175 | 0.861 |
| 1Mean Hospital Stay (days)Mean ± SD | 7.2 ± 1.5 | 7.4 ± 1.2 | 0.713 | 0.756 |
| 2Sexn,% | ||||
| F | 23 (51.1%) | 20 (47.6%) | 0.106 | 0.745 |
| M | 22 (48.9%) | 22 (52.4%) | ||
| 2ASAn,% | ||||
| ASA I | 15 (33.3%) | 14 (33.3%) | ||
| ASA II | 11 (24.4%) | 10 (23.8%) | 0.031 | .999 |
| ASA III | 9 (20%) | 9 (21.4%) | ||
| ASA IV | 10 (22.2%) | 9 21.4%) | ||
| 1Student t test: 2Pearson Chi-Square. | ||||
Student t test.
Pearson Chi-Square.
Preoperative and postoperative twice daily complete blood counts were noted. 45 patients did not receive any C-tx (Control Group) and 42 patients received C-tx both preoperatively and postoperatively (Treatment Group). The C-tx device is composed of a server and c-pad (Figure 1). The c-server drives the c-pad by use of the selected c-protocol. The device enables controlled-temperature modulation with cooling at a specific temperature (11°C) for a prolonged time [21]. Not to allow cold-induced vasodilatation, the device progressively increases the temperature during treatment. Compression was not adjusted as a part of the cTreatment protocol only cold therapy was perfortmed. All of the patients operated had advanced osteoarthritis of the knee. Operations were carried out by 2 orthopaedic surgeons specifically experienced in the field of arthroplasty. Patients in either treatment or control group were blinded for the other group. For the treatment group c-pads were applied to the operated knees preoperatively for 90 minutes until they were taken to the operation hall and postoperatively for 6 hours on surgery day starting just after the operation, and for 2 hours for subsequent 2 days. Control group received standard cold therapy with ice packages 8 times for 15 minutes with 45-minute intervals for the operation day and postoperative 2nd day. Control group did not take any cold therapy before the operation. All of the patients received elastic bandages for compression, same analgesic medication, diclofenac sodium 3 ml (Diclomec) twice daily and 100 mg Tramadol hydrochloride (Contramal) once, at 6 hours postoperatively.
Figure 1.
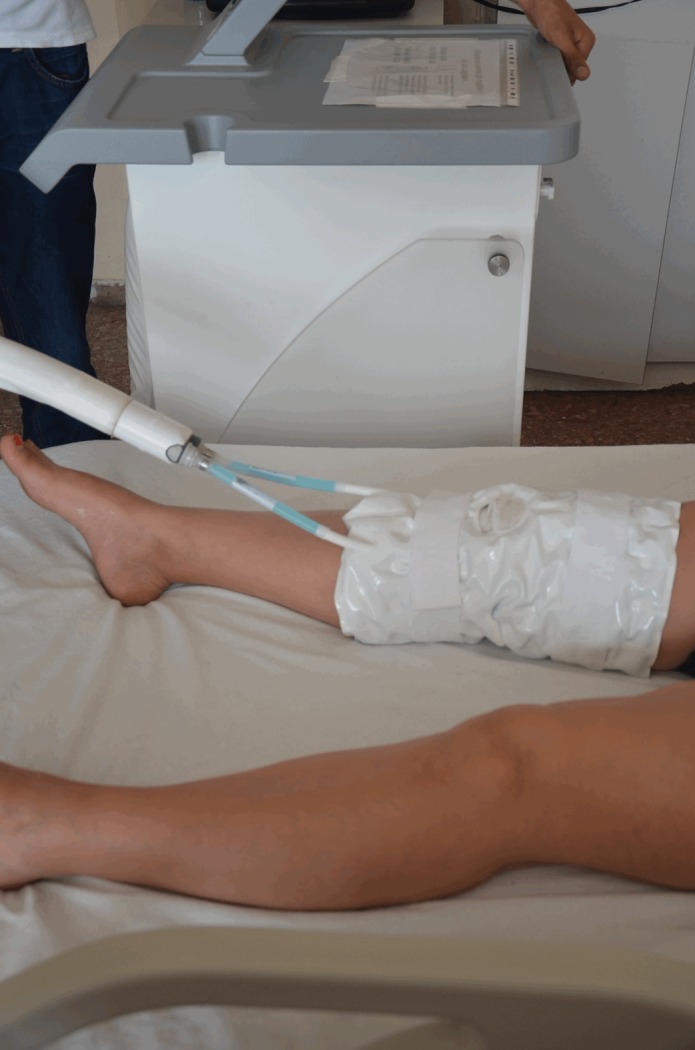
The C-tx device is composed of a server and c-pad.
Postoperative 24 hours later amount of bloody drainage and verbal rating pain scores were noted. All patients verbally rated the intensity of their knee pain using a 0 to 10 numeric scale (0: no pain, 10: worst pain). 2 patients from treatment group and 7 patients from control group received blood transfusion. Decision for blood transfusion was made by an anaesthesician who was blinded to both treatment arms; following the guidelines of the AABB (American Association of Blood Banks). For patients who did not have concomitant active bleeding or coronary syndrome, Hb levels of 8 g/dl or lower was threshold for blood transfusion [22].
Statistical analysis
During the assessment of the data obtained in the study, IBM SPSS 22 program was used for statistical analysis. During the assessment of the study data, conformity of the parameters to the normal distribution was assessed by the Kolmogorov-Smirnov test. During the evaluation of the study data, along with the descriptive statistical methods, parameters with normal distribution for the comparison of quantitative data were evaluated using Student’s t-test. The qualitative data were evaluated using Chi-square test and Fisher’s Exact test. Significance was accepted at P<0.05 level. According to the Power and Sample programme used; Δ 0.8 SD: 1.2 was accepted for postoperative Hb value, sample size was found to be 36 minimally for power value of 0.80 and α:0.05.
Results
Preoperative, postoperative 48th hour mean hemoglobin values, hemoglobin decrease percentages, and the amount of bloody drainage measured 24 hours postoperatively of the treatment and control group are shown on Table 2. We found significant difference among both groups regarding the preoperative and postoperative hemoglobin levels, amount of bloody drainage (P<0.001). Decrease in hemoglobin levels compared to preoperative values was measured in percentages not to bring any bias regarding different preoperative hemoglobin levels however we did not find any statistically significant difference in need of blood transfusion between both groups (Fischer’s exact test, P=0.091) (Table 3). Mean verbally rated pain scores were 6.1 in C-tx group, and 6.6 in control group (P> 0.05), respectively. We had 2 superficial wound infections in the treatment group and 5 superficial wound infections in the control group. Superficial wound infections were diagnosized by taking wound swabs. Superficial wound infections may be related to the application of ice packages.
Table 2.
Preoperative, postoperative 48th hour mean hemoglobin values, hemoglobin decrease percentages, and the amount of bloody drainage measured 24 hours postoperatively of the treatment and control group
| Group | Patients (N) | Mean ± SD | t | P | |
|---|---|---|---|---|---|
| Preoperative Hemoglobin (g/dl) | Control | 45 | 13.12 ± 1.30 | 1.273 | 0.207 |
| Treatment | 42 | 12.76 ± 1.32 | |||
| Postoperative Hemoglobin (g/dl) | Control | 45 | 9.91 ± 1.25 | -5.100 | <0.001 |
| Treatment | 42 | 11.22 ± 1.14 | |||
| Drop in Hemoglobin (%) | Control | 45 | 24.47 ± 5.10 | 13.258 | <0.001 |
| Treatment | 42 | 11.93 ± 3.52 | |||
| Drainage from hemovac (cc) | Control | 45 | 319.78 ± 60.66 | 8.983 | <0.001 |
| Treatment | 42 | 210.24 ± 52.43 |
Student’s t test.
Table 3.
No significant difference between both groups regarding postoperative blood transfusion
| Transfusion need | P | ||
|---|---|---|---|
|
| |||
| Hb≥8 g/dl | Hb≤8 g/dl | ||
|
| |||
| n (%) | n (%) | ||
| Control Group | 37 (82.2%) | 8 (17.8%) | .091 |
| Treatment Group | 40 (95.2%) | 2 (4.8%) | |
Fisher’s Exact Test.
Discussion
Cold therapy is frequently used after orthopedic operations and there are numerous studies to explore the effects of cold therapy [16,18,19,21,23]. C-treatment has become popular and started to be used in some orthopedics clinics in recent years. The treatment was carried out by a medical device providing automatic controlled energy exchange and a pad applied to the operation site. Potential risks of the treatment may be too cold or too long application of c-pads or applying with too much compression that may compromise skin circulation. Abnormal reactions to cold such as Reynaud phenomenon may potentially lead to tissue damage. It’s thought that patients who have marked response to cold such as cold urticeria, cryoglobulinemia, paroxysmal cold hemoglobinuria may not be suitable for c-treatment [24-28].
Hypothermia potentially leads to immunosuppression, thus cold therapy may increase risk of infection [27,31]. Adequate post-operative pain relief following total knee arthroplasty is very important to optimal postoperative recovery and earlier patient mobilization with the aim of decreasing the incidence of the development of thromboembolism; however there are many side effects of pain medication. Pain relief options for major orthopedic operations have limitations due to associated side effects that often require additional treatment. Side-effects of the opiate pain medication include lethargy, sedation, respiratory depression and, potential addiction [28-30]. The side effects of non-steroidal anti-inflammatory agents include gastric symptoms and may cause peptic ulcers [31]. Verbal rating pain scores were noted only 24 hours postoperatively, it would be better to take these scores additionally at postoperative 36 and 48 hours too. As a limitation of the study walking capacities of the patients together with range of motion values could also be additionally observed.
Any decrease in body temperatures of the patients during C-tx was not seen. We had 2 superficial wound infections however there were 5 superficial wound infections in the control group that were all successfully treated by parenteral antibiotherapy. Postoperative drainage from the wound is also a potential danger for superficial and deep infection [29]. Superficial wound infections of the treatment group were less than the control group, which may be due to vasoconstriction and decrease of drainage from the wound.
Our results demonstrate that c-treatment applied both pre and postoperatively after total knee arthroplasty significantly reduces peri and postoperative hemorrhage without any significant decrease in postoperative pain. Clinically important blood loss requiring blood transfusion is not uncommon in clinical practice [30] however we did not find any significant difference between both treatment and control groups regarding need for blood transfusion (P>0.05). Deep venous thrombosis and pulmonary embolism may follow total knee arthroplasty and may cause mortality and morbidity. Incidence of venous thrombosis and pulmonary embolus can be reduced by preoperative and postoperative anticoagulation, postoperative devices and postoperative early mobilization. Preoperative anticoagulation is effective in prevention of venous thrombosis however it may increase perioperative bleeding [31,33-36]. In one recent study, advanced cryotherapy was not found to accelerate early recovery after total knee arthroplasty and no statistically significant difference was found between c-tx group and control group in means of VAS or secondary outcome measures [34]. C-treatment performed preoperatively may be effective in decreasing perioperative hemorrhage due to vasoconstriction and let safer anticoagulation.
According to our results, c-treatment performed preoperatively and postoperatively for total knee arthroplasty is effective in decreasing perioperative and postoperative hemorrhage, however it’s not superior to classical cold therapy in case of postoperative pain control and does not significantly reduce blood transfusion.
Acknowledgements
We would like to thank Ebru Osmanoğlu Akyol PhD who is Head of Varyans Statistical Consulting in Istanbul, Turkey for her valuable contribution on the statistical analysis section in this study.
Disclosure of conflict of interest
None.
References
- 1.Ahmed I, Chan JK, Jenkins P, Brenkel I, Walmsley P. Estimating the transfusion risk following total knee arthroplasty. Orthopedics. 2012;35:e1465–71. doi: 10.3928/01477447-20120919-13. [DOI] [PubMed] [Google Scholar]
- 2.Illingworth KD, Mihalko WM, Parvizi J, Sculco T, McArthur B, Bitar YE, Saleh KJ. How to Minimize Infection and Thereby Maximize Patient Outcomes in Total Joint Arthroplasty: A Multicenter Approach: AAOS Exhibit Selection. J Bone Joint Surg Am. 2013;95:e50. doi: 10.2106/JBJS.L.00596. [DOI] [PubMed] [Google Scholar]
- 3.Villadsen A, Overgaard S, Holsgaard-Larsen A, Christensen R, Roos EM. Postoperative effects of neuromuscular exercise prior to hip or knee arthroplasty: a randomised controlled trial. Ann Rheum Dis. 2014;73:1130–7. doi: 10.1136/annrheumdis-2012-203135. [DOI] [PubMed] [Google Scholar]
- 4.Drago L, De Vecchi E, Romano’ CL, Vassena C, Banfi G. Behaviour of perioperative values of haemoglobin, haematocrit and red blood cells in elderly patients undergoing lower limb arthroplasty: a retrospective cohort study on non-transfused patients. Int J Immunopathol Pharmacol. 2013;26:427–33. doi: 10.1177/039463201302600215. [DOI] [PubMed] [Google Scholar]
- 5.Perazzo P, Viganò M, De Girolamo L, Verde F, Vinci A, Banfi G, Romagnoli S. Blood management and transfusion strategies in 600 patients undergoing total joint arthroplasty: an analysis of pre-operative autologous blood donation. Blood Transfus. 2013;11:370–6. doi: 10.2450/2013.0197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansour J, Graf K, Lafferty P. Bleeding disorders in orthopedic surgery. Orthopedics. 2012;35:1053–62. doi: 10.3928/01477447-20121120-09. [DOI] [PubMed] [Google Scholar]
- 7.Tai TW, Chang CW, Lai KA, Lin CJ, Yang CY. Effects of tourniquet use on blood loss and soft-tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94:2209–15. doi: 10.2106/JBJS.K.00813. [DOI] [PubMed] [Google Scholar]
- 8.Wang GJ, Hungerford DS, Savory CG, Rosenberg AG, Mont MA, Burks SG, Mayers SL, Spotnitz WD. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: a brief note on a randomized prospective trial. J Bone Joint Surg Am. 2001;83-A:1503–5. doi: 10.2106/00004623-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hu DJ, Kane MA, Heymann DL. Transmission of HIV, hepatitis B virus, and other bloodborne pathogens in health care settings: a review of risk factors and guidelines for prevention. World Health Organization. Bull World Health Organ. 1991;69:623–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Perlas A, Kirkham KR, Billing R, Tse C, Brull R, Gandhi R, Chan VW. The Impact of Analgesic Modality on Early Ambulation Following Total Knee Arthroplasty. Reg Anesth Pain Med. 2013;38:334–9. doi: 10.1097/AAP.0b013e318296b6a0. [DOI] [PubMed] [Google Scholar]
- 11.Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total kneearthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274–7. doi: 10.1016/j.arth.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Dowell D, Kunins HV, Farley TA. Opioid analgesics--risky drugs, not risky patients. JAMA. 2013;309:2219–20. doi: 10.1001/jama.2013.5794. [DOI] [PubMed] [Google Scholar]
- 13.Kirsh K, Peppin J, Coleman J. Characterization of prescription opioid abuse in the United States: focus on route of administration. J Pain Palliat Care Pharmacother. 2012;26:348–61. doi: 10.3109/15360288.2012.734905. [DOI] [PubMed] [Google Scholar]
- 14.Spiller HA, Gorman SE, Villalobos D, Benson BE, Ruskosky DR, Stancavage MM, Anderson DL. Prospective multicenter evaluation of tramadol exposure. J Toxicol Clin Toxicol. 1997;35:361–4. doi: 10.3109/15563659709043367. [DOI] [PubMed] [Google Scholar]
- 15.Nuki G. Pain control and the use of non-steroidal analgesic anti-inflammatory drugs. Br Med Bull. 1990;46:262–78. doi: 10.1093/oxfordjournals.bmb.a072390. [DOI] [PubMed] [Google Scholar]
- 16.Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007;41:365–9. doi: 10.1136/bjsm.2006.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer HB, Simanski CJ, Sharp C, Bonnet F, Camu F, Neugebauer EA, Rawal N, Joshi GP, Schug SA, Kehlet H PROSPECT Working Group. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63:1105–23. doi: 10.1111/j.1365-2044.2008.05565.x. [DOI] [PubMed] [Google Scholar]
- 18.Schaser KD, Stover JF, Melcher I, Lauffer A, Haas NP, Bail HJ, Stöckle U, Puhl G, Mittlmeier TW. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61:642–9. doi: 10.1097/01.ta.0000174922.08781.2f. [DOI] [PubMed] [Google Scholar]
- 19.Barry S, Wallace L, Lamb S. Cryotherapy after total knee replacement:a survey of current practice. Physiother Res Int. 2003;8:111–20. doi: 10.1002/pri.279. [DOI] [PubMed] [Google Scholar]
- 20.Altman DG, Bland JM. Statistics notes. Treatment allocation in controlled trials: why randomise? BMJ. 1999;318:1209. doi: 10.1136/bmj.318.7192.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19:334–337. doi: 10.1016/j.arth.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Int Med. 2012;157:49. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 23.Deal DN, Tipton J, Rosencrance E, Curl WW, Smith TL. Ice reduces edema. A study of microvascular permeability in rats. J Bone Joint Surg Am. 2002;84-A:1573–8. [PubMed] [Google Scholar]
- 24.Herrera E, Sandoval MC, Camargo DM, Salvini TF. Motor and sensory nerve conduction are affected differently by ice pack, ice massage, and cold water immersion. Phys Ther. 2010;90:581–91. doi: 10.2522/ptj.20090131. [DOI] [PubMed] [Google Scholar]
- 25.Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17:718–22. doi: 10.1054/arth.2002.33562. [DOI] [PubMed] [Google Scholar]
- 26.Naidu S, Baskerville PA, Goss DE, Roberts VC. Raynaud’s phenomenon and cold stress testing: a new approach. Eur J Vasc Surg. 1994;8:567–73. doi: 10.1016/s0950-821x(05)80592-4. [DOI] [PubMed] [Google Scholar]
- 27.Polderman KH. Hypothermia, immune suppression and SDD: can we have our cake and eat it? Crit Care. 2011;15:144. doi: 10.1186/cc10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–46. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 29.Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–8. doi: 10.2106/JBJS.F.00163. [DOI] [PubMed] [Google Scholar]
- 30.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement: effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am. 1991;73:1037. [PubMed] [Google Scholar]
- 31.Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689. doi: 10.1002/bjs.1800800607. [DOI] [PubMed] [Google Scholar]
- 32.Howard AW, Aaron SD. Low molecular weight heparin decreases proximal and distal deep venous thrombosis following total kneearthroplasty. A meta-analysis of randomized trials. Thromb Haemost. 1998;79:902–6. [PubMed] [Google Scholar]
- 33.Kim KI, Kang DG, Khurana SS, Lee SH, Cho YJ, Bae DK. Thromboprophylaxis for deep vein thrombosis and pulmonary embolism after total joint arthroplasty in a low incidence population. Knee Surg Relat Res. 2013;25:43–53. doi: 10.5792/ksrr.2013.25.2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumptionafter knee arthroplasty? Clin Orthop Relat Res. 2014;472:3417–23. doi: 10.1007/s11999-014-3810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosque J Jr, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228–33. doi: 10.3928/01477447-20120222-12. quiz 234-5. [DOI] [PubMed] [Google Scholar]
- 36.Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants. A Review of the Pharmacology, Dosing, and Complications. Curr Emerg Hosp Med Rep. 2013;1:83–97. doi: 10.1007/s40138-013-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


